TLR4/NF-κB信号转导通路介导吡格列酮在内脂素诱导内皮细胞炎症损伤过程中作用机制的探讨
何晓乐,刘 军,张航向,王 宁,徐 荣,杨 洁,王晓明*
TLR4/NF-κB信号转导通路介导吡格列酮在内脂素诱导内皮细胞炎症损伤过程中作用机制的探讨
何晓乐1,刘 军2,张航向1,王 宁1,徐 荣1,杨 洁3,王晓明1*
(1第四军医大学西京医院老年病科,西安 710032;2兰州军区兰州总医院骨科中心,兰州 730000;3兰州军区28分部西关干休所,西安 710000)
观察吡格列酮对内脂素诱导人脐静脉内皮细胞(HUVECs)炎症损伤过程中的影响,探讨吡格列酮改善内皮功能的信号转导机制。将HUVECs随机分组,给予不同浓度的内脂素进行诱导,运用蛋白印迹法(Western blotting)检测各组细胞Toll样受体4(TLR4)、细胞间黏附分子-1(ICAM-1)、核因子-κB(NF-κB)和NK-κB抑制蛋白α(IκB-α)的表达;再给予过氧化物酶体增殖物激活受体γ(PPARγ)激动剂吡格列酮,观察各组指标表达变化。与正常对照组相比,内脂素呈浓度依赖性地增加ICAM-1含量,下调IκB-α蛋白的表达,同时上调TLR4的表达,差异具有统计学意义(<0.05),且当内脂素浓度为1×10-5mol/L时效果最显著。而吡格列酮呈浓度依赖性地抑制内脂素所诱导的上述效应,差异具有统计学意义(<0.05),当吡格列酮浓度为20μmol/L时效果最显著。吡格列酮对内脂素诱导的HUVECs炎症损伤有保护作用,其机制可能与抑制TLR4/NF-κB信号转导通路有关。
吡格列酮;内脂素;内皮细胞;炎症;TLR4/NF-κB信号通路
Toll样受体4(Toll-like receptor 4,TLR4)是天然免疫系统的一种模式识别受体,在免疫效应细胞单核/巨噬细胞和树突状细胞中均有表达[1]。TLR4与其相应配体结合上调核因子-κB(nuclear factor-κB,NF-κB)的表达和免疫反应基因的激活,进而调控多种炎性因子、趋化因子、黏附因子等分泌,参与动脉粥样硬化(atherosclerosis,AS)的发生、发展,TLR4在斑块的稳定性破坏方面起重要作用[2]。内脂素(visfantin)是Fukuhara等[3]最新发现的由一种脂肪细胞分泌的细胞因子,研究表明该因子与炎性反应及2型糖尿病均有密切关系。在周桂建等[4]的研究中指出,冠心病患者中的血清内脂素水平显著升高,是冠心病的危险因子之一。吡格列酮(pioglitazone)作为目前临床广泛应用的经典噻唑烷二酮类(thiazolidinediones,TZDs)胰岛素增敏剂类药物,在控制血糖的同时具有改善多种心血管危险因素的作用[5],但其对AS发展前期血管内皮细胞损伤过程中的影响目前尚不清楚,TLR4/NF-κB信号通路是否参与吡格列酮对内脂素诱导的内皮细胞的损伤过程,国内外均无报道。本研究用吡格列酮干预内脂素诱导的人脐静脉内皮细胞(human umbilical vein endothelial cells,HUVECs),探讨吡格列酮改善内皮细胞炎症损伤的分子机制。
1 材料与方法
1.1 药品和试剂
HUVECs细胞株(第四军医大学免疫教研室),核因子κB抑制蛋白α(inhibitor of nuclear factor κB-α,IκB-α)抗体(江苏碧云天生物技术研究所);鼠抗兔二抗、二甲亚砜(DMSO)、胰蛋白酶(均Sigma公司,美国);DMEM低糖培养液(HycLone公司,美国);小牛血清(杭州四季青公司);吡格列酮(Cayman公司,美国)。
1.2 HUVECs的培养
