花药绒毡层发育和花粉母细胞减数分裂相关基因研究进展
刘伟华,邱 博,罗红兵
(湖南农业大学农学院,长沙 410128)
花药绒毡层发育和花粉母细胞减数分裂相关基因研究进展
刘伟华,邱 博,罗红兵*
(湖南农业大学农学院,长沙 410128)
植物雄性不育是自然界普遍存在的现象,也是利用杂种优势的重要途径之一。在花药发育过程中,花粉囊壁发育和减数分裂与花粉的育性有重要关系。主要从绒毡层发育和花粉母细胞减数分裂两个方面对近些年来植物花药发育基因调控网络进行综述,以期进一步了解和利用雄性不育性。
植物;花药;雄性不育;绒毡层;减数分裂
植物生殖器官的正常发育对植物的繁衍具有重要的作用。在农业生产中,虽然雄性不育会引起大面积减产,造成重大损失,但由于植物雄性不育突变比雌性不育突变更为普遍[1],雄性不育也成为利用杂种优势的一个重要途径。植物雄性不育的利用能简化制种程序,省去人工去雄的繁琐过程和去雄不彻底的麻烦,提高杂种生产效率和杂种质量。花药发育成熟包括一系列的细胞分裂和分化,需要众多基因参与调控,阐明花药发育相关基因在分子水平的变化对研究雄性不育机理和利用雄性不育具有一定意义。
1 花药发育的过程
以拟南芥为例,花药发育可分为14个时期[2]:第1期L1、L2和L3三个细胞层组成了花药原基,L1发育为表皮;第2期,位于花药4个角表皮下L2层的孢原细胞经过平周分裂形成初生周缘细胞层和初生造孢细胞层;在第3期,初生周缘细胞经过平周和圆周分裂在造孢细胞周围由内至外依次形成绒毡层、中层和药室内壁3层细胞。在第4、5期,造孢细胞分裂为许多花粉母细胞,绒毡层、中层、药室内壁和表皮构成花粉囊壁。第6期中层逐渐降解,绒毡层细胞出现液泡化,胼胝质积累,花粉母细胞进行减数分裂并在第7期形成四分体小孢子。第8期时,绒毡层开始解体,胼胝质溶解释放出小孢子,随后小孢子在第9期形成初生外壁和液泡。第10、11期绒毡层完全降解,单核花粉粒经非均等有丝分裂形成2-胞花粉粒。第12期时,每个花药形成4个花粉囊,花粉粒发育为3-胞花粉粒。在13、14期时花药开裂散出成熟花粉粒(图1)。
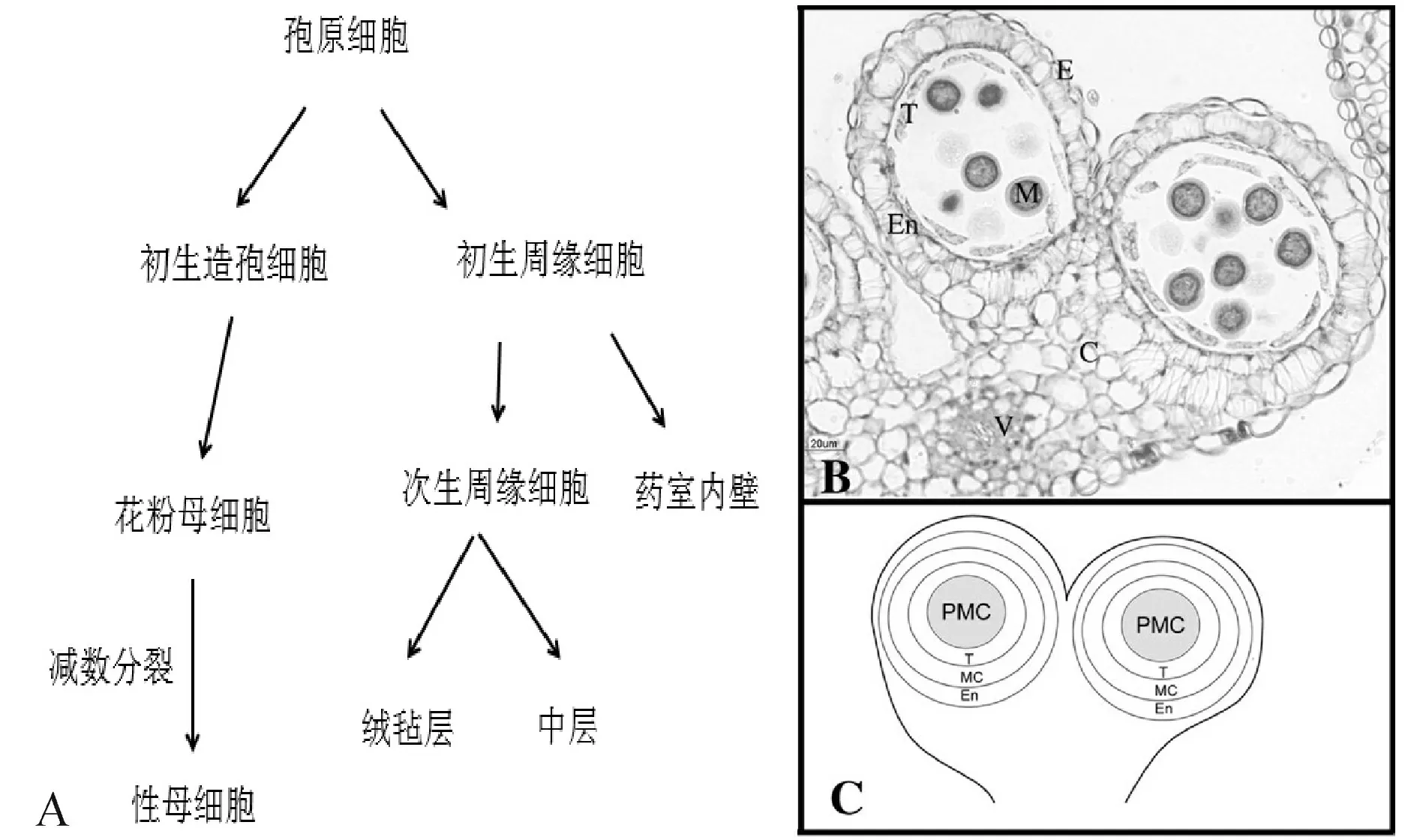
图1 花药发育过程与结构[5]注:PS.初生造孢细胞,PP.初生周缘细胞,PMC.花粉母细胞,M.小孢子,SPC.次生周缘细胞,En.药室内壁,T.绒毡层,Mc.中层,C.药隔基本组织,V.药隔维管束。
从花药原基分化到成熟花粉粒的形成,花药绒毡层发育、花粉母细胞减数分裂等是形成可育花粉的关键。例如,玉米msca1[3]突变体的孢原细胞未分裂为初生造孢细胞和初生周缘细胞,造成雄性不育;MAC1[4](MAIZEMULTIPLEARCHESPORIALCELLS1)基因在花药发育早期具有调控细胞分化的作用,其发生突变会形成过多的孢原细胞,导致花药发育异常。
2 绒毡层发育相关基因
绒毡层位于花药壁的最内侧,能分泌酶降解胼胝质释放小孢子,能为花粉壁合成提供物质,也能为花粉粒的发育提供营养[6~8]。绒毡层的正常发育对调控花粉育性具有重要的作用。
