连花清瘟对细颗粒物致大鼠肺部炎性损伤的拮抗作用研究
许 宁,平 芬,徐 鑫,诸葛铭宁,张凤蕊,韩书芝
·中医·中西医结合研究·
连花清瘟对细颗粒物致大鼠肺部炎性损伤的拮抗作用研究
许 宁,平 芬,徐 鑫,诸葛铭宁,张凤蕊,韩书芝
目的 探讨细颗粒物(PM2.5)急性暴露对大鼠肺部炎性损伤的作用,及低、中、高剂量连花清瘟对肺部炎性损伤的拮抗作用。方法 2013年6—12月选取48只健康成年Wistar大鼠,采用随机数字表法分为空白对照组、0.9%氯化钠溶液对照组、PM2.5染尘组及低、中、高剂量连花清瘟组,每组8只。由石家庄市环境监测中心提供PM2.5,制备PM2.5悬液。空白对照组大鼠无任何干预措施;0.9%氯化钠溶液对照组大鼠给予气管滴注0.9%氯化钠溶液(1 ml/kg);PM2.5染尘组大鼠给予气管滴注PM2.5悬液1 ml/kg(7.5 mg/kg);低、中、高剂量连花清瘟组大鼠首先分别连续灌胃给予低(2 g/kg)、中(4 g/kg)、高(8 g/kg)剂量连花清瘟溶液10 ml/kg,共4 d,第4天灌胃给药后分别气管滴注PM2.5悬液1 ml/kg(7.5 mg/kg),24 h后处死。光镜下观察肺组织病理形态变化,酶联免疫吸附试验(ELISA)法测定各组大鼠支气管肺泡灌洗液(BALF)及血清中白介素(IL)1、IL-6、肿瘤坏死因子α(TNF-α)水平。结果 空白对照组及0.9%氯化钠溶液对照组大鼠肺组织病理切片光镜下未见异常表现;PM2.5染尘组大鼠肺组织病理切片光镜下可见炎性细胞渗出,肺间质纤维组织增生,间质水肿;低、中、高剂量连花清瘟组大鼠肺组织病理切片光镜下可见随连花清瘟剂量增加,肺泡腔内炎性细胞渗出逐渐减轻。0.9%氯化钠溶液对照组大鼠BALF及血清中IL-1、IL-6、TNF-α水平与空白对照组比较,差异均无统计学意义(P>0.05);PM2.5染尘组大鼠BALF中IL-1水平较0.9%氯化钠溶液对照组升高,血清中IL-1水平、BALF及血清中IL-6、TNF-α水平较空白对照组和0.9%氯化钠溶液对照组升高(P<0.05);高剂量连花清瘟组大鼠BALF中IL-1水平较空白对照组、0.9%氯化钠溶液对照组、PM2.5染尘组、低剂量连花清瘟组、中剂量连花清瘟组降低,BALF和血清中IL-6水平较空白对照组、0.9%氯化钠溶液对照组、PM2.5染尘组、低剂量连花清瘟组降低,BALF中TNF-α水平和血清中IL-1水平较PM2.5染尘组、低剂量连花清瘟组、中剂量连花清瘟组降低,血清中TNF-α水平较PM2.5染尘组、低剂量连花清瘟组降低(P<0.05)。结论 PM2.5急性暴露可以导致大鼠肺部炎性损伤,连花清瘟对肺部炎性损伤有拮抗作用。
肺炎;颗粒物;支气管肺泡灌洗液;白细胞介素;肿瘤坏死因子α
许宁,平芬,徐鑫,等.连花清瘟对细颗粒物致大鼠肺部炎性损伤的拮抗作用研究[J].中国全科医学,2015,18(27):3355-3359.[www.chinagp.net]
Xu N,Ping F,Xu X,et al.Antagonistic effects of Lianhuaqingwen on rat′s pulmonary inflammatory injury induced by airborne fine particulate matters[J].Chinese General Practice,2015,18(27):3355-3359.