HUVECs用含10%小牛血清的DMEM细胞培养液在37%、5%CO2的培养箱内培养2~3d。待细胞融合后,用0.25%胰蛋白酶进行消化。镜下观察到细胞收缩变圆时弃去消化液,加入培养液以终止胰蛋白酶的作用。用滴管吹打壁上的细胞,使其完全脱落并分离。根据实验需要按5×105个/ml接种于培养板或培养瓶中。待细胞融合后,换用无血清的DMEM培养液培养24h,使细胞同步化,然后即可进行分组实验。
1.3 细胞分组及处理
取对数生长期细胞,以每孔2×105/ml接种于多聚赖氨酸包被的96孔板中,于37℃、5% CO2培养箱中进行孵育,24h内细胞达到80%~90%融合后,用含体积分数0.01%磷酸盐缓冲液(phosphate buffer saline,PBS)的DMEM孵育细胞24h,使细胞同步化。将接种孔分为3组。(1)正常对照组:在培养液中孵育HUVECs 24h;(2)内脂素干预组:给予不同浓度的内脂素(1×10−7、l×10−6和1×10-5mol/L)后孵育24h;(3)吡格列酮干预组:给予浓度为1×10-5mol/L的内脂素预孵2h后,加入不同浓度的吡格列酮(10和20μmol/L),孵育24h。
1.4 蛋白印迹法检测HUVECs中TLR4的表达
弃去培养液,用冷PBS轻轻漂洗一遍后,随即加入细胞裂解液和蛋白酶抑制剂以裂解细胞。刮下细胞,置于Eppendorf离心管,并用破膜仪反复抽打细胞3次以剪切细胞内核酸,使蛋白充分溶出。静置于冰上20min以沉淀蛋白,再以12 000转/min、4℃离心15min。取上清液并移至另一Eppendorf离心管中。按照4∶1的体积比加入蛋白上样缓冲液,随后煮沸10min,置-20℃保存。蛋白定量按照试剂盒说明进行。蛋白上样量为20μg,12%聚丙烯酰胺凝胶电泳使用浓缩胶80V或分离胶120V电泳分离蛋白,转膜至聚偏二氟乙烯(polyvinylidene fluoride,PVDF)膜;采用5g脱脂奶粉封闭2h;加入1∶1000稀释的兔抗人TLR4多克隆抗体和内标条带一抗的TBST缓冲液孵育过夜;磷酸盐吐温缓冲液振荡洗膜10min×3次,加入1∶5000稀释的二抗,室温下孵育1h,再次洗膜。采用ECL底物化学发光显色后曝光显影,避光晾干后扫描存盘,并用电脑软件进行条带灰度值的分析。
1.5 蛋白印迹法测定HUVECs中IκB-α蛋白和细胞间黏附分子−1(intercellular cell adhesion molecule-1,ICAM-1)含量表达
收集各组细胞加入细胞裂解液提取总蛋白,并用Bradford法测定蛋白质浓度,补上样缓冲液使总量为80μl,再加入100μl溴酚蓝染液煮沸4min。然后各取10μl样品,SDS-PAGE凝胶进行电泳分离,半干法将蛋白转移至硝酸纤维素膜,封闭、洗膜,加入1∶600稀释的兔抗大鼠IκB-α、ICAM-1一抗,4℃过夜,用洗膜液漂洗后加入1∶5000稀释的二抗,摇床1h,TBST缓冲液冲洗3遍,每次5min,加入ECL荧光液显色,上机检测。用目标条带与内标条带的积分吸光度值(integrated absorbance,IA)的比值的百分率表示IκB-α和ICAM-1的表达水平。
1.6 统计学处理
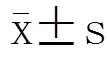
2 结 果
2.1 内脂素对HUVECs TLR4表达变化的影响
与对照组相比,内脂素干预组随着浓度(1×10-7、1×10-6和1×10-5mol/L)升高,TLR4的表达升高(<0.05),且在内脂素浓度为1×10-5mol/L时作用最为明显,TLR4的表达最高。提示内脂素呈浓度依赖性地上调TLR4的表达(图1)。

图1 内脂素对HUVECs中TLR4蛋白表达的影响