2.1 受体蛋白激酶参与调控绒毡层发育
类受体蛋白激酶在植物的生命活动中具有重要作用[9],许多受体蛋白激酶在绒毡层发育中起调控作用。
Zhao等人[10]发现,ems1(EXCESSMICROSPOROCYTES1)突变体绒毡层细胞发育为花粉母细胞导致绒毡层缺失,形成过多花粉母细胞。但在ems1中,SPL/NZZ(SPOROCYTELESS/NOZZLE)基因表达未受影响,说明EMS1位于SPL/NZZ的调控下游。EXS[11](EXTRASPOROGENOUSCELLS)能调控孢原细胞的正常分裂,其突变后孢原细胞分裂为异常的造孢细胞,次生周缘细胞解体或受到药室内壁和造孢细胞团挤压而无法形成绒毡层细胞,导致绒毡层缺失。tpd1(TAPETUMDETERMINANT1)突变体的绒毡层前体细胞发育为花粉母细胞,导致了绒毡层缺失,并且TPD1与EMS1/EXS可能存在互补协同关系,TPD1基因调控细胞分裂也需要EMS1/EXS基因参与[12,13]。Jia[14]等人的研究也表明TPD1作为EMS1的配体,参与调控花药的发育。SERK1、SERK2(SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1/2)能感受由花粉母细胞向外传递的信号,参与调控绒毡层的发育。在serk1serk2双突变体中绒毡层前体细胞发育为花粉母细胞,通过减数分裂形成四分体小孢子,导致绒毡层缺失,其表型与ems1/exs和tpd1突变体相似[15]。bam1、bam2[16](BARELYANYMERISTEM1/2)突变体在花药发育早期的细胞分裂中出现异常,双突变体的孢原细胞未分裂形成初生周缘细胞,导致绒毡层、中层和药室内壁等结构缺失[17]。ER(ERECTA)、ERL1、ERL2(ERECTA-LIKE1/2)和MPK3MPK6(MITOGEN-ACTIVATED PROTEIN KINASE3/6)可能在同一途径中调控花药发育,er-105erl1-2erl2-1三突变体和mpk3/+mpk6/-突变体的表型相似,表现为孢原细胞分裂异常,延迟了绒毡层和中层发育形成。在mpk3/+mpk6/-突变体中,EMS1、TPD1等基因的表达未受影响,在ems1突变体中MPK3/6的表达水平变化也不明显,这表明MPK3/MPK6与EMS1、TPD1等基因的调控途径可能不同[18]。
拟南芥AG(AGAMOUS)[19]基因能激活SPL/NZZ基因调控花药发育,而SPL/NZZ转录因子主要在花药发育早期的孢原细胞分裂中起作用[20,21]。在spl/nzz双突变体中,孢原细胞分裂异常,导致绒毡层缺失,没有花粉母细胞的发育和花粉囊壁形成。Wijeratne[22]等人的研究发现AG与SPL/NZZ可能也存在正负调节,AG正向调节SPL/NZZ的表达,而SPL/NZZ基因可能抑制AG基因的表达。水稻中MSP1[23](MULTIPLESPOROCYTE1)的突变会引起绒毡层完全缺失,形成过多的花粉母细胞,与ems1/exs突变体类似,表现出雄性不育。
