氮输入对土壤甲烷产生、氧化和传输过程的影响及其机制
胡敏杰,仝川*,邹芳芳
(1.福建师范大学湿润亚热带生态-地理过程教育部重点实验室,亚热带湿地研究中心,地理科学学院,福建 福州 350007;2.福建农林大学安溪茶学院,福建 福州 350002)
氮输入对土壤甲烷产生、氧化和传输过程的影响及其机制
胡敏杰1,仝川1*,邹芳芳2
(1.福建师范大学湿润亚热带生态-地理过程教育部重点实验室,亚热带湿地研究中心,地理科学学院,福建 福州 350007;2.福建农林大学安溪茶学院,福建 福州 350002)
随着人为氮输入的增加,外源氮成为影响土壤甲烷产生、氧化和传输过程的重要因素。土壤甲烷排放受氮素有效性的调节,氮输入会改变土壤初始环境和甲烷排放规律,最终影响甲烷排放量。综述了氮输入对土壤甲烷产生、氧化和传输过程的影响及机制。研究表明,1)氮输入对甲烷排放通量的影响存在促进、抑制和不显著3种情况,这主要是甲烷产生、氧化和传输过程的变化引起的;2)氮输入对甲烷产生过程的影响受产甲烷底物和产甲烷微生物活性的控制,氮输入通过增加土壤有机碳的含量为甲烷产生提供了丰富的底物,同时底物理化性质和植被覆盖度的变化使得这种影响复杂化,氮输入既可促进又可抑制产甲烷菌的活性,并且这种作用受氮形态的影响;3)氮输入对甲烷氧化过程的影响主要是通过刺激或抑制甲烷氧化菌的活性实现的,氮形态的不同也使得这种变化更为复杂;4)氮输入对甲烷传输过程的影响主要受植物通气组织的数量以及传输效率的控制,并且在不同生态系统这种控制作用差异较大。综上所述,氮输入对土壤甲烷产生、氧化和传输过程的影响及机制具有明显的复杂性和不确定性,今后研究中应综合考虑氮输入对甲烷排放关键过程的影响,并侧重于探讨氮输入对相关微生物群落结构、丰度和活性的影响,同时注重对各个生态系统的协同研究,确定氮输入影响下各个生态系统对全球甲烷排放的贡献率。
机制;甲烷产生;甲烷氧化;微生物;氮输入
自工业革命以来,由于化石燃料的燃烧、牲畜的饲养以及水稻的栽培,全球甲烷排放通量已经增加了2倍[1]。同时,由于甲烷吸收长波辐射的效率是CO2的20~30倍,有甲烷参与的化学过程也促进了O3和CO2等的形成[2],因此甲烷成为仅次于CO2的重要温室气体。相关研究已经证实,土壤是大气甲烷最重要的源或汇[3]。土壤中甲烷净源或汇功能主要是由厌氧环境下产甲烷菌产生的甲烷和有氧环境下甲烷氧化菌引起的甲烷损耗间的平衡决定的[4]。其中,土壤中甲烷的产生主要有2个过程:1)微生物将有机化合物水解为CO2、H2和乙酸;2)厌氧条件下,产甲烷菌以H2作为H供体还原CO2形成CH4或将乙酸脱甲基形成CH4[5-6]。而甲烷产生过程生产的甲烷有30%~90%在有氧条件下又被甲烷氧化菌氧化了[7],并且最终排放通量还受传输过程的调节。因此,土壤甲烷排放是一个复杂的生物地球化学过程,净甲烷通量是甲烷产生、氧化和传输过程综合作用的结果[8]。
人类活动(如化石燃料的燃烧、氮肥施用)以及生物固氮作用等输入和积累的氮素随地表径流、干湿沉降等多种途径进入生态系统,已经导致生态系统外源氮输入的增加[9-10]。氮素是控制土壤生物反应最重要的因子[11]。氮输入会引起生态系统服务功能的变化,如改变了群落结构,使水生生态系统富营养化等[12]。越来越多的证据表明,生态系统氮输入改变了土壤微生物和植被的生理机能,直接影响温室气体的生产与消耗进程[13]。氮输入对土壤甲烷排放的影响可能有2个方面:1)以NH4+形式富集的氮可以通过土壤好氧微生物减少甲烷的消耗[14];2)氮输入通过增加产甲烷微生物所需有机碳的供给,可以提高甲烷的产生,但氮输入也可以减缓产甲烷微生物的活性,最终降低或增加甲烷排放。由此可知,氮输入对甲烷产生与氧化等生物化学过程的影响极其复杂,其方向和大小受生态系统类型、输入氮的化学形态以及环境条件等的影响[15]。虽然氮输入背景下,甲烷源/汇功能及其通量变化等已成为全球变化研究中的重要一环[16],但其过程和机理还有许多不明晰之处。因此,全面梳理氮输入对土壤甲烷排放关键过程的影响及机制,有助于了解甲烷排放通量对外源氮输入的响应,可为准确估算全球甲烷排放,减少人类活动对气候的影响提供策略支持。
1 氮输入对甲烷排放通量的影响
目前关于氮输入对甲烷产生过程的影响存在促进、抑制和影响不显著3种情况(表1),这可能与输入氮的形态、浓度以及土壤特性等的不同有关。Yao等[17]在水稻田的研究发现,输入尿素降低了甲烷排放,而Zhang等[18]在相邻区域水稻田的氮肥添加实验则发现,甲烷排放通量随氮肥输入量的增加而升高。Liu和Greaver[15]运用Meta-analysis方法研究也发现,对草地、湿地、厌氧的农业系统平均而言,氮输入30~400 kg N/(hm2·a),使甲烷排放通量显著增加了95%。但Whalen和Reeburgh[19]的研究却发现,森林土壤施氮后,其甲烷排放并没有显著变化,他们认为这可能是森林土壤氮含量并未达到饱和。可见,外源氮输入对甲烷排放具有复杂性和不确定性,这可能是甲烷产生、氧化和传输过程综合影响的结果。
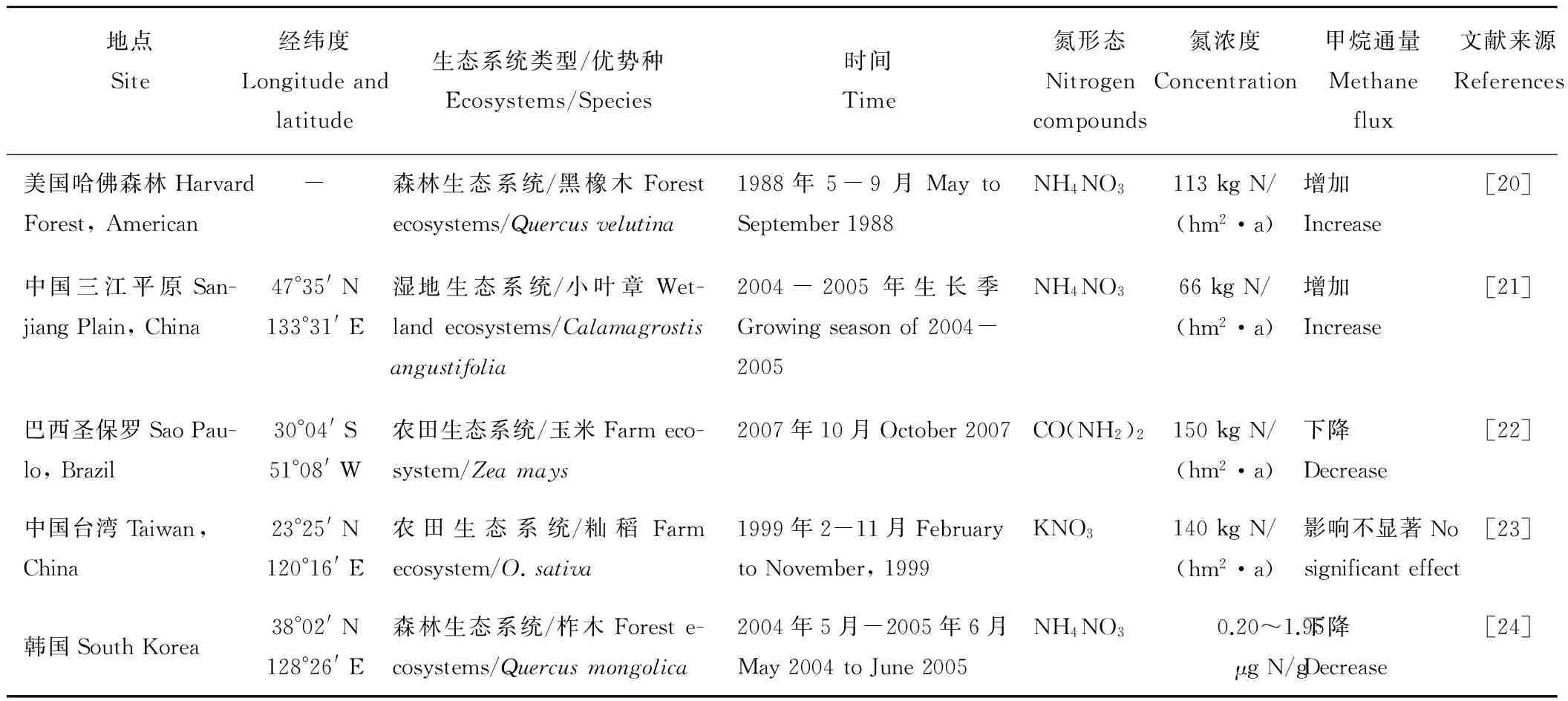
表1 氮输入对土壤甲烷排放通量影响
2 氮输入对甲烷产生的影响及机理
2.1 土壤产甲烷底物对甲烷产生的影响
甲烷产生是甲烷排放的先决条件。土壤中甲烷的产生是在厌氧条件下由产甲烷菌作用于产甲烷底物的产物,有机底物是产甲烷菌唯一的C源和能量来源。底物量的丰富程度直接决定了土壤微生物和酶的活性以及功能的发挥。土壤中产甲烷底物一般由土壤中固有的有机物质以及生物残留或由外源输入的有机物质构成[25]。大多数研究认为,外源氮输入增加了土壤中氮素的有效性,这就相应地提高了植被的生产力和产甲烷菌所需有机底物的有效性[26],使得产甲烷微生物具有更多可利用的底物,促进甲烷的产生。如Zhang等[27]对泥炭沼泽的研究发现,大气氮沉降增加了泥炭土壤氮的有效性,这会提高初级生产力和矿化速率,从而促进甲烷等温室气体的排放。Darby和Turner[28]也认为氮输入增加了地上和地下生物量,相应地输入到土壤中的植被枯落物增加,提高了土壤中的有机碳含量。一般而言,具有较高初始C∶N的底物,其分解速率会随着氮的增加而增高,在微生物作用下形成有机物质,从而为甲烷产生提供更多的有机底物[29]。Aronson和Helliker[14]对非湿地(non-wetland)土壤的研究也发现,高氮处理下净甲烷排放率明显增加,他们认为这是因为高氮输入刺激了许多微生物过程,提高了分解速率,间接地为甲烷产生提供所需底物。氮输入引起的土壤有机质本身、新鲜的植物枯落物以及根系分泌物等的增加,也为土壤甲烷产生提供了丰富的底物[30]。此外,短期的氮输入刺激了土壤微生物的活性,导致根系分泌物的快速分解,也促进了土壤有机质的分解。在受氮限制环境中,氮输入还可以通过减轻氮的限制作用,刺激土壤有机底物的产生[31]。但是也有研究认为,氮输入虽然可以通过C底物供应的增加[32]而促进了土壤微生物活性,但也可通过增加土壤毒害作用而抑制了微生物活性[33]。Pregitzer等[34]发现,长期的氮输入增加了植被的死亡率,他们认为这可能是因为氮输入增加了土壤酸性。同时,氮输入对底物的影响还与植被覆盖率有关。Granberg等[35]在一个贫瘠的瑞典沼泽地研究发现,氮输入增加了莎草(Cyperusrotundus)的覆盖率,但减少了甲烷的排放。这可能表明在莎草丰富的泥炭土壤中,根的分布和C的分配可能改变了,这间接地减少了产甲烷菌的底物有效性,降低了莎草控制甲烷释放的能力。此外,在硝态氮还原过程中,其底物利用热量的效率明显优于甲烷产生过程,导致产甲烷底物浓度下降到产甲烷菌无法利用的程度,抑制了甲烷的生成[36]。
2.2 土壤产甲烷微生物活性变化对甲烷产生的影响
