单核铁(Ⅲ)配合物[Fe(hmb)2(N3)(H2O)]的合成及其晶体结构
赵儒霞,张海洋,张淑华
(桂林理工大学a.广西环境友好电磁化学功能物质重点实验室;b.化学与生物工程学院,广西桂林541004)
金属配合物因其在光、电、磁化学和分离、吸附、催化等领域所具有的巨大应用前景而备受青睐[1-5]。铁配合物因其特异的磁学性质而成为研究的热点[6-7]。在前人研究的基础上,笔者继续合成铁磁配合物以探索其构筑特点及磁学特性。在配合物的构筑中,分子间的弱相互作用——氢键成为配合物向多维空间结构拓展的一座桥梁[8-10]。在此,配体的选择成为决定性的因素。配体的给体基团性质、配体的齿数、配体点间的间距、配体间的连接基团以及配体异构等诸多因素都可能对配合物的最终结构产生影响[11]。3-甲氧基水杨醛配体苯环上连有—CHO、—OH、—OR基团,使其具有丰富的配位模式,且苯环及苯环上的氢原子为分子间作用力及氢键的产生提供了有利条件,因而成为理想的选择。笔者等已报道了系列水杨醛衍生物的配合物[12-15],本研究选用3-甲氧基水杨醛、叠氮化钠与六水合氯化铁在室温下合成了单核铁配合物[Fe(hmb)2(N3)(H2O)],通过元素分析、X射线单晶衍射对其进行了表征,确定了配合物的结构。
1 实验部分
1.1 试剂与仪器
Agilent G8910A CCD单晶衍射仪,美国安捷伦公司;Perkin-Elmer 240Q元素分析仪,美国珀金埃尔默股份有限公司。使用的试剂均为分析纯。
1.2 配合物的合成
将FeCl3·6H2O(0.75 mmol,0.203 g)、叠氮化钠(1.5 mmol,0.097 5 g)和3-甲氧基水杨醛(0.75 mmol,0.114 g)溶于10 mL乙腈溶液中,搅拌10 min后加入三乙胺调节pH为7.0,继续搅拌20 min后,将得到的溶液于室温下静置3 d后,过滤并洗涤得到黑色的块状晶体即[Fe(hmb)2(N3)(H2O)]。产量0.083 g,产率52.93%(以Hhmb为基础计算)。元素分析:C,45.92;N,10.08;H,3.88%。理论值(C16H16FeN3O7,Mr=418.17):C,45.95;N,10.05;H,3.86%。反应方程式及配体Hhmb的结构如图1所示。

图1 反应方程式及配体结构式Fig.1 Reaction formula equation and ligand formula
1.3 晶体结构解析