2.2 bHLH转录因子调控绒毡层发育
碱性螺旋-环-螺旋(basic Helix-Loop-Helix,bHLH)结构域转录因子主要参与调控植物生化过程和发育进程[24~27]。DYT1[28](DYSFUNCTIONALTAPETUM1)在花药的绒毡层中大量表达,位于EMS1/EXS、SPL/NZZ下游,AMS、MS1上游。其发生突变会导致绒毡层细胞过度液泡化,丧失正常功能,花粉母细胞外围的胼胝质形成受到影响。此外,DYT1也参与调控下游基因MYB35、MS1等的表达[29]。
水稻中UDT1、TDR、ETA1和bHLH142都编码bHLH转录因子,前者与绒毡层的分化形成有关,后三者与绒毡层的解体退化有关。UDT1[30](UNDEVELOPEDTAPETUM1)突变体在减数分裂时绒毡层细胞高度液泡化,花粉母细胞不分裂为小孢子,逐渐解体,造成雄性不育。TDR1[31](TAPETUMDEGENERATIONRETARDATION1)的表达与绒毡层的解体有关,其发生突变导致绒毡层与中层延迟解体,引起小孢子败育,导致雄性不育的发生。此外,在udt1突变体中TDR1基因的转录量减少,而在tdr1突变体中UDT1的转录不受影响,表明UDT1可能位于TDR1上游位置。ETA1[32](ETERNALTAPETUM1)基因直接调控与细胞PCD有关的AP25和AP37(ASPARTIC PROTEASE 25/37)两个天冬氨酸蛋白酶的表达,从而调控绒毡层的解体退化。bHLH142[33]与花药绒毡层PCD调控相关,突变体表现出绒毡层延迟解体,花粉母细胞不进行减数分裂,引起完全雄性不育。玉米ms32[34](MALESTERILITY32)突变体无法形成正常功能的绒毡层,绒毡层前体细胞分裂形成体细胞层,并逐渐膨大液泡化,并在花粉母细胞发育时解体,导致绒毡层缺失,引起不育。
2.3 MYB结构域蛋白调控绒毡层发育
生物中含有MYB结构域的蛋白是一个较大的蛋白家族,根据MYB结构域基序数目差异分为MYB1R、R2R3MYB和3RMYB等亚族,其中R2R3MYB转录因子是最大的一个亚类[35]。
拟南芥中AtMYB33、AtMYB65[36]是两个功能冗余的类GAMYB转录因子,在调控花药绒毡层活动中起作用。双突变体myb33myb65表现出绒毡层细胞过度液泡化、膨胀肥大,并向内挤压小孢子,造成败育。AtMYB103[37,38]突变后会引起绒毡层过早解体,小孢子畸形、退化,导致雄性不育。该基因也能调控下游胼胝质解体基因A6,小孢子外壁形成基因MS1、MS2的表达。TDF1(TAPETALDEVELOPMENTANDFUNCTION1)编码的R2R3 MYB转录因子主要在绒毡层细胞、花粉母细胞中参与转录调控。在tdf1突变体中,绒毡层畸形发育,胼胝质未降解释放小孢子,引起雄配子败育。Zhu[39]等人的研究表明,该基因还与上游的AtMYB103、MYC型转录因子AMS[40]、下游的DYT1协同参与调控绒毡层的发育。
2.4 PHD-finger结构域蛋白调控绒毡层活动