甲烷是在土壤中由产甲烷微生物通过有机分解而生产。氮输入对土壤产甲烷微生物活性的影响较为复杂,一般认为有促进和抑制两种作用[37]。Bodelier等[38]认为,硝酸盐减少了产甲烷作用,因为当微生物在厌氧环境下氧化有机底物(如乙酸)时,可以使用硝酸盐作为电子受体,而产甲烷菌无法与这些硝酸盐还原剂竞争底物,使得甲烷产生减少。由于氮输入促进了生态系统的新陈代谢过程,微生物随着新陈代谢作用获得了更多的能量,这也将减缓产甲烷微生物的活性[39]。但Siciliano等[40]则认为,甲烷的产生主要取决于产甲烷菌的活性,在氮富集环境下,更高的枯落物输入减缓了C对微生物的限制作用,提高了产甲烷菌的活性,从而产生了更多的甲烷。不同形态氮的影响也是不一致的,如(NH4)2SO4添加下甲烷的排放就明显低于尿素,这是因为SO42-作为有机质氧化的电子受体,通过与产甲烷菌竞争产甲烷底物而抑制甲烷的产生[41]。而NaNO3对排放的影响取决于硝酸盐含量的多少,当NO3-含量高时会抑制甲烷的产生,反之则促进了甲烷的产生。也有研究认为,氮输入引起的土壤产甲烷菌pH值的变化以及H2S的毒害作用等也抑制了产甲烷菌的活性,减少了甲烷的产生[42]。
3 氮输入对甲烷氧化的影响及机理
3.1 不同氮形态对甲烷氧化的影响及机理
输入氮化学形态和水平的不同对甲烷氧化过程的影响是不同的[43]。甲烷氧化菌在低渗透压下具有最佳的氧化活性,氮肥(如KNO3,NH4Cl,NH4NO3)通过增加渗透压,已经展示对甲烷氧化菌活性的抑制作用[44]。Crill等[45]通过不同氮肥的施加实验发现,NH4Cl对甲烷氧化的抑制作用明显高于KNO3,这是因为NH4+是甲烷氧化的竞争性抑制剂,通过与甲烷单氧酶的竞争,减少了甲烷的氧化。而尿素的抑制作用更弱,这主要是因为输入的尿素需要经过土壤微生物的分解后才能缓慢释放氮素。铵盐对甲烷氧化的抑制作用在多种土壤类型中都得到证实[46-47],这种抑制机制非常复杂,不仅包括由NH4+引起的甲烷单氧酶、氨氧化菌以及甲烷氧化菌间的竞争性抑制作用,也包括由NH4+氧化引起的羟胺和亚硝酸盐间的非竞争性抑制作用。此外,由于甲烷单氧酶对底物的竞争以及铵盐氧化产生的亚硝酸盐的毒害作用也抑制了甲烷的氧化[48],各离子的毒害作用依次为[49]:NH4+ 3.2 土壤甲烷氧化微生物活性变化对甲烷氧化的影响 在有氧土壤表层,好氧的甲烷氧化菌能够消耗超过90%的产生于深层厌氧层的甲烷[14]。甲烷氧化菌的活性既能被氮激活,又可被氮抑制。根据生理学、形态学特征,可将甲烷氧化菌分为两类,即Ⅰ型(Type Ⅰ)甲烷氧化菌和Ⅱ型(Type Ⅱ)甲烷氧化菌[55]。研究发现,氮输入刺激了滨海湿地土壤甲烷的氧化,因为此处甲烷氧化菌群落主要以Ⅰ型甲烷氧化菌为主导[56]。相反,氮输入抑制了森林[57]和农业[58]土壤Ⅱ型甲烷氧化菌活性。氮对不同类型甲烷氧化菌的抑制/促进作用是因为不同类型甲烷氧化菌间的竞争,在氮富集条件下Ⅱ型甲烷氧化菌固定分子态氮的能力更强,从而降低了它们对铵盐和硝酸盐的需求,导致Ⅰ型甲烷氧化菌在竞争中占有优势[59]。不同环境下,Ⅰ型和Ⅱ型甲烷氧化菌所起作用也是不一样的,Ⅰ型甲烷氧化菌喜好相对稳定的环境,而Ⅱ型甲烷氧化菌则常产生于波动强烈的环境下(如水稻土)[60]。大多数研究认为,氮输入抑制了甲烷的氧化活性。Gupta等[61]研究发现,在湿地土壤中专性好氧型甲烷氧化菌可以使用分子氧将甲烷氧化成CO2和细胞碳,在土壤表层和植物氧释放的根际这些微生物最为活跃,从而限制甚至抑制甲烷氧化菌活性。Shukla 等[62]认为,铵盐对甲烷氧化的抑制作用在一定程度上可以解释为是离子或盐的影响,阳离子的添加引起的土壤氨的生理盐胁迫和离子交换都可能引起土壤甲烷氧化的下降,而氨氧化代谢产生的硝酸盐和亚硝酸盐对产甲烷菌的毒害作用是其他可能的原因。由于好氧甲烷氧化的控制与氧气和甲烷有关,因此最大的甲烷氧化率往往发生在最适宜甲烷氧化菌生存的地方[63]。此外,由于甲烷和氨具有相似的基因结构,当土壤中具有丰富的铵态氮时,甲烷氧化菌的氧化底物会从甲烷转换为氨气[64]。但这种转换仅仅发生在氨气浓度显著高于甲烷的土壤中[65]。 相反,氮输入也会促进甲烷氧化。由于硝化细菌同样可以氧化消耗甲烷,氮输入刺激了硝化细菌的生长,从而促进了土壤对甲烷的氧化吸收[66]。也有研究显示,氮输入没有显著影响滨海湿地甲烷氧化能力和甲烷通量,这是因为虽然氮输入影响了甲烷氧化菌群落结构,但并没有干扰甲烷氧化菌群落的整体活性。关于甲烷氧化菌对N的耐受性在其他土壤类型中也得到相似的结论[67]。较高的甲烷有效性也会抵消由铵盐以及其他物理化学和生物机制(pH的变化、土壤水势、离子吸附、中间体的毒害作用等)引起的对甲烷氧化的竞争抑制作用[68]。氮输入时间的长短也影响了甲烷氧化作用,因为自然状态下氮素首先输入到土壤表层,由于受土层的阻隔,氮输入初期对土壤吸收甲烷的影响较弱。此外,分类结构和微生物群落活性的变化引起的甲烷氧化和氮周转过程的变化是其他可能的原因[69]。 在评估氮输入对甲烷排放的影响时,具有通气组织的植物(如维管束植物)对甲烷排放的影响是研究的重点。已有研究显示,土壤或植物根部产生的甲烷有很大部分是通过植物体传输到大气中的,其传输量约占甲烷传输总量的50%~90%[70]。虽然植物传输通常是让土壤中的甲烷通过体内的通气组织进入大气,但不同类型植物采取的甲烷传输机制是不同的,主要有对流传输和扩散传输两种。一般而言,对流传输机制植物的甲烷输送效率要高于扩散传输机制的植物[71]。植物通气组织的数量以及传输效率显著影响着甲烷的排放。Saarnio和Silvola[72]在泥炭沼泽的研究发现,最高的甲烷排放一般是与维管束植物有关,因为维管束植物拥有发达的通气组织,甲烷分子通过植物的通气组织传输到大气中,可避免土壤甲烷氧化微生物的氧化作用。但发达的通气组织也可以在植物根部形成好氧区域,大气中的氧气通过植株的通气组织进入根部,从而加速甲烷的氧化[73]。由此可见,植物体对甲烷的传输过程具有复杂性和不确定性。一般而言,植物体传输甲烷的效率主要与温度[74-75]、湿度[76]、通气组织[77]以及生长期[78]等有关,湿度越低,温度、风速越高,通气组织越多都将提高植物体传输甲烷的能力。土壤甲烷排放也与植被物种构成有关。Bubier[79]研究了苔藓植物物种与甲烷通量间的关系发现,Sphagnumfuscum表层的甲烷通量低于S.angustifolium占优势的区域,而Myliaanomala覆被的区域其甲烷通量更高。Tong等[80]对闽江河口湿地的研究也表明,单株植物甲烷传输能力是不同的。 一般而言,氮输入促进了植物的生长,相应地也增加了植物的通气组织数量[81],这种影响在湿地生态系统中表现得尤为明显[82]。维管束植物在将C分配到产甲烷区中也有重要作用[83]。由此可见,植物群落与甲烷排放间存在密切的关系,氮输入通过促进植物生产力和生物量,影响了甲烷的排放。通常湿地植物都具有发达的通气组织,而干旱及营养元素贫瘠的区域具有通气组织的植物较少,这就使得湿地生态系统成为甲烷的重要排放源。此外,植物的生理活动也影响了底物质量和氧化剂数量等调控甲烷氧化的因素,控制甲烷排放。 甲烷排放是甲烷产生、氧化和传输过程的最终表现,外源氮的输入使得这些过程更加复杂、多变。综上所述,国内外相关学者已就氮输入对甲烷产生、氧化和传输过程及其机理进行了较为深入的研究,对甲烷产生与氧化的微生物过程也有了初步的描述,但在机理的探究上还存在许多不确定性,目前研究中尚存在以下问题和不足: 1)关于氮输入对甲烷排放的影响因素和机理的探究尚显不足,尤其是氮输入对土壤微生物以及酶的活性等方面的研究较少,难以从根源解释相关问题。同时,关于氮与其他因素之间的耦合研究也较欠缺。 2)现有研究多为短期的、小区域的研究,缺乏长期、连续以及多区域协同研究,难以保证数据的准确性、连续性和可比性。