环境因素在呼吸系统疾病中起着不可忽视的作用,流行病学研究显示,环境中细颗粒物(PM2.5)浓度与呼吸系统及心血管系统疾病的发病率、病死率密切相关[1-2],其具体机制尚未明确,而肺部炎性损伤可能是PM2.5暴露诱导肺损伤的重要机制。PM2.5随呼吸进入肺泡内,作用于肺泡巨噬细胞,巨噬细胞在肺部炎性反应中发挥着重要作用,PM2.5通过诱导其释放大量细胞因子[3]、激活并增加核因子(NF)κB相关的基因表达,使毛细血管通透性增加、中性粒细胞浸润[4],从而造成肺甚至其他器官的炎性损伤[5]。连花清瘟有外疏卫表,清热解毒,宣肺泄热之效,因其确切的临床疗效,已被广泛应用于多种流感病毒感染引起的呼吸系统疾病[6]。本研究采集石家庄市PM2.5,制备PM2.5悬液,观察PM2.5暴露后大鼠肺组织病理变化及染尘大鼠支气管肺泡灌洗液(BALF)及血清中促炎性细胞因子水平的变化,探讨PM2.5对大鼠肺部炎性损伤的作用,并通过观察连花清瘟干预后染尘大鼠BALF及血清中促炎性细胞因子水平的变化,进一步探讨其对PM2.5所致大鼠肺部炎性损伤的拮抗作用。
1 材料与方法
1.1 PM2.5悬液配制 PM2.5由石家庄市环境监测中心提供,采用TEOM1405D双通道颗粒物监测仪收集,洗脱PM2.5,冷冻干燥,使用前以灭菌注射用水配制成浓度为7.5 mg/ml PM2.5悬液,超声震荡混匀。连花清瘟溶液为连花清瘟干膏粉41.73 g、20.87 g、10.43 g分别加入羟甲基纤维素钠溶液,配制成80%、40%、20%的高、中、低剂量连花清瘟溶液。
1.2 实验动物分组及给药方法 2013年6—12月选取48只雄性Wistar大鼠(河北医科大学实验动物中心提供),体质量180~200 g,采用随机数字表法分为2个对照组(空白对照组及0.9%氯化钠溶液对照组)和4个实验组(PM2.5染尘组及低、中、高剂量连花清瘟组),每组8只。空白对照组大鼠不给予任何干预措施,0.9%氯化钠溶液对照组大鼠给予气管滴注0.9%氯化钠溶液(1 ml/kg)1次,PM2.5染尘组大鼠给予气管滴注PM2.5悬液1 ml/kg(7.5 mg/kg)1次,低、中、高剂量连花清瘟组大鼠给予连花清瘟溶液10 ml/kg(连花清瘟2 g/kg、4 g/kg、8 g/kg)灌胃,共给药4 d,第4天灌胃给药后给予气管滴注PM2.5悬液,剂量及次数同PM2.5染尘组。
1.3 标本采集 丙戊酸钠腹腔注射麻醉下腹主动脉采集动脉血5 ml后放血处死大鼠(空白对照组直接处死,0.9%氯化钠溶液对照组气管滴注0.9%氯化钠溶液24 h后处死,各实验组均染尘24 h后处死)。所取动脉血分离血清,3 000 r/min离心15 min(离心半径8 cm);以磷酸盐缓冲液(PBS)进行支气管肺泡灌洗,2.5 ml/次,反复3次,所得BALF离心取上清液,1 000 r/min离心10 min(离心半径8 cm),于-80 ℃冰箱保存。取大鼠部分右肺,甲醛溶液固定24 h后,常规石蜡包埋,切片,苏木素-伊红(HE)染色,光镜下观察。
1.4 指标检测 采用酶联免疫吸附试验(ELISA)法测定(试剂盒购自美国BG公司,用Multiskan型全自动酶标仪测OD值)BALF及血清中促炎性细胞因子白介素(IL)1、IL-6、肿瘤坏死因子α(TNF-α)水平,操作步骤按试剂盒说明书进行。
2 结果
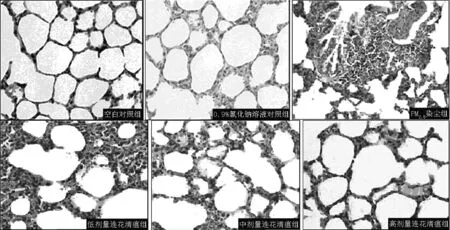