PHD-finger结构域转录因子在玉米、拟南芥中最先发现,后来在人类中也发现存在该类结构域的转录因子。拟南芥的MS1[41~43](MALESTERILITY1)与含PHD-finger结构域的蛋白具有很强同源性。在ms1突变体中,小孢子能从四分体中正常释放,但随后绒毡层细胞高度液泡化,花粉外壁无法形成,进而退化降解,导致败育。PTC1[44](PERSISTENT TAPETAL CELL1)是水稻中一个PHD-finger蛋白,在花药发育中该基因在绒毡层细胞和小孢子中都有表达,但其突变体的绒毡层细胞出现一种类似细胞凋亡的状态,导致绒毡层延迟解体,小孢子败育,花粉粒形态异常。
综合上述对DYT1、TDF1、MYB35以及下游AMS、MS1和AtMYB103的调控表达关系,在调控绒毡层发育中可能存在两个分支:一条是通过DYT1调控TDF1,进而调控下游基因表达;另一条是通过DYT1调控MYB35,进而调控下游基因表达(图2)。

图2 拟南芥花药发育部分基因调控网络
3 花粉母细胞减数分裂相关基因
与动物直接形成雌、雄配子不同,植物雄蕊中的花粉母细胞经过减数分裂Ⅰ期和Ⅱ期,形成胼胝质包围的四分体小孢子,随后胼胝质解体释放小孢子。小孢子经过两次有丝分裂:第一次有丝分裂形成2-胞花粉粒;第二次有丝分裂时,生殖细胞再分裂形成两个生殖细胞,最后形成成熟的3-胞花粉粒[45]。
减数分裂前期Ⅰ同源染色体的联会配对关系到后期Ⅰ同源染色体的有序分离。拟南芥RCK(ROCK-N-ROLLERS)[46]基因参与调控减数分裂同源染色体的联会和二价体的形成。其发生突变后同源染色体联会和交叉紊乱,二价体数目减少,出现单价体,影响花粉母细胞正常的减数分裂,导致花粉败育。ZYP1[47]基因编码的蛋白与其他物种中的联会复合体横丝蛋白具有相似的结构。zyp1突变体没有形成联会复合体,严重延迟了减数分裂Ⅰ期的进程。水稻ZEP1[48]基因编码的横丝蛋白是联会复合体的中心元件,与ZYP1同源。在突变体中,前期Ⅰ同源染色体交叉正常,但联会复合体未能正常装配,影响减数分裂正常发生。CRC1(CENTRAL REGION COMPONENT 1)[49]联会复合体蛋白与ZEP1共同构成联会复合体中心组件。crc1突变体在减数分裂的偶线期同源染色体未配对,形成24个单体,后期染色体不均等分离,形成不同染色体数目的小孢子,造成败育。水稻OsDMC1[50]基因失去功能后会影响减数分裂前期I的同源染色体联会配对,出现单体,从而引起小孢子染色体数目差异,导致败育。此外,与同源染色体配对联会相关的基因还有PAIR1[51]、PAIR2[52]和PAIR3[53]等,在各突变体中,同源染色体配对异常形成单价体,导致二价体减少,造成后期Ⅰ染色体分离紊乱。