现有研究多局限于单一生态系统的研究,缺乏多生态系统间的综合研究,导致研究结论缺乏普适性。此外,目前关于氮输入对甲烷排放影响的争议在很大程度上源于测定方法的差异,缺乏标准的测定方法和体系。 3)氮输入对土壤甲烷排放通量的影响,势必会对全球变暖潜力产生促进作用,但目前相关组织和机构对全球变暖的评估和估算中却没有着重考虑这一因素,这不利于对全球变暖的控制和调节。 为此,未来可在以下几方面进行重点研究和探讨: 1)加强对甲烷产生与氧化过程中微生物机制的研究,探讨氮输入对相关微生物群落结构、丰度和活性的影响,明确微生物活动在甲烷产生与氧化过程中的地位与作用。 2)加强对多生态系统的整合研究,综合研究氮输入对湿地、森林和草地等生态系统甲烷排放通量及其关键过程的影响,为全面估算氮输入对全球甲烷贡献率提供参考和数据支持。 3)改进研究方法,建立标准体系,注重室内培养与野外原位实验的结合,并利用最新手段(如稳定同位素技术、高通量测序等)和机理模型,明晰氮输入对甲烷产生与氧化过程中促进/抑制作用的临界值,以及甲烷产生与氧化过程对甲烷排放通量变化的贡献,为全球甲烷减排提供依据。 [1] Dlugokencky E J, Walter B P, Masarie K A,etal. Measurements of an anomalous global methane increase during 1998. Geophysical Research Letters, 2001, 28(3): 499-502. [2] Smith K R, Desai M A, Rogers J V,etal. Joint CO2and CH4accountability for global warming. Proceedings of the National Academy of Sciences, 2013, 110(31): 2865-2874. [3] Hergoualc’h K A, Verchot L V. Changes in soil CH4fluxes from the conversion of tropical peat swamp forests: a meta-analysis. Journal of Integrative Environmental Sciences, 2012, 9(2): 93-101. [4] Bodelier P L E, Laanbroek H J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiology Ecology, 2004, 47(3): 265-277. [5] Thauer R K, Kaster A K, Seedorf H,etal. Methanogenic archaea: ecologically relevant differences in energy conservation. Nature Reviews Microbiology, 2008, 6(8): 579-591. [6] Bhullar G S, Iravani M, Edwards P J,etal. Methane transport and emissions from soil as affected by water table and vascular plants. BMC Ecology, 2013, 13(1): 1-9. [7] Bosse U, Frenzel P. Activity and distribution of methane-oxidizing bacteria in flooded rice soil microcosms and in rice plants (Oryzasativa). Applied and Environmental Microbiology, 1997, 63(4): 1199-1207. [8] Sun W L, Sun Z G, Sun W G,etal. The methane oxidation potential of soils intidal marshes of the Yellow River Estuary and its responses to import of organic matter. Acta Prataculturae Sinica, 2014, 23(1): 104-112. [9] Ding W X, Cai Z C. Methane emission from mires and its influencing factors. Scientia Geographica Sinica, 2002, 22(5): 619-625. [10] Galloway J N, Townsend A R, Erisman J W,etal.Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science, 2008, 320: 889-892. [11] Zhang L H, Song C C, Zheng X H,etal. Effects of nitrogen on the ecosystem respiration, CH4and N2O emissions to the atmosphere from the freshwater marshes in northeast China. Environmental Geology, 2007, 52(3): 529-539. [12] Lu M, Zhou X, Luo Y,etal. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agriculture, Ecosystems & Environment, 2011, 140(1): 234-244. [13] Mosier A R, Halvorson A D, Reule C A,etal. Net global warming potential and greenhouse gas intensity in irrigated cropping systems in northeastern Colorado. Journal of Environmental Quality, 2006, 35(4): 1584-1598. [14] Aronson E L, Helliker B R. Methane flux in non-wetland soils in response to nitrogen addition: a meta-analysis. Ecology, 2010, 91(11): 3242-3251. [15] Liu L, Greaver T L. A review of nitrogen enrichment effects on three biogenic GHGs: the CO2sink may be largely offset by stimulated N2O and CH4emission. Ecology Letters, 2009, 12(10): 1103-1117. [16] Krause K, Niklaus P A, Schleppi P. Soil-atmosphere fluxes of the greenhouse gases CO2, CH4and N2O in a mountain spruce forest subjected to long-term N addition and to tree girdling. Agricultural and Forest Meteorology, 2013, 181: 