晶体的X射线衍射数据由Agilent G8910A CCD面探衍射仪在室温下用Mo-Kα辐射(λ=0.071 073 nm),以ω-θ扫描方式在2.98°≤θ≤25.01°收集,并使用SADABS程序进行LP因子校正和吸收校正[16]。用帕特森法确定金属离子位置,差值傅里叶法求出全部非氢原子坐标,理论加氢法得到氢原子位置,并用最小二乘法对结构进行修正。最大残余峰为1 849 e·nm-3,在距离O7 0.163 2 nm、距离H7D 0.128 5 nm的位置。计算工作在PC机上用SHELXS—97[17]和SHLEXL—97[17]程序完成。晶体学参数见表1,部分键长、键角的数据见表2。
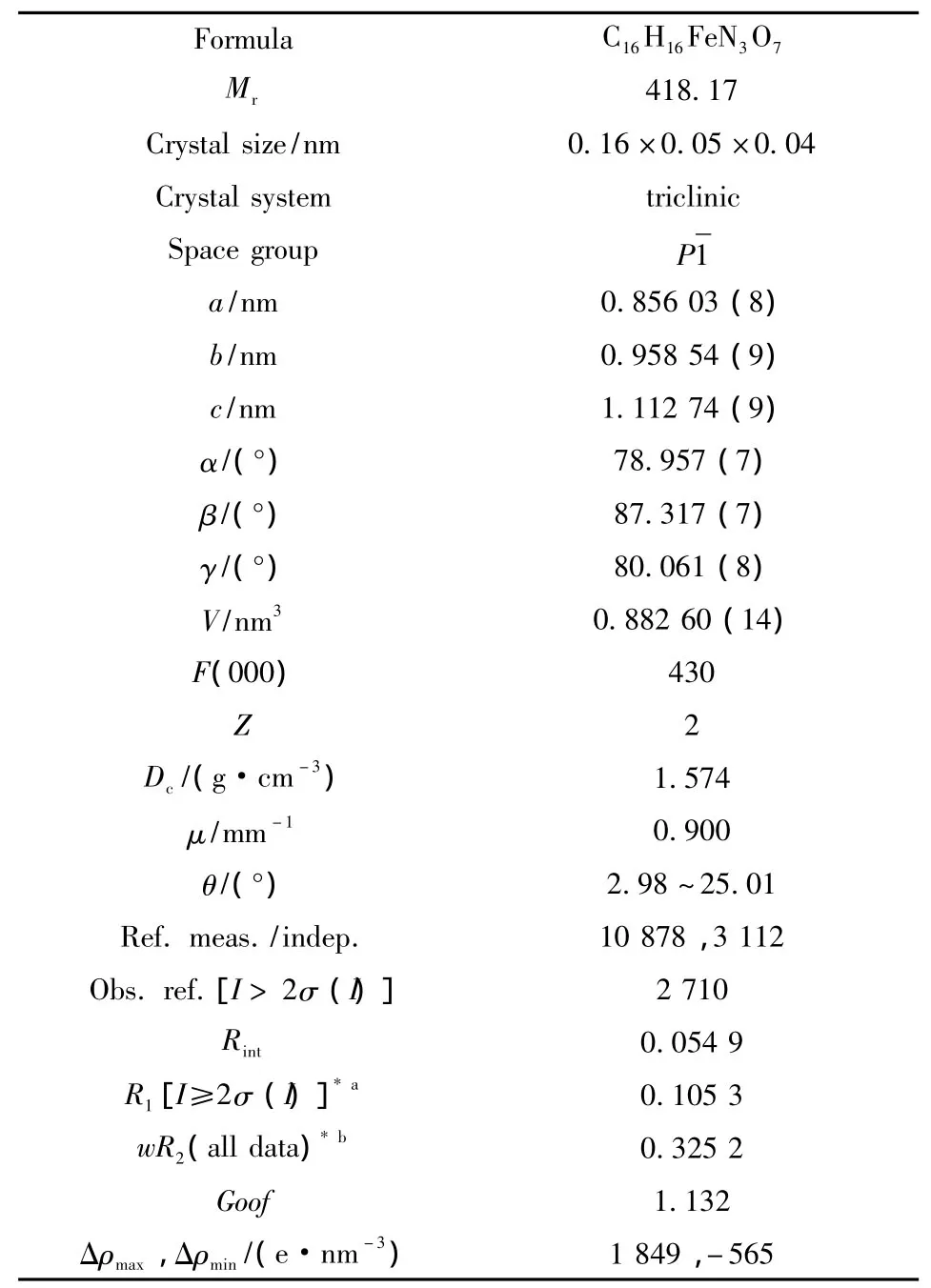
表1 配合物的晶体学参数Table 1 Crystallographic data for the complex

表2 配合物的部分键长、键角Table 2 Metal-ligand bond lengths and angles of the complex
2 结果与讨论
2.1 配合物的结构

图2 配合物晶体的结构Fig.2 Crystal structure of the complex
配合物在b轴方向上依靠分子间氢键(C5—H5…N1i(对称码:(i)1-x,-y,-z),0.354 7 nm,∠166.40°;C8—H8…N1ii(对称码:(ii)1-x,1-y,-z),0.338 2 nm,∠144.95°)形成一维双链状结构,双链之间依靠π…π共轭作用(π…π间距离为0.380 0 nm)[23-24]及分子间氢键作用(O7—H7D…O3iii(对称码:(iii)1-x,-y,1-z),0.300 2 nm,∠143.92°;C16—H16…O1iv(对称码:(iv)1-x,1-y,1-z),0.386 8 nm,∠136.64°;O7—H7D…C7iii(对称码:(iii)1-x,-y,1-z),0.384 1 nm,∠155.50°;O7—H7…C9iii(对称码:(iii)1-x,-y,1-z),0.3844nm,∠138.70°)[25-26]。在bc面上构成二维平面结构(图3b),其二维结构进一步通过分子间氢键(C12—H12…N2v(对称码:(V)-x,1-y,1-z),0.352 8 nm,∠145.58°)形成三维空间结构,在ac面的堆积如图3a所示。
3 结论
以3-甲氧基水杨醛、叠氮化钠与六水合氯化铁在室温下反应合成了具有八面体构型的单核铁配合物。该配合物属于三斜晶系空间群,通过分子间氢键作用连接成一维双链状结构,双链间进一步通过π…π共轭作用及分子间氢键作用构筑成三维立体结构。