纺锤体在染色体正常分离的过程中具有重要作用。MPS1[54]是拟南芥中一种含有卷曲螺旋结构域的蛋白,与纺锤体的形成有关。mps1突变体在减数分裂过程中纺锤体畸形,纺锤丝牵引异常,染色体不均等分离,造成败育。CTF7/ECO1(CHROMOSOME TRANSMISSION FIDELITY 7/ESTABLISHMENT OF COHESION 1)[55]主要参与DNA修复、有丝分裂和减数分裂。该基因变异阻碍黏连蛋白的形成,造成减数分裂时花粉母细胞的染色体结构异常,导致突变体花药发育不良,花粉败育发生。水稻的DTM1(DEFECTIVETAPETUMANDMEIOCYTES1)[56]基因编码一个内质网膜蛋白,与早期的绒毡层发育和后期的减数分裂有关。其突变造成花粉母细胞减数分裂过程缓慢,多数减数分裂细胞保持在细线期或偶线期,从而影响花粉母细胞的减数分裂进程。
4 展望
目前在模式植物拟南芥和水稻中发现和鉴定出许多花药发育的相关基因,这些基因准确的时空表达以及基因构成的调控网络对花药结构的形成和花粉育性具有重要作用。在基因调控网络中某些基因产物的功能是互为冗余的,其中一个基因失去功能后,另一个基因产物能够代替或部分代替该基因的功能使调控过程不受影响。但是多数基因发生突变后会对网络下游基因的表达产生影响,从而影响花药结构发育进程,最终影响到花粉育性。
目前,随着科学技术不断进步和分子生物学的深入发展,生物实验技术在基因克隆、基因测序、基因功能验证的应用不断更新和日趋成熟,已在多种植物中发现和鉴定出许多与植物花粉育性相关的基因和其在调控网络中的相对位置。这些基因产物可能直接参与花药结构的形成,或参与信号的传导间接参与调控其他基因表达。但基因与基因间的表达调控作用多数是通过突变体下游基因表达量的变化来间接说明,对其中分子水平的调控机理仍不明晰。而且有关植物花药发育的许多基因尚未被发掘,甚至有些基因可能通过多条途径参与调控。这对植物花药发育调控大网络的完善和雄性不育的实际应用,尤其在作物育种上的应用造成一定困难。希望在不久的将来,在前人研究的基础上能对植物花药发育的基因调控网络有系统的研究和阐述,以期能够在作物生产中更好利用植物雄性不育特性。
[1] Wang D,Skibbe DS,Walbot V.Maize Male sterile 8 (Ms8),a putative β-1,3-galactosyltransferase,modulates cell division,expansion,and differentiation during early maize anther development[J]. Plant Reprod,2013,26(4):329-338.
[2] Sanders PM,Bui AQ,Weterings K,et al.Anther developmental defects inArabidopsisthalianamale-sterile mutants[J]. Sexual Plant Reproduction,1999,11:297-322.
[3] Chaubal R,Anderson JR,Trimnell MR,et al.The transformation of anthers in the msca1 mutant of maize[J]. Planta,2003,216(5):778-788.