Figure 1 Effect of visfantin on TLR4 expression in HUVECs HUVECs: human umbilical vein endothelial cells; TLR4: Toll-like receptor 4. Compared with control group,*<0.05; compared with visfantin 1×10-7mol/L group,#<0.05; compared with visfantin 1×10-6mol/L group,△<0.05
2.2 内脂素对HUVECs ICAM-1表达变化的影响
与对照组相比,内脂素干预组随着浓度(1×10-7、1×10-6和1×10-5mol/L)升高,ICAM-1的表达升高(<0.05),且在内脂素浓度为1×10-5mol/L时作用最为明显,ICAM-1的表达最高。提示内脂素呈浓度依赖性地上调ICAM-1的表达(图2)。
2.3 内脂素对HUVECs IκB-α表达变化的影响
与对照组相比,内脂素干预组随着浓度(1×10-7、1×10-6和1×10-5mol/L)升高,IκB-α表达降低(<0.05),且在内脂素浓度为1×10-7mol/L时作用最为明显,IκB-α的表达最低。提示内脂素呈浓度依赖性地下调IκB-α的表达(图3)。

图2 内脂素对HUVECs中ICAM-1表达的影响
Figure 2 Effect of visfantin on ICAM-1 expression in HUVECs HUVECs: human umbilical vein endothelial cells; ICAM-1: intercellular cell adhesion molecule-1. Compared with control group,*<0.05;compared with visfantin 1×10-7mol/L group,#<0.05; compared with visfantin 1×10-6mol/L group,△<0.05
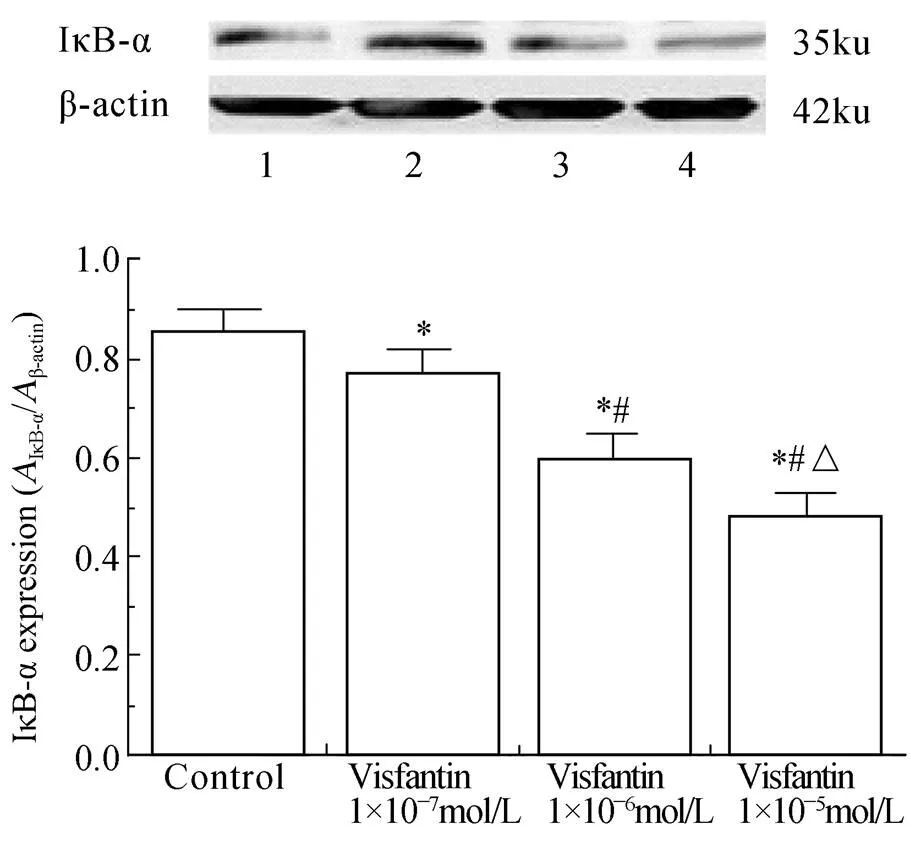
图3 内脂素对HUVECs中IκB-α表达的影响
Figure 3 Effect of visfantin on IκB-α expression in HUVECs HUVECs: human umbilical vein endothelial cells; IκB-α: inhibitor of nuclear factor κB-α. Compared with control group,*<0.05; compared with visfantin 1×10-7mol/L group,#<0.05; compared with visfantin 1×10-6mol/L group,△<0.05
2.4 吡格列酮对内脂素诱导的HUVECs TLR4表达变化的影响
与对照组比较,10μmol/L 吡格列酮干预组的HUVECs TLR4表达升高(<0.05);在20μmol/L 吡格列酮干预组中,TLR4的表达下降(<0.05),提示吡格列酮干预浓度为10μmol/L时,中和作用较为明显。但和1×10-5mol/L的内脂素干预组比较,吡格列酮干预组随着吡格列酮浓度(10和20μmol/L)升高,TLR4的表达下降(<0.05),且在吡格列酮浓度为20μmol/L时效果最为显著,提示吡格列酮呈浓度依赖性地抑制内脂素对HUVECs TLR4表达的上调作用(图4)。

图4 吡格列酮对内脂素诱导的HUVECs中TLR4表达变化的影响
Figure 4 Effect of pioglitazone on TLR4 expression in HUVECs induced by visfantin HUVECs: human umbilical vein endothelial cells; TLR4: Toll-like receptor 4. Compared with control group,*<0.05; compared with visfantin 1×10-5mol/L group,#<0.05; compared with pioglitazone 10μmol/L +visfantin 1×10-5mol/L group,△<0.05