61-68. [17] Yao Z S, Zheng X H, Dong H B,etal. A 3-year record of N2O and CH4emissions from a sandy loam paddy during rice seasons as affected by different nitrogen application rates. Agriculture, Ecosystems & Environment, 2012, 152: 1-9. [18] Zhang L, Jacob D J, Knipping E M,etal. Nitrogen deposition to the United States: distribution, sources, and processes. Atmospheric Chemistry and Physics Discussions, 2012, 12(1): 241-282. [19] Whalen S C, Reeburgh W S. Effect of nitrogen fertilization on atmospheric methane oxidation in boreal forest soils. Chemosphere-Global Change Science, 2000, 2(2): 151-155. [20] Gulledge J, Hrywna Y, Cavanaugh C,etal. Effects of long-term nitrogen fertilization on the uptake kinetics of atmospheric methane in temperate forest soils. FEMS Microbiology Ecology, 2004, 49(3): 389-400. [21] Zhang L H, Song C C, Nkrumah P N. Responses of ecosystem carbon dioxide exchange to nitrogen addition in a freshwater marshland in Sanjiang Plain, Northeast China. Environmental Pollution, 2013, 180: 55-62. [22] Zanatta J A, Bayer C, Vieira F C B,etal. Nitrous oxide and methane fluxes in South Brazilian Gleysol as affected by nitrogen fertilizers. Revista Brasileira de Ciência do Solo, 2010, 34(5): 1653-1665. [23] Liou R M, Huang S N, Lin C W. Methane emission from fields with differences in nitrogen fertilizers and rice varieties in Taiwan paddy soils. Chemosphere, 2003, 50(2): 237-246. [24] Jang I, Lee S, Zoh K D,etal. Methane concentrations and methanotrophic community structure influence the response of soil methane oxidation to nitrogen content in a temperate forest. Soil Biology and Biochemistry, 2011, 43(3): 620-627. [25] Liu D Y, Ding W X, Yuan J J,etal. Substrate and/or substrate-driven changes in the abundance of methanogenic archaea cause seasonal variation of methane production potential in species-specific freshwater wetlands. Applied Microbiology and Biotechnology, 2014, 98(10): 4711-4721. [26] Nykänen H, Vasander H, Huttunen J T,etal. Effect of experimental nitrogen load on methane and nitrous oxide fluxes on ombrotrophic boreal peatland. Plant and Soil, 2002, 242(1): 147-155. [27] Zhang L H, Song C C, Wang D X,etal. Effects of exogenous nitrogen on freshwater marsh plant growth and N2O fluxes in Sanjiang Plain, Northeast China. Atmospheric Environment, 2007, 41(5): 1080-1090. [28] Darby F A, Turner R E. Effects of eutrophication on salt marsh root and rhizome biomass accumulation. Marine Ecology Progress Series, 2008, 363: 63-70. [29] Kong Y H, Nagano H, Kátai J,etal. CO2, N2O and CH4production/consumption potentials of soils under different land-use types in central Japan and eastern Hungary. Soil Science and Plant Nutrition, 2013, 59(3): 455-462. [30] Silvola J, Saarnio S, Foot J,etal. Effects of elevated CO2and N deposition on CH4emissions from European mires. Global Biogeochemical Cycles, 2003, 17(2):1-37. [31] Wu L Q, Ma K, Li Q,etal. Composition of archaeal community in a paddy field as affected by rice cultivar and N fertilizer. Microbial Ecology, 2009, 58(4): 819-826. [32] Cao C C, Qi Y C, Dong Y S,etal. Effects of nitrogen deposition on critical fractions of soil organic in terrestrial ecosystems.Acta Prataculturae Sinica, 2014, 23(2):323-332. [33] Treseder K K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies.Ecology Letters, 2008, 