[4] Wang CJ,Nan GL,Kelliher T,et al.Maize multiple archesporial cells 1 (mac1),an ortholog of rice TDL1A,modulates cell proliferation and identity in early anther development[J]. Development,2012,139(14):2594-2603.
[5] Wilson ZA,Zhang DB.FromArabidopsisto rice:pathways in pollen development[J]. J Exp Bot,2009,60(5):1479-1492.
[6] Wu HM,Cheun AY.Programmed cell death in plant reproduction[J]. Plant Mol Biol,2000,44(3):267-281.
[7] Zhu J,Lou Y,Xu X,et al.A genetic pathway for tapetum development and function inArabidopsis[J]. J Integr Plant Biol,2011,53(11):892-900.
[8] Wang D,Oses-Prieto JA,Li KH,et al.The male sterile 8 mutation of maize disrupts the temporal progression of the transcriptome and results in the mis-regulation of metabolic functions[J]. Plant J,2010,63(6):939-951.
[9] Johnson KL,Ingram GC.Sending the right signals:regulating receptor kinase activity[J]. Curr Opin Plant Biol,2005,8(6):648-656.
[10] Zhao DZ,Wang GF,Speal B,et al.The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in theArabidopsisanther [J]. Genes Dev,2002,16(15):2021-2031.
[11]Canales C,Bhatt AM,Scott R,et al.EXS,a putative LRR receptor kinase,regulates male germline cell number and tapetal identity and promotes seed development inArabidopsis[J]. Curr Biol,2002,12(20):1718-1827.
[12]Yang SL,Xie LF,Mao HZ,et al.Tapetum determinant1 is required for cell specialization in theArabidopsisanther[J]. Plant Cell,2003,15(12):2792-2804.
[13]Yang SL,Jiang L,Puah CS,et al.Overexpression of TAPETUM DETERMINANT 1 alters the cell fates in theArabidopsiscarpel and tapetum via genetic interaction with excess microsporocytes 1/extra sporogenous cells[J]. Plant Physiol,2005,139(1):186-191.
[14]Jia G,Liu X,Owen HA,et al.Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase[J]. Proc Natl Acad Sci USA,2008,105(6):2220-2225.
[15]Albrecht C,Russinova E,Hecht V,et al.TheArabidopsisthalianaSOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES 1 and 2 control male sporogenesis[J]. Plant Cell,2005,17(12):3337-3349.
[16]Hord CL,Chen C,Deyoung BJ,et al.The BAM1/BAM2 receptor-like kinases are important regulators ofArabidopsisearly anther development[J]. Plant Cell,2006,18(7):1667-1680.
[17]DeYoung BJ,Bickle KL,Schrage KJ,et al.The CLAVATA1-related BAM1,BAM2 and BAM3 receptor kinase-like proteins are required for meristem function inArabidopsis[J]. Plant J,2006,45(1):1-16.
[18]Hord CL,Sun YJ,Pillitteri LJ,et al.Regulation ofArabidopsisearly anther development by the mitogen-activated protein kinases,MPK3 and MPK6,and the ERECTA and related receptor-like kinases[J]. Mol Plant,2008,1(4):645-658.
[19]Ito T,Wellmer F,Yu H,et al.The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS[J]. Nature,2004,430:356-360.
[20]Yang WC,Ye D,Xu J,et al.The SPOROCYTELESS gene ofArabidopsisis required for initiation of sporogenesis and encodes a novel nuclear protein[J]. Genes Dev,1999,13(16):2108-2117.
[21]Schiefthaler U,Balasubramanian S,Sieber P,et al.Molecular analysis of NOZZLE,a gene involved in pattern formation and early sporogenesis during sex organ development inArabidopsisthaliana[J]. Proc Natl Acad Sci USA,1999,96(20):11664-11669.
[22]Wijeratne AJ,Zhang W,Sun Y,et al.Differential gene expression inArabidopsiswild-type and mutant anthers:insights into anther cell differentiation and regulatory networks[J]. Plant J,2007,52(1):14-29.
[23]Nonomura K,Miyoshi K,Eiguchi M,et al.TheMSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice[J]. Plant Cell,2003,15(8):1728-1739.