2.5 吡格列酮对内脂素诱导的HUVECs ICAM-1表达变化的影响
与对照组比较,10μmol/L吡格列酮干预组的HUVECs ICAM-1表达升高(<0.05),在20μmol/L吡格列酮干预组中,ICAM-1的表达下降(<0.05),提示吡格列酮干预浓度为10μmol/L时,中和作用较为明显。但与1×10-5mol/L的内脂素干预组比较,吡格列酮干预组随着吡格列酮浓度(10和20μmol/L)升高,ICAM-1的表达下降(<0.05),且在吡格列酮浓度为20μmol/L时效果最为显著,提示吡格列酮呈浓度依赖性地抑制内脂素对HUVECs ICAM-1表达的上调作用(图5)。
2.6 吡格列酮对内脂素诱导的HUVECs IκB-α表达变化的影响
与对照组和1×10-5mol/L内脂素干预组比较,吡格列酮干预组随着吡格列酮浓度(10和20μmol/L)升高,IκB-α表达升高(<0.05),且在吡格列酮浓度为20μmol/L时效果最为显著,提示吡格列酮呈浓度依赖性地抑制内脂素对HUVECs IκB-α表达的下调作用(图6)。

图5 吡格列酮对内脂素诱导的HUVECs中ICAM-1表达的影响
Figure 5 Effect of pioglitazone on ICAM-1 expression in HUVECs induced by visfantin HUVECs: human umbilical vein endothelial cells; ICAM-1: intercellular cell adhesion molecule-1. Compared with control group,*<0.05;compared with visfantin 1×10-5mol/L group,#<0.05; compared with pioglitazone 10umol/L +visfantin 1×10-5mol/L group,△<0.05

图6 吡格列酮对内脂素诱导的HUVECs中IκB-α表达的影响
Figure 6 Effect of pioglitazone on IκB-α expression in HUVECs induced by visfantin HUVECs: human umbilical vein endothelial cells; IκB-α: inhibitor of nuclear factor κB-α. Compared with control group,*<0.05; compared with visfantin 1×10-5mol/L group,#<0.05; compared with pioglitazone 10μmol/L +visfantin 1×10-5mol/L group,△<0.05
3 讨 论
糖代谢障碍和高血压往往共同发生,高血压的发病率在糖尿病人群中显著增高,2型糖尿病伴高血压患者往往发展成为糖尿病并发AS、甚至加快老年多器官功能不全疾病的发展。究其本质,炎性因子的过度表达是关键基础,血管内皮细胞损伤是直接因素[6]。近年来的动物实验和临床实验均显示,肿瘤坏死因子α、白细胞介素-6等炎性因子水平可影响胰岛素受体敏感性[7],故而糖尿病患者体内炎性因子浓度也明显高于正常人,表明慢性炎症是2型糖尿病伴高血压患者的重要发病基础。不断有学者发现内脂素有多种生理功能,包括炎性反应、胰岛素抵抗等[8]。Gheibi等[9]发现,内脂素水平与炎性标志物有显著关联,可诱导C−反应蛋白、白细胞介素−1β等多种炎性因子的表达。Bułdak等[10]研究提示血清内脂素水平与冠状动脉病变程度呈正相关,说明内脂素与炎性反应密切相关。Lewis等[11]研究发现,内脂素水平随冠状动脉病变支数和Gensini积分的增加而升高,是促进斑块不稳定化中起关键作用的酶。Sato等[12]研究证实内脂素可通过激活NF-κB途径上调人单核细胞中基质金属蛋白酶(matrix metallo proteinases,MMPs)中的MMP-2和MMP-9的表达及活性。