2.1 大鼠肺组织病理所见 空白对照组及0.9%氯化钠溶液对照组大鼠肺组织病理切片光镜下未见异常表现;PM2.5染尘组大鼠肺组织病理切片光镜下可见肺泡间隔增厚,肺泡腔缩小,炎性细胞渗出,小支气管壁增厚,管腔内杯状细胞增生,周围炎性细胞浸润,肺间质纤维组织增生,间质水肿,毛细血管充血,小血管管壁增厚,周围可见嗜酸粒细胞浸润;低、中、高剂量连花清瘟组大鼠肺组织病理切片光镜下可见随连花清瘟剂量增加,肺泡腔内炎性细胞渗出逐渐减轻(见图1)。
2.2 各组大鼠BALF及血清中促炎性细胞因子水平变化 各组大鼠BALF及血清中IL-1、IL-6、TNF-α水平比较,差异均有统计学意义(P<0.05);其中0.9%氯化钠溶液对照组大鼠BALF及血清中IL-1、IL-6、TNF-α水平与空白对照组比较,差异均无统计学意义(P>0.05);PM2.5染尘组大鼠BALF中IL-1水平较0.9%氯化钠溶液对照组升高,血清中IL-1水平、BALF及血清中IL-6、TNF-α水平较空白对照组和0.9%氯化钠溶液对照组升高,差异均有统计学意义(P<0.05);低剂量连花清瘟组大鼠BALF中IL-6水平较PM2.5染尘组降低,血清中IL-1水平、BALF及血清中TNF-α水平较空白对照组和0.9%氯化钠溶液对照组升高,差异均有统计学意义(P<0.05);中剂量连花清瘟组大鼠BALF中IL-6水平较空白对照组、0.9%氯化钠溶液对照组、PM2.5染尘组、低剂量连花清瘟组降低,BALF中TNF-α水平和血清中IL-1水平较空白对照组和0.9%氯化钠溶液对照组升高、较PM2.5染尘组降低,血清中IL-6水平较PM2.5染尘组、低剂量连花清瘟组降低,差异均有统计学意义(P<0.05);高剂量连花清瘟组大鼠BALF中IL-1水平较空白对照组、0.9%氯化钠溶液对照组、PM2.5染尘组、低剂量连花清瘟组、中剂量连花清瘟组降低,BALF和血清中IL-6水平较空白对照组、0.9%氯化钠溶液对照组、PM2.5染尘组、低剂量连花清瘟组降低,BALF中TNF-α水平和血清中IL-1水平较PM2.5染尘组、低剂量连花清瘟组、中剂量连花清瘟组降低,血清中TNF-α水平较PM2.5染尘组、低剂量连花清瘟组降低,差异均有统计学意义(P<0.05,见表1)。
注:PM2.5=细颗粒物

图1 各组大鼠肺组织病理切片光镜下表现(HE染色,×200)
注:与空白对照组比较,aP<0.05;与0.9%氯化钠溶液对照组比较,bP<0.05;与PM2.5染尘组比较,cP<0.05;与低剂量连花清瘟组比较,dP<0.05;与中剂量连花清瘟组比较,eP<0.05;PM2.5=细颗粒物,BALF=支气管肺泡灌洗液,IL-1=白介素1,IL-6=白介素6,TNF-α=肿瘤坏死因子α
3 讨论
PM2.5可随空气进入小气管和肺泡并持续作用,当其到达肺泡时,被气管纤毛系统清除并且/或者被巨噬细胞吞噬[7]。Maciejczyk等[8]研究报道,PM2.5被巨噬细胞吞噬过程中可通过与之相关的编码转录因子、炎性相关因子基因转录水平的增高诱导大量促炎性细胞因子释放,破坏细胞因子网络平衡,从而调节和始动肺局部炎性反应,诱导肺炎性损伤。另一方面,PM2.5对肺机械屏障作用的破坏使肺部清除异物作用下降,进一步诱导更严重的炎性反应,导致促炎性细胞因子水平的进一步升高[9]。本研究中肺组织病理切片光镜下显示,PM2.5可导致大鼠肺泡腔内炎性细胞渗出,细支气管黏膜损伤,炎性细胞浸润,肺间质水肿,毛细血管充血,而Riva等[10]及曲红梅等[11]研究也表明,PM2.5暴露的肺组织病理切片也可见肺泡塌陷及单核细胞、中性粒细胞浸润、淋巴细胞增生。