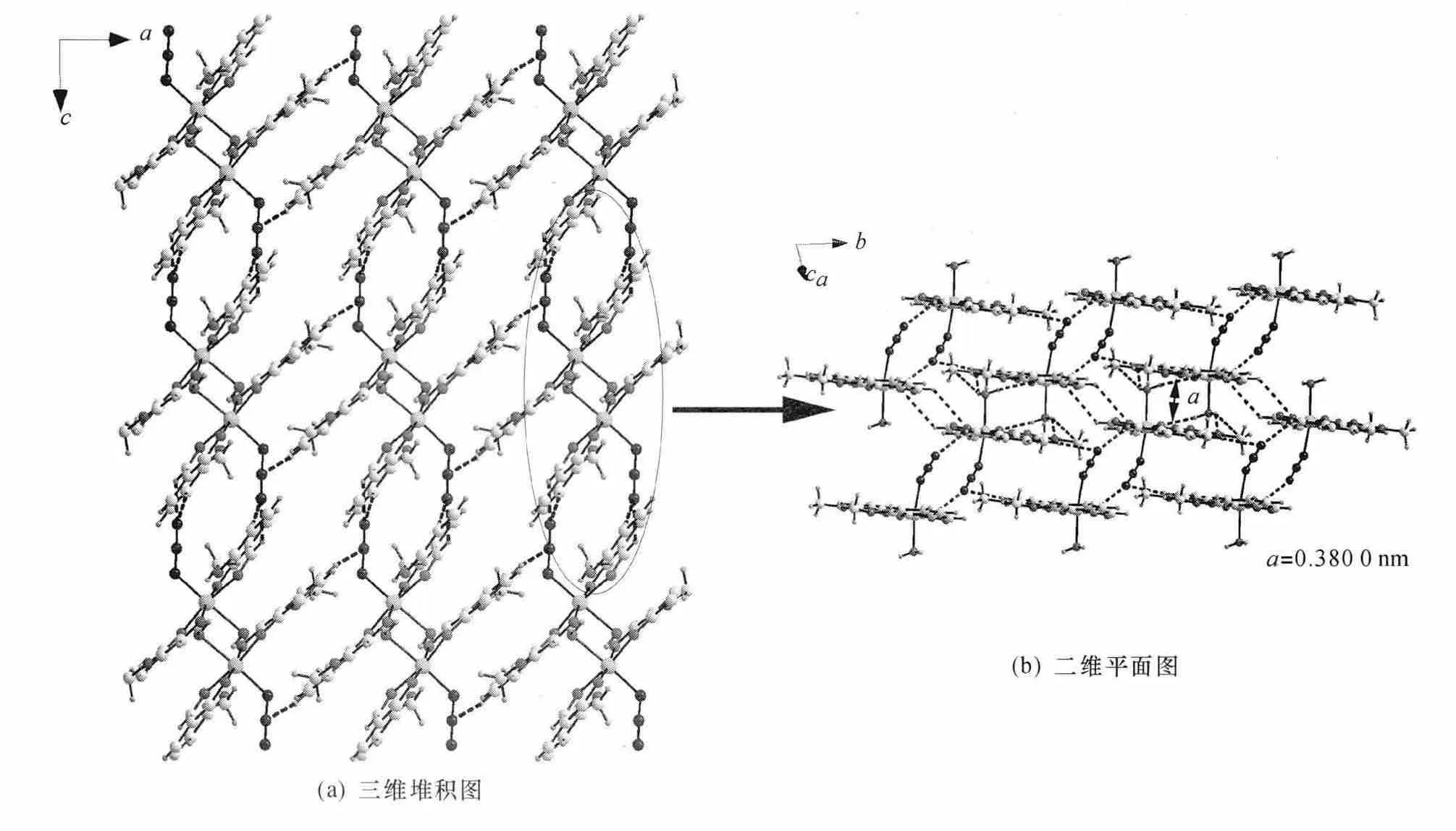
图3 配合物空间结构Fig.3 Packing map of the complex
[1]Bujoli B,Lane S M,Nonglaton G,et al.Metal phosphonates applied to biotechnologies:A novel approach to oligonucleotide microarrays[J].Chem.Eur.J.,2005,11(7):1980-1988.
[2]Zhang S H,Song Y,Liang H,et al.Microwave-assisted synthesis,crystal structure and properties of a disc-like heptanuclear Co(II)cluster and a heterometallic cubanic Co(II)cluster[J].Cryst.Eng.Comm,2009,11(5):865-872.
[3]Pelayo-Vázquez J B,González F J,Leyva M A,et al.A ruthenium carbonyl cluster containing a hydroquinone ligand:A layered structure with a polymetallic species.Structure and electrochemical characterization[J].J.Organomet.Chem.,2012,716:289-293.
[4]Zhao Y,Chen K,Fan J,et al.Structural modulation of silver complexes and their distinctive catalytic properties[J].Dalton Trans.,2014,43(5):2252-2258.
[5]Bozoklu G,Gateau C,Imbert D,et al.Metal-controlled diastereoselective self-assembly and circularly polarized luminescence of a chiral heptanuclear europium wheel[J].Journal of the American Chemical Society,2012,134(20):8372-8375.