[24]Heisler MG,Atkinson A,Bylstra YH,et al.a gene that controls development of carpel margin tissues inArabidopsis,encodes a bHLH protein[J]. Development,2001,128(7):1089-1098.
[25]Chinnusamy V,Ohta M,Kanrar S,et al.ICE1:a regulator of cold-induced transcriptome and freezing tolerance inArabidopsis[J]. Genes Dev,2003,17(8):1043-1054.
[26]Andrade-Zapata I,Baonza A.The bHLH factors extramacrochaetae and daughterless control cell cycle in drosophila imaginal discs through the transcriptional regulation of the cdc25 phosphatase string[J]. PLoS Genet,2014,10(3):e1004233.
[27]Andriankaja ME,Danisman S,Mignolet-Spruyt LF,et al.Transcriptional coordination between leaf cell differentiation and chloroplast development established by TCP20 and the subgroup Ib bHLH transcription factors[J]. Plant Mol Biol,2014,85(3):233-245.
[28]Zhang W,Sun Y,Timofejeva L,et al.Regulation ofArabidopsistapetum development and function by DYSFUNCTIONAL TAPETUM 1 (DYT1) encoding a putative bHLH transcription factor[J]. Development,2006,133(16):3085-3095.
[29]Feng B,Lu D,Ma X,et al.Regulation of theArabidopsisanther transcriptome by DYT1 for pollen development[J]. Plant J,2012,72(4):612-624.
[30]Jung KH,Han MJ,Lee YS,et al.Rice Undeveloped Tapetum 1 is a major regulator of early tapetum development[J]. Plant Cell,2005,17(10):2705-2722.
[31]Li N,Zhang DS,Liu HS,et al.The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development[J]. Plant Cell,2006,18(11):2999-3014.
[32]Niu N,Liang W,Yang X,et al.EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice[J]. Nat Commun,2013,4:1445.
[33]Ko SS,Li MJ,Sun-Ben Ku M,et al.The bHLH142 transcription factor coordinates with TDR1 to modulate the expression of EAT1 and regulate pollen development in rice[J]. Plant Cell,2014,26(6):2486-2504.
[34]Moon J,Skibbe D,Timofejeva L,et al.Regulation of cell divisions and differentiation by MALE STERILITY 32 is required for anther development in maize[J]. Plant J,2013,76(4):592-602.
[35]Stracke R,Werber M,Weisshaar B.The R2R3-MYB gene family inArabidopsisthaliana[J]. Curr Opin Plant Biol,2001,4(5):447-456.
[36]Millar AA,Gubler F.TheArabidopsisGAMYB-like genes,MYB33 and MYB65,are microRNA-regulated genes that redundantly facilitate anther development[J]. Plant Cell,2005,17(3):705-721.
[37]Zhang ZB,Zhu J,Gao JF,et al.Transcription factor AtMYB103 is required for anther development by regulating tapetum development,callose dissolution and exine formation inArabidopsis[J]. Plant J.2007,52(3):528-538.
[38]Higginson T,Li SF,Parish RW.AtMYB103 regulates tapetum and trichome development inArabidopsisthaliana[J]. Plant J,2003,35(2):177-192.
[39]Zhu J,Chen H,Li H,et al.Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation inArabidopsis[J]. Plant J,2008,55(2):266-277.
[40]Sorensen AM,Krber S,Unte US,et al.TheArabidopsisABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor[J]. Plant J,2003,33(2):413-423.
[41]Wilson ZA,Morroll SM,Dawson J,et al.TheArabidopsisMALESTERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis,with homology to the PHD-finger family of transcription factors[J]. Plant J,2001,28(1):27-39.
[42]Fernández Gómez J,Wilson ZA.A barley PHD finger transcription factor that confers male sterility by affecting tapetal development[J]. Plant Biotechnol J,2014,12(6):765-777.
[43]Ito T,Shinozaki K.TheMALESTERILITY1 gene ofArabidopsis,encoding a nuclear protein with a PHD-finger motif,is expressed in tapetal cells and is required for pollen maturation[J]. Plant Cell Physiol,2002,43(11):1285-1292.