TLR4在机体的免疫防御中起重要作用,作为联系免疫反应和炎性反应之间的桥梁,与AS的发展密切相关[13]。TLR4介导的信号转导主要通过髓样分化蛋白88依赖途径和非依赖途径产生大量的炎性因子[14]。已有研究证实,代谢综合征患者体内游离脂肪酸和脂肪炎性因子等水平上升可激活TLR4引起炎性反应,进而阻碍胰岛素信号转导,心血管疾病患者体内TLR4水平也高于正常水平[15]。对AS患者的研究显示,TLR4可影响AS相关炎性因子的合成与释放,提示TLR4很可能是AS防治的标靶[16]。NF-κB是重要的炎性介质转录因子,是由p65和p50构成的同源或异源二聚体[17]。Junker等[18]报道NF-κB参与多种炎性介质及细胞因子的基因调控。炎性因子可激活核因子B抑制蛋白激酶,使IκB-α分子磷酸化,启动一系列炎性反应相关基因的转录程序,诱导更多的炎性因子高度表达[19]。Daeian等[20]的体外研究结果提示NF-κB不仅介导TLR4的下游细胞转导,且可诱导TLR4的表达。
有报道揭示在2型糖尿病患者尤其是肥胖患者的血清中内脂素水平较高[21],我们推测其与2型糖尿病患者继发高血压甚至诱发AS相关。在本实验中,内脂素具有上调HUVECs TLR4的表达的作用,且随着浓度增加,效果愈加明显,内脂素呈浓度依赖性地下调HUVECs IκB-α表达。综上,提示内脂素对TLR4/NF-κB信号通路具有激活作用;同时我们发现内脂素参与炎性因子的调节,呈浓度依赖性地上调ICAM-1的表达。实验结果表明,内脂素通过促炎作用和激活TLR4/NF-κB信号通路参与内皮细胞的损伤过程,为患者AS发生发展的重要因素之一。
吡格列酮是胰岛素增敏剂TZDs类药物,能通过激活过氧化物酶体增殖物激活受体(peroxisome proliferators activated receptors,PPARs)而发挥其生物学作用[22],其中的受体亚型PPARγ是一个重要的细胞分化转录因子,调节糖和脂质代谢,调控血管紧张素Ⅱ的Ⅰ型受体mRNA的表达[23],在炎性表达、内皮细胞凋亡、心血管疾病的形成机制中发挥重要的作用[24]。有报道称,PPARγ受体激动剂通过诱导NO的释放对血管内皮具有保护作用[25]。本实验发现,吡格列酮可抑制内脂素对HUVECs TLR4表达的上调作用和对IκB-α表达的下调作用,并抑制内脂素对ICAM-1表达的上调作用,在浓度为20μmol/L时效果较好,提示吡格列酮呈浓度依赖性地对内脂素具有抑制作用和降低炎性表达功能,从而保护内皮细胞损伤,降低患者高血压和AS发病率。
综上所述,吡格列酮可浓度依赖性地抑制内脂素诱导的内皮细胞损伤,其作用点和抑制TLR4/NF-κB信号通路的激活和调节炎性因子表达相关,为我们今后临床治疗提供新的依据。但对血管内皮细胞的保护作用是否涉及其他通路有待于下一步的研究。
[1] Wu JF, Chen CH, Ni YH,. Toll-like receptor and hepatitis B virus clearance in chronic infected patients: a long-term prospective cohort study in Taiwan[J]. J Infect Dis, 2012, 206(5): 662−668.