在巨噬细胞释放的大量细胞因子中,IL-1、IL-6、TNF-α等为早期主要的促炎性细胞因子[12],在肺的炎性反应中起着重要的作用。这些促炎性细胞因子除了趋化嗜酸粒细胞、中性粒细胞等炎性细胞向肺组织迁移,还可增高其活性,使之分泌炎性细胞因子,进一步趋化炎性细胞,形成炎性细胞与炎性细胞因子之间的恶性循环[13]。TNF-α由活化的单核巨噬细胞等产生,在各种炎性损伤中,TNF-α是最早释放并发挥关键作用的细胞因子。本研究制备的大鼠PM2.5染尘模型BALF及血清中促炎性细胞因子TNF-α水平较空白对照组和0.9%氯化钠溶液对照组均明显升高。而Ding等[14]在PM2.5致新生大鼠肺炎症及氧化应激的研究中也发现,短期暴露于PM2.5即可诱发肺部炎性反应,表现在TNF-α水平升高,而TNF-α可通过与其特异性受体(TNF-R1和TNF-R2)的结合诱发炎性反应,使肺部出现炎性损伤。IL-6主要由单核巨噬细胞及血管内皮细胞等产生,与宿主炎性反应和疾病严重程度相关,血清IL-6水平常作为细胞因子级联反应激活的标志。IL-1主要由单核细胞产生。本研究结果显示,大鼠急性暴露于PM2.5可引起BALF及血清中IL-6、IL-1水平明显升高,与Phipps等[9]研究肺炎链球菌感染的大鼠香烟暴露组较空气暴露组肺匀浆中IL-1、IL-6、TNF-α水平均增加的结果一致。
连花清瘟主要药理作用及机制有:提高细胞免疫功能,抑制慢性阻塞性肺疾病(COPD)气管炎症,降低病毒感染后的肺指数,抑制病毒感染后的肺部炎性损害,抗甲型人流感病毒[15]。其主要成分为连翘、金银花、麻黄、杏仁、石膏、板蓝根、绵马贯众、鱼腥草、广藿香、大黄、红景天、薄荷脑、甘草,从中药组分来看符合中医治疗炎性疾病的特点,除了具有明确的抗病毒作用,对急性肺部感染效果显著,还具有一定的免疫调节作用[16]。本研究结果显示,高剂量连花清瘟组BALF及血清中促炎性细胞因子IL-1、IL-6、TNF-α水平较PM2.5染尘组均明显下降,且病理结果提示,炎性反应明显减轻,表明连花清瘟对PM2.5急性暴露所致肺炎性损伤有拮抗作用。而夏敬文等[17]研究连花清瘟对COPD大鼠模型气管炎症的影响,染尘组血清、肺组织匀浆及BALF中IL-8和TNF-α水平较对照组明显升高,而连花清瘟治疗组血清及BALF中上述细胞因子水平显著降低,也提示连花清瘟对气管炎症的抑制作用。
综上所述,本实验证实PM2.5急性暴露可导致大鼠肺部炎性损伤,而中、高剂量连花清瘟对这种炎性损伤具有拮抗作用,但其具体调节机制还有待进一步研究。
利益冲突声明:本课题未涉及任何厂家及相关雇主或其他经济组织直接或间接的经济或利益的赞助。无利益冲突。
[1]Lepeule J,Laden F,Dockery D,et al.Chronic exposure to fine particles and mortality:an extended follow-up of the Harvard Six Cities Study from 1974 to 2009[J].Environ Health Perspect,2012,120(7):965-970.