[44]Li H,Yuan Z,Vizcay-Barrena G,et al.PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice[J]. Plant Physiol,2011,156(2):615-630.
[45]Li T,Gong C,Wang T.RA68 is required for postmeiotic pollen development inOryzasativa[J]. Plant Mol Biol,2010,72(3):265-277.
[46]Chen C,Zhang W,Timofejeva L,et al.TheArabidopsisROCK-N-ROLLERSgene encodes a homolog of the yeast ATP-dependent DNA helicase MER3 and is required for normal meiotic crossover formation[J]. Plant J,2005,43(3):321-334.
[47]Higgins JD,Sanchez-Moran E,Armstrong SJ,et al.TheArabidopsissynaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over[J]. Genes Dev,2005,19(20):2488-2500.
[48]Wang M,Wang K,Tang D,et al.The central element protein ZEP1 of the synaptonemal complex regulates the number of crossovers during meiosis in rice[J]. Plant Cell,2010,22(2):417-430.
[49]Miao C,Tang D,Zhang H,et al.Central region component1,a novel synaptonemal complex component,is essential for meiotic recombination initiation in rice[J]. Plant Cell,2013,25(8):2998-3009.
[50]Deng ZY,Wang T.OsDMC1 is required for homologous pairing inOryzasativa[J]. Plant Mol Biol,2007,65(1-2):31-42.
[51]Nonomura K,Nakano M,Fukuda T,et al.The novel geneHOMOLOGOUSPAIRINGABERRATIONINRICEMEIOSIS1 of rice encodes a putative coiled-coil protein required for homologous chromosome pairing in meiosis[J]. Plant Cell,2004,16(4):1008-1020.
[52]Nonomura KI,Nakano M,Murata K,et al.An insertional mutation in the ricePAIR2 gene,the ortholog ofArabidopsisASY1,results in a defect in homologous chromosome pairing during meiosis [J]. Mol Genet Genomics,2004,271(2):121-129.
[53]Yuan W,Li X,Chang Y,et al.Mutation of the rice gene PAIR3 results in lack of bivalent formation in meiosis[J]. Plant J,2009,59(2):303-315.
[54]Jiang H,Wang FF,Wu YT,et al.MULTIPOLARSPINDLE1 (MPS1),a novel coiled-coil protein ofArabidopsisthaliana,is required for meiotic spindle organization[J]. Plant J,2009,59(6):1001-1010.
[55]Bolaos-Villegas P,Yang X,Wang HJ,et al.ArabidopsisCHROMOSOME TRANSMISSION FIDELITY 7 (AtCTF7/ECO1) is required for DNA repair,mitosis and meiosis[J]. Plant J,2013,75(6):927-940.
[56]Yi J,Kim SR,Lee DY,et al.The rice geneDEFECTIVETAPETUMANDMEIOCYTES1 (DTM1) is required for early tapetum development and meiosis[J]. Plant J,2012,70(2):256-270.
Advances in Genes Related to Tapetum Development and Microsporocyte Meiosis in Anther
LIU Wei-hua,QIU Bo,LUO Hong-bing*
(College of Agronomy,Hunan Agricultural University,Changsha,Hunan 410128,China)
Male sterility in plant is common phenomenon in nature,which is one of important ways for heterosis utilization.There exists vital relevance between pollen fertility and both anther layers development and meiosis during the process of anther development.This paper introduced the gene regulatory expression network of anther development from aspects of tapetal development and meiosis of pollen mother cells in order to further investigate and utilize the male sterility.
plant;anther;male sterility;tapetum;meiosis
2015-03-21
刘伟华(1990-),男,福建南平人,硕士研究生,Email:lwillhall@163.com。
*通信作者:罗红兵,教授,博士生导师,主要从事玉米种质创新的新技术及应用研究,Email:hbluo48@sohu.com。
“十二五”农村领域国家科技计划(2011BAD35B01);湖南省科技计划(2013TP4096);长沙市科技计划(K1406002-61)。
Q78
A
1001-5280(2015)03-0311-06
10.3969/j.issn.1001-5280.2015.03.23