[2] Barnes MA, Roychowdhury S, Nagy LE,. Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1[J]. Redox Biol, 2014, 8(2): 929−935.
[3] Fukuhara A, Matsuda M, Nishizawa M,. Visfantin: a protein secreted by visceral fat that mimics the effects of insulin[J]. Science, 2005, 307(5708): 426−430.
[4] Zhou GJ, Zhang NR, Zhang H,. Study on the relationship between serum level of visfantin and coronary heart disease[J]. Clin Med China, 2011, 27(4): 384−386. [周桂建, 张宁汝, 张 恒, 等. 内脂素与冠心病关系的探讨[J]. 中国综合临床, 2011, 27(4): 384−386.]
[5] Breckwoldt MO, Chen JW, Stangenberg L,. Tracking the inflammatory response in strokeby sensing the enzyme myeloperoxidase[J]. Proc Natl Acad Sci USA, 2008, 105(47): 18584−18589.
[6] Neumann A, Weill A, Ricordeau P,. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study[J]. Diabetologia, 2012, 55(7): 1953−1962.
[7] Zhou LY, Wang ZM, Gao YB,. Stimulation of hepatoma cell invasiveness and metastatic potential by proteins secreted from irradiated nonparenchymal cells[J]. Int J Radiat Oncol Biol Phys, 2012, 84(3): 822−828.
[8] Liu C, Gao F, Li B,. TLR4 knockout protects mice from radiation-induced thymic lymphoma by downregulation of IL-6 and miR-21[J]. Leukemia, 2011, 25(9): 1516−1519.
[9] Gheibi S, Aboutaleb N, Khaksari M,. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia[J]. J Mol Neurosci, 2014, 54(2): 264−270.
[10] Bułdak RJ, Bułdak Ł, Polaniak R,. Visfantin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: anstudy[J]. Oncol Rep, 2013, 29(2): 771−778.
[11] Lewis JD, Ferrara A, Peng T,. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study[J]. Diabetes Care, 2011, 34(4): 916−922.
[12] Sato K, Awasaki Y, Kandori H,. Suppressive effects of acid-forming diet against the tumorigenic potential of pioglitazone hydrochloride in the urinary bladder of male rats[J]. Toxicol Appl Pharmacol, 2011, 251(13): 234−244.
[13] Yang X, Chan JC. Comment: analyses using time-dependent pioglitazone usage in Cox models may lead to wrong conclusions about its association with cancer[J]. Diabetes Care, 2011, 34(8): 136−137.
[14] Shyu KG, Wang BW, Lin CM,. Cyclic stretch enhances the expression of Toll-like receptor 4 gene in cultured cardiomyocytes via p38 MAP kinase and NF-kappaB pathway[J]. J Biomed Sci, 2010, 17: 15.
[15] Carracedo-Martnez E. Comment on: prescribing of rosiglitazone and pioglitazone following safety signals analysis of trends in dispensing patterns in the Netherlands from 1998 to 2008[J]. Drug Saf, 2014, 37(12): 1069−1070.
[16] Xu H, Wu Q, Dang S,. Alteration of CXCR7 expression mediated by TLR4 promotes tumor cell proliferation and migration in human colorectal carcinoma[J]. PLoS One, 2011, 6(12): e27399.