[2]Balakrishnan K,Sambandam S,Ramaswamy P,et al.Establishing integrated rural-urban cohorts to assess air pollution-related health effects in pregnant women,children and adults in Southern India:an overview of objectives,design and methods in the Tamil Nadu Air Pollution and Health Effects(TAPHE) study[J].BMJ Open,2015,5(6):e008090.
[3]He W,Qu T,Yu Q,et al.LPS induces IL-8 expression through TLR4,MyD88,NF-kappaB and MAPK pathways in human dental pulp stem cells[J].Int Endod J,2013,46(2):128-136.
[4]Shukla A,Timblin C,BeruBe K,et al.Inhaled particulate matter causes expression of nuclear factor(NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro[J].Am J Respir Cell Mol Biol,2000,23(2):182-187.
[5]Rodríguez-Cotto RI,Ortiz-Martínez MG,Jiménez-Vélez BD.Organic extracts from African dust storms stimulate oxidative stress and induce inflammatory responses in human lung cells through Nrf2 but not NF-κB[J].Environ Toxicol Pharmacol,2015,39(2):845-856.
[6]Zhao P,Yang HZ,Lv HY,et al.Efficacy of Lianhuaqingwen capsule compared with oseltamivir for influenza a virus infection:a meta-analysis of randomized,controlled trials[J].Altern Ther Health Med,2014,20(2):25-30.
[7]Donaldson K,Stone V.Current hypotheses on the mecha-nisms of toxicity of ultra fine particles[J].Ann Ist Super Sanita,2003,39(3):405-410.
[8]Maciejczyk P,Chen LC.Effects of subchronic exposures to concentrated ambient particles(CAPs) in mice.Ⅷ.Source-related daily variations in vitro responses to CAPs[J].Inhal Toxicol,2005,17(4/5):243-253.
[9]Phipps JC,Aronoff DM,Curtis JL,et al.Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae[J].Infect Immun,2010,78(3):1214-1220.
[10]Riva DR,Magalhães CB,Lopes AA,et al.Low dose of fine particulate matter(PM2.5) can induce acute oxidative stress,inflammation and pulmonary impairment in healthy mice[J].Inhal Toxicol,2011,23(5):257-267.
[11]Qu HM,Niu JP,Kui FR,et al.Study on rat pulmonary toxicity induced by PM2.5in atmosphere of Lanzhou city[J].Chinese Journal of Public Health,2006,22(5):598-599.(in Chinese) 曲红梅,牛静萍,魁发瑞,等.大气中PM2.5致大鼠呼吸道急性损伤作用[J].中国公共卫生,2006,22(5):598-599.
[12]Laskin DL,Mainelis G,Turpin BJ,et al.Pulmonary effects of inhaled diesel exhaust in young and old mice:a pilot project[J].Res Rep Health Eff Inst,2010(151):3-31.
[13]Culptt SV,Rogers DF,Shah P,et al.Impaired inhibition by dexamethasone of cytokine release by alveolar macroph-ages from patients with chronic obstructive pulmonary disease[J].Am J Respir Crit Care Med,2003,167(1):24-31.
[14]Ding LR,Wang K,Fahmy B,et al.Airborne fine particulate matter induced pulmonary inflammation as well as oxidative stress in neonate rats[J].Chin Med J(Engl),2010,123(20):2895-2900.
[15]Duan ZP,Jia ZH,Zhang J,et al.Natural herbal medicine Lianhuaqingwen capsule anti-influenza A(H1N1) trial:a randomized,double blind,positive controlled clinical trial[J].Chin Med J(Engl),2011,124(18):2925-2933.
[16]莫红缨,杨子峰,郑劲平,等.连花清瘟胶囊防治流感病毒FM1感染小鼠的实验研究[J].中药材,2008,31:1230-1233.