11(10): 1111-1120. [34] Pregitzer K S, Burton A J, Zak D R,etal. Simulated chronic nitrogen deposition increases carbon storage in Northern Temperate forests. Global Change Biology, 2008, 14(1): 142-153. [35] Granberg G, Sundh I, Svensson B H,etal. Effects of temperature, and nitrogen and sulfur deposition, on methane emission from a boreal mire. Ecology, 2001, 82(7): 1982-1998. [36] Patra A K, Yu Z T. Combinations of nitrate, saponin, and sulfate additively reduce methane production by rumen cultures in vitro while not adversely affecting feed digestion, fermentation or microbial communities. Bioresource Technology, 2014, 155: 129-135. [37] Fang H J, Cheng S L, Yu G R,etal. Microbial mechanisms responsible for the effects of atmospheric nitrogen deposition on methane uptake and nitrous oxide emission in forest soils: a review. Acta Ecologica Sinica, 2014, 34(17): 4799-4806. [38] Bodelier P L E, Roslev P, Henckel T,etal. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature, 2000, 403: 421-424. [39] Templer P H, Pinder R W, Goodale C L. Effects of nitrogen deposition on greenhouse-gas fluxes for forests and grasslands of North America. Frontiers in Ecology and the Environment, 2012, 10(10): 547-553. [40] Siciliano A, Ruggiero C, De Rosa S. A new integrated treatment for the reduction of organic and nitrogen loads in methanogenic landfill leachates. Process Safety and Environmental Protection, 2013, 91(4): 311-320. [41] Cai Z C, Xing G X, Yan X Y,etal. Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant and Soil, 1997, 196(1): 7-14. [42] Banger K, Tian H, Lu C. Do nitrogen fertilizers stimulate or inhibit methane emissions from rice fields. Global Change Biology, 2012, 18(10): 3259-3267. [43] Ding W X, Cai Z C. Effect of nitrogen fertilizers on methane oxidation in soils by methanotrophs. Chinese Journal of Eco-Agriculture, 2003, 11(2): 50-53. [44] King G M, Schnell S. Effects of ammonium and non-ammonium salt additions on methane oxidation byMethylosinustrichosporiumOB3b and Maine forest soils. Applied and Environmental Microbiology, 1998, 64(1): 253-257. [45] Crill P M, Martikainen P J, Nykanen H,etal. Temperature and N fertilization effects on methane oxidation in a drained peatland soil. Soil Biology and Biochemistry, 1994, 26(10): 1331-1339. [46] Van der Nat F, De Brouwer J, Middelburg J J,etal. Spatial distribution and inhibition by ammonium of methane oxidation in intertidal freshwater marshes. Applied and Environmental Microbiology, 1997, 63(12): 4734-4740. [47] Zhu G, Jetten M S M, Kuschk P,etal. Potential roles of anaerobic ammonium and methane oxidation in the nitrogen cycle of wetland ecosystems. Applied Microbiology and Biotechnology, 2010, 86(4): 1043-1055. [48] Veillette M, Viens P, Ramirez A A,etal. Effect of ammonium concentration on microbial population and performance of a biofilter treating air polluted with methane. Chemical Engineering Journal, 2011, 171(3): 1114-1123. [49] Ettwig K F, Butler M K, Le Paslier D,etal. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature, 2010, 464: 543-548. [50] He P J, Yang N, Fang W J,etal. Interaction and independence on methane oxidation of landfill cover soil among three impact factors: water, oxygen and ammonium. Frontiers of Environmental Science & Engineering in China, 2011, 5(2): 175-185. [51] Xu X, Inubushi K. Responses of ethylene and methane consumption to temperature and pH in temperate volcanic forest soils. European Journal of Soil Science, 2009, 60(4): 489-498. [52] Aronson E L, Dubinsky E A, Helliker B R. Effects of nitrogen addition on soil microbial diversity and methane cycling capacity depend on drainage conditions in a pine forest soil. Soil Biology and Biochemistry, 2013, 62: 119-128. [53] Bodelier P L E, Hahn A P, Arth I R,etal. Effects of ammonium-based fertilization on microbial processes involved in methane emission from soils planted with rice. Biogeochemistry, 2000, 51(3): 225-257. [54] Krüger M, Eller G, Conrad R,etal. Seasonal variation in pathways of CH4production and in CH4oxidation in rice fields determined by stable carbon isotopes and specific inhibitors. Global Change Biology, 2002, 8(3): 265-280. [55] Semrau J D, DiSpirito A A, Yoon S. Methanotrophs and copper. FEMS Microbiology Reviews, 2010, 34(4): 496-531. [56] Siljanen H M P, Saari A, Krause S,etal. Hydrology is reflected in the functioning and community composition of methanotrophs in the littoral wetland of a boreal lake. FEMS Microbiology Ecology, 2011, 75(3): 430-445. [57] Mohanty S R, Bodelier P L E, Floris V,etal. Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest soils. Applied and Environmental Microbiology, 2006, 72(2): 1346-1354. [58] Cébron A, Bodrossy L, Stralis-Pavese N,etal. Nutrient amendments in soil DNA stable isotope probing experiments reduce the observed methanotroph diversity. Applied and Environmental Microbiology, 2007, 73(3): 798-807. [59] Krause S, Meima-Franke M, Hefting M M,etal. Spatial patterns of methanotrophic communities along a hydrological gradient in a riparian wetland. FEMS Microbiology Ecology, 2013, 86(1): 59-70. [60] Stapleton L M, Crout N M J, Säwström C,etal. Microbial carbon dynamics in nitrogen amended Arctic tundra soil: measurement and model testing. Soil Biology and Biochemistry, 2005, 37(11): 2088-2098. [61] Gupta V, Smemo K A, Yavitt J B,etal. Stable isotopes reveal widespread anaerobic methane oxidation across latitude and peatland type. Environmental Science & Technology, 2013, 47(15): 8273-8279. [62] Shukla P N, Pandey K D, Mishra V K. Environmental determinants of soil methane oxidation and methanotrophs. Critical Reviews in Environmental Science and Technology, 2013, 43(18): 1945-2011. [63] Sindern A, Ricken T, Bluhm J,etal. Bacterial methane oxidation in landfill cover layers-a coupled FE multiphase description. PAMM, 2013, 13(1): 193-194. [64] Dubey S K. Spatio-kinetic variation of methane oxidizing bacteria in paddy soil at mid-tillering: effect of N-fertilizers. Nutrient Cycling in Agroecosystems, 2003, 65(1): 53-59. [65] Yang N, Lü F, He P,etal. Response of methanotrophs and methane oxidation on ammonium application in landfill soils. Applied Microbiology and Biotechnology, 2011, 92(5): 1073-1082. [66] Chan A S K, Parkin T B. Methane oxidation and production activity in soils from natural and agricultural ecosystems. Journal of Environmental Quality, 2001, 30(6): 1896-1903. [67] Bykova S, Boeckx P, Kravchenko I,etal. Response of CH4oxidation and methanotrophic diversity to NH4+and CH4mixing ratios. Biology and Fertility of Soils, 2007, 43(3): 341-348. [68] Segarra K E A, Comerford C, Slaughter J,etal. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochimica et Cosmochimica Acta, 2013, 115: 15-30. [69] Kravchenko I K. Methane oxidation in boreal peat soils treated with various nitrogen compounds. Plant and Soil, 2002, 242(1): 157-162. [70] Duan X N, Wang X K, Cheng L,etal. Methane emission from aquatic vegetation zones of Wuliangsu Lake, Inner Mongolia. Environmental Science, 2007, 28(3): 456-459. [71] Whiting G J, Chanton J P. Control of the diurnal pattern of methane emission from emergent aquatic macrophytes by gas transport mechanisms. Aquatic Botany, 1996, 54(2-3): 237-253. [72] Saarnio S, Silvola J. Effects of increased CO2and N on CH4efflux from a boreal mire: a growth chamber experiment. Oecologia, 1999, 119(3): 349-356. [73] Jia Z J, Cai Z C. Effects of rice plants on methane emission from paddy fields. Chinese Journal of Applied Ecology, 2003, 14(11): 2049-2053. [74] Garnet K N, Megonigal J P, Litchfield C,etal. Physiological control of leaf methane emission from wetland plants. Aquatic Botany, 2005, 81(2): 141-155. [75] Hang J F, Tong C, Liu Z X,etal. Plant-mediated methane transport and emission from aSpartinaalternifloramarsh. Chinese Bulletin of Botany, 2011, 46(5): 534-543. [76] Arkebauer T J, Chanton J P, Verma S B,etal. Field measurements of internal pressurization inPhragmitesaustralis(Poaceae) and implications for regulation of methane emissions in amid latitude prairie wetland. American Journal of Botany, 2001, 88(4): 653-658. [77] Aulakh M S, Wassmann R, Rennenberg H,etal. Pattern and amount of aerenchyma aelate to variable methane transport capacity of different rice cultivars. Plant Biology, 2000, 2(2): 182-194. [78] Kim J N, Verma S B, Billesbach D P. Seasonal variation in methane emission from a temperatePhragmites-dominated marsh: effect of growth stage and plant-mediated transport. Global Change Biology, 1999, 5(4): 433-440. [79] Bubier J L. The relationship of vegetation to methane emission and hydrochemical gradients in northern peatlands. Journal of Ecology, 1995, 83: 403-420. [80] Tong C, Wang W Q, Huang J F,etal. Invasive alien plants increase CH4emissions from a subtropical tidal estuarine wetland. Biogeochemistry, 2012, 111(1-3): 677-693. [81] Joabsson A, Christensen T R. Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Global Change Biology, 2001, 7(8): 919-932. [82] Adam Langley J, Mozdzer T J, Shepard K A,etal. Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Global Change Biology, 2013, 19(5): 1495-1503. [83] Ström L, Ekberg A, Mastepanov M,etal. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Global Change Biology, 2003, 9(8): 1185-1192. 参考文献: [8] 孙万龙, 孙志高, 孙文广, 等. 黄河口潮滩湿地土壤CH4氧化潜力及其对有机物输入的响应. 草业学报, 2014, 23(1): 104-112. [9] 丁维新, 蔡祖聪. 沼泽甲烷排放及其主要影响因素. 地理科学, 2002, 22(5): 619-625. [32] 曹丛丛, 齐玉春, 董云社, 等. 氮沉降对陆地生态系统关键有机碳组分的影响. 草业学报, 2014, 23(2): 323-332. [37] 方华军, 程淑兰, 于贵瑞, 等. 大气氮沉降对森林土壤甲烷吸收和氧化亚氮排放的影响及其微生物学机制. 生态学报, 2014, 34(17): 4799-4806. [43] 丁维新, 蔡祖聪. 氮肥对土壤氧化甲烷的影响研究. 中国生态农业学报, 2003, 11(2): 50-53. [70] 段晓男, 王效科, 陈琳, 等. 乌梁素海湖泊湿地植物区甲烷排放规律. 环境科学, 2007, 28(3): 456-459. [73] 贾仲君, 蔡祖聪. 水稻植株对稻田甲烷排放的影响. 应用生态学报, 2003, 14(11): 2049-2053. [75] 黄佳芳, 仝川, 刘泽雄, 等. 沼泽湿地互花米草植物体传输与排放甲烷特征. 