[6]Caneschi A,Cornia A,Fabretti A C,et al.Structure and magnetic properties of a dodecanuclear twisted-ring iron(Ⅲ)cluster[J].AngewandteChemieInternationalEdition,1999,38(9):1295-1297.
[7]Weber B,Jäger E-G.Structure and magnetic properties of iron(Ⅱ/Ⅲ)complexes with N2O2-2coordinating schiff base like ligands[J].European Journal of Inorganic Chemistry,2009(4):465-477.
[8]Qin Z Q,Jennings M C,Puddephatt R J.Crosslinking a palladium(Ⅱ)polymer gives a laminated sheet structure[J].Chemical Communications,2002(4):354-355.
[9]Muthu S,Yip J H K,Vittal J J.Coordination networks of Ag(Ⅰ)and N,N'-bis(3-pyridinecarboxamide)-1,6-hexane:Structures and anion exchange[J].Journal of the Chemical Society,Dalton Transactions,2002(24):4561-4568.
[10]Burchell T J,Eisler D J,Puddephatt R J.Self-assembly using dynamic coordination chemistry and hydrogen bonding:Mercury(Ⅱ)macrocycles,polymers and sheets[J].Inorg.Chem.,2004,43(18):5550-5557.
[11]Hong M C,Chen R,Liang W P.Inorganic Chemistry of 21th Century[M].Beijing:Science Press,2005.