[17]Xia JW,Chen XD,Zhang J,et al.Experimental investigation of the effect of Lianhuaqingwen Capsule on the rat models of chronic obstructive pulmonary disease[J].Fudan University Journal of Medical Sciences,2008,35(3):441-444.(in Chinese) 夏敬文,陈小东,张静,等.连花清瘟胶囊对慢性阻塞性肺病的治疗作用[J].复旦学报:医学版,2008,35(3):441-444.
(本文编辑:陈素芳)
Antagonistic Effects of Lianhuaqingwen on Rat′s Pulmonary Inflammatory Injury Induced by Airborne Fine Particulate Matters
XUNing,PINGFen,XUXin,etal.
DepartmentofEmergency,HebeiGeneralHospital,Shijiazhuang050051,China
Objective To investigate the pulmonary inflammatory injury in rats induced by acute exposure to PM2.5and to explore the antagonistic effect of low,medium,and high dose of Lianhuaqingwen to the inflammatory injury.Methods From June to December in 2013,selected 48 healthy adult Wistar rats.Using random number table method,divided them into control group,sodium chloride group,PM2.5group,low-dose group,medium-dose group and high-dose group,with 8 rats in each group.Airborne fine particulate matters were provided by Shijiazhuang Environmental Monitoring Center,and PM2.5suspension liquid was prepared.Control group was not given any intervention;sodium chloride group was given trachea instillation of 0.9% sodium chloride solution (1 ml/kg);PM2.5group was given trachea instillation of 1 ml/kg(7.5 mg/kg) PM2.5suspension liquid;low-dose,medium-dose and high-dose groups were respectively given gavage with 2 g/kg,4 g/kg and 8 g/kg Lianhuaqingwen solution of 10 ml/kg for 4 days,and the three groups were given trachea instillation of 1 ml/kg(7.5 mg/kg) PM2.5suspension liquid on the fourth day and were killed 24 hours later.Pathological morphologic changes were observed under light microscope,and ELISA was employed to examine the levels of IL-1,IL-6 and TNF-α in BALF and serum.Results In control group and sodium chloride group,no abnormal manifestations in pulmonary tissue slices were observed under light microscope;in PM2.5group,exudation of inflammatory cells,interstitial proliferation of fibrous tissue and interstitial edema were noted in pulmonary tissue slices under light microscope;in the three Lianhuaqingwen groups,we found the exudation of inflammatory cells in alveolar space reduced with the increase of the dosage of Lianhuaqingwen.Sodium chloride group and control group were not significantly different in the levels of IL-1,IL-6 and TNF-α in BALF and serum(P>0.05);PM2.5group was higher than sodium chloride group in the level of IL-1 in BALF,and PM2.5group was higher than control group and sodium chloride group in the level of IL-1 in serum and the levels of IL-6 and TNF-α in BALF and serum (P<0.05);high-dose group was lower than control group,sodium chloride group,PM2.5group,low-dose group and medium group in the level of IL-1 in BALF,lower than control group,sodium chloride group,PM2.5group and low-dose group in the level of IL-6 in BALF and serum,lower than PM2.5group,low-dose group and medium-dose group in the level of TNF-α in BALF and the level of IL-1 in serum,and lower than PM2.5group and low-dose group in the level of TNF-α in serum(P<0.05).Conclusion The acute exposure to PM2.5can induce pulmonary inflammatory injury in rats,Lianhuaqingwen could produce antagonistic effects to the inflammatory injury.
Pneumonia;Particulate matter;Bronchoalveolar lavage fluid;Interleukin;Tumor necrosis factor-alpha
050051河北省石家庄市,河北省人民医院急诊科(许宁,徐鑫,诸葛铭宁),呼吸内二科(平芬,张凤蕊,韩书芝)
平芬,050051河北省石家庄市,河北省人民医院呼吸内二科;E-mail:pingfen2003@126.com
R 563.1
A
10.3969/j.issn.1007-9572.2015.27.021
2015-01-21;
2015-07-21)