植物学报, 2011, 46(5): 534-543. Effects of nitrogen input on CH4production, oxidation and transport in soils, and mechanisms: a review HU Min-Jie1, TONG Chuan1*, ZOU Fang-Fang2 1.KeyLaboratoryofHumidSub-tropicalEco-geographicalProcessoftheMinistryofEducation,ResearchCentreofWetlandsinSubtropicalRegion,SchoolofGeographicalSciences,FujianNormalUniversity,Fuzhou350007,China; 2.AnxiTeaCollege,FujianAgricultureandForestryUniversity,Fuzhou350002,China Methane is an important component of carbon output in anaerobic soil. Minor changes to the soil carbon cycle will cause significant changes in the metabolic processes involving methane, which in turn can be markedly affected by exogenous nitrogen input. With increase in anthropogenic nitrogen inputs, exogenous nitrogen becomes an important factor in soil methane production, oxidation, and transmission processes. Methane emissions are regulated by nitrogen availability. Nitrogen inputs can change the background environment and methane emission mechanisms in soil, and consequently influence methane emission fluxes. Research into effects of nitrogen input on CH4production and the mechanisms of N effects on oxidation and transport processes in soils are reviewed in this paper. The important findings in the literature are: 1) The effects of nitrogen input on CH4fluxes in soils can be positive, negative or neutral, due to the range of effects of added N on methane production, oxidation, and transport processes; 2) The effects of nitrogen input on methane production processes are controlled by methanogenic substrates and methanogenic microbial activities. Nitrogen input provides rich substrates for methane production by increasing soil organic carbon content. The changes in the physical and chemical properties of substrates and vegetation cover make this effect complicated. Nitrogen input can also either promote or inhibit the activity of methanogens, depending on the form of nitrogen supplied; 3) The effects of nitrogen input on methane oxidation processes mainly arise from stimulation or inhibition of the activities of methanotrophs; 4) The effects of nitrogen input on methane transport processes depend mostly on the number of aerenchyma vessels and on transport efficiencies, and the degree of dependence varied greatly in different ecosystems. Overall, the effects of nitrogen input on soil CH4production, oxidation, and transport process are complicated and the mechanisms are uncertain. Future research should focus on the effects of nitrogen input on the critical processes determining methane emissions, on investigation of the effects of nitrogen input on microbial community structures, abundance and activities, and on collaborative research in a range of ecosystems. The goal of future research should be to determine the contribution of various ecosystems to global methane emissions at specific levels of nitrogen input. mechanisms; methane production; methane oxidation; microorganism; nitrogen input 10.11686/cyxb2014313 http://cyxb.lzu.edu.cn 2014-07-14;改回日期:2014-09-12 国家自然科学基金项目(41071148),福建省教育厅项目(JA13469),福建师范大学创新团队项目和福建师范大学地理科学学院研究生创新基金项目资助。 胡敏杰(1988-),男,安徽合肥人,在读博士。E-mail: mjhu0014@163.com *通讯作者Corresponding author. E-mail: tongch@fjnu.edu.cn 胡敏杰,仝川,邹芳芳. 氮输入对土壤甲烷产生、氧化和传输过程的影响及其机制. 草业学报, 2015, 24(6): 204-212. Hu M J,Tong C,Zou F F. Effects of nitrogen input on CH4production, oxidation and transport in soils, and mechanisms: a review. Acta Prataculturae Sinica, 2015, 24(6): 204-212.4 氮输入对甲烷传输的影响及机理
5 研究不足与展望