[12]Zhang S H,Li N,Ge C M,et al.Structures and magnetism of{Ni2Na2},{Ni4}and{Ni6ⅡNiⅢ}2-hydroxy-3-alkoxybenzaldehyde clusters[J].Dalton Trans.,2011,40(12):3000-3007.
[13]Ge C M,Zhang S H,Feng C,et al.A heterotetranuclear metal complex{Ni2Na2}with azido bridges[J].Zeitschrift für Anorganische und Allgemeine Chemie,2011,637(1):112-116.
[14]Zhang S H,Zhao R X,Li H P,et al.Structural variation from heterometallic cluster-based 1D chain to heterometallic tetranuclear cluster:Syntheses,structures and magnetic properties[J].Journal of Solid State Chemistry,2014,216:30-35.
[15]Zhang S H,Zhang Y D,Zou H H,et al.A family of cubane cobalt and nickel clusters:Syntheses,structures and magnetic properties[J].Inorganica Chimica Acta,2013,396:119-125.
[16]Blessing R H.An empirical correction for absorption anisotropy[J].Acta Crystallographica Section A:Foundations of Crystallography,1995,51(1):33-38.
[17]Sheldrick G M.A short history of SHELX[J].Acta Crystallographica Section A:Foundations of Crystallography,2007,64(1):112-122.
[18]Lu M F,Chen T T,Wang M,et al.A new μ3-oxo-centered tri-nuclear carboxyl bridged iron(Ⅲ)complex with thio-methyl groups in the periphery:Structural,spectroscopic and electrochemical studies[J].J.Mol.Struct.,2014,1060:131-137.
[19]Dutta A K,Maji S K,Dutta S,et al.Synthesis,structural and magnetic properties of oxo-,chloroacetato-bridged tetranuclear iron(Ⅲ)complex[J].J.Mol.Struct.,2012,1029(12):68-74.
[20]Halcrow M A.Jahn-Teller distortions in transition metal compounds,and their importance in functional molecular and inorganic materials[J].Chem.Soc.Rev.,2013,42(4):1784-1795.
[21]Sreekanth A,Fun H K,Kurup M R P.Structural and spectral studies of an iron(Ⅲ)complex[Fe(Pranthas)2][FeCl4]derived from 2-acetylpyridine-N(4),N(4)-(butane-1,4-diyl)thiosemicarbazone(HPranthas)[J].J.Mol.Struct.,2005,737(1):61-67.
[22]Min K S,Arif A M,Miller J S.Synthesis,structure and magnetic properties of an oxo-bridged dinuclear iron(Ⅲ)complex[(TPyA)FFeⅢOFeⅢF(TPyA)](BF4)20.5MeOH(TPyA=tris(2-pyridylmethyl)amine)containing the FFeⅢOFeⅢF linkage[J].Inorg.Chim.Acta.,2007,360(6):1854-1858.
[23]Ghosh A K,Ghoshal D,Ribas J,et al.Higher dimensional networks of Mn(Ⅱ)azide/cyanate using flexible N-donor ligands:Synthesis,crystal structure and magnetic properties[J].J.Mol.Struct.,2006,796(1-3):195-202.
[24]Guo W,He J Q,Li Z C,et al.Chain-like assembly of threonine-based cyclophanes through π-π interaction and C—H…O hydrogen bond[J].Tetrahedron Letters,2004,45(29):5763-5766.
[25]Desiraju G R.The C—H…O hydrogen bond:Structural implications and supramolecular design[J].Accounts of Chemical Research,1996,29(9):441-449.
[26]Yu S S,Zhou H,Duan H B.Three-dimensional hydrogen bonding framework in the crystal structure of a zinc(Ⅱ)complex of imidazole multicarboxylic acid[J].Synthesis and Reactivity in Inorganic,Metal-Organic,and Nano-Metal Chemistry,2013,43(10):1521-1524.