[17] Wang S, Astsaturov IA, Bingham CA,. Effective antibody therapy induces host-protective antitumor immunity that is augmented by TLR4 agonist treatment[J]. Cancer Immunol Immunother, 2012, 61(1): 49−61.
[18] Junker Y, Zeissig S, Kim SJ,. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of Toll-like receptor 4[J]. J Exp Med, 2012, 209(13): 2395−2408.
[19] Eiró N, González-Reyes S, González L,. Duodenal expression of Toll-like receptors and interleukins are increased in both children and adult celiac patients[J]. Dig Dis Sci, 2012, 57(9): 2278−2285.
[20] Daeian N, Radfar M, Jahangard-Rafsanjani Z,. Selenium supplementation in patients undergoing hematopoietic stem cell transplantation: effects on pro-inflammatory cytokines levels[J]. Daru, 2014, 22: 51.
[21] Dkhil MA, Abdel-Baki AA, Wunderlich F,. Dietary selenium affects intestinal development ofin mice[J]. Parasitol Res, 2014, 113(1): 267−274.
[22] Gheibi S, Aboutaleb N, Khaksari M,. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia[J]. J Mol Neurosci, 2014, 54(2): 264−270.
[23] Kipp AP, Banning A, van Schothorst EM,. Marginal selenium deficiency down-regulates inflammation-related genes in splenic leukocytes of the mouse[J]. J Nutr Biochem, 2012, 23(9): 1170−1177.
[24] Burk RF, Olson GE, Hill KE,. Maternal-fetal transfer of selenium in the mouse[J]. FASEB J, 2013, 27(8): 3249−3256.
[25] Guo M, Lv T, Liu F,. Dietary selenium influences calcium release and activation of MLCK in uterine smooth muscle of rats[J]. Biol Trace Elem Res, 2013, 154(1): 127−133.
(编辑: 刘子琪)
TLR4/NF-κB signal pathway mediates pioglitazone protecting human vascular endothelial cells against visfantin-induced injury
HE Xiao-Le1, LIU Jun2, ZHANG Hang-Xiang1, WANG Ning1, XU Rong1, YANG Jie3, WANG Xiao-Ming1*
(1Department of Geriatrics, Xijing Hospital, the Fourth Military Medical University, Xi’an 710032, China;2Center of Orthopedics, Lanzhou General Hospital, Lanzhou Military Command, Lanzhou 730000, China;3Xiguan Post for Retired Cadres, the 28th Subcommand, Lanzhou Military Command, Xi’an 710000, China)
To determine the effect of pioglitazone on visfantin-induced inflammatory injury in human vascular endothelial cells and investigate the underlying signal pathway of pioglitazone in improving endothelial functions.Human umbilical vein endothelial cells (HUVECs) were treated by different concentrations of visfantin. Then Western blotting was used to detect the expression of Toll-like receptor4 (TLR4), intercellular cell adhesion molecule-1 (ICAM-1), nuclear factor-κB (NF-κB) and inhibitor of NK- κB-α (IκB-α). Their expression levels were measured again after the cells were respectively exposed to the agonist of peroxisome proliferator activated receptor gamma (PPARγ), pioglitazone.Compared with the control group, visfantin enhanced the expression of ICAM-1 in a dose-dependent manner, and also induced TLR4 up-regulation and IκB-α down-regulation (<0.05), with visfantin at dose of 1×10-5mol/L showing the strongest effect. However, pioglitazone inhibited the above effects of visfatin in a dose-dependent manner, with dose of 20 μmol/L having the maximal effect.Pioglitazone exerts protective effect on visfantin-induced inflammatory injury in human vascular endothelial cells, which may be due to its blocking TLR4/NF-κB signal pathway.
pioglitazone; visfantin; endothelial cells; inflammation; TLR4/NF-κB signal pathway
(81370927)(2013JM4009).
R741
A
10.11915/j.issn.1671-5403.2015.04.069
2015−02−03;
2015−03−12
国家自然科学基金(81370927);陕西省自然科学基金(2013JM4009)
王晓明, E-mail: xmwang@fmmu.edu.cn

