中国南海群海绵Agelasmauritiana的化学成分研究
方凤凯,汤 华,刘宝姝,庄春林,孙 鹏,张 文(第二军医大学药学院海洋药物研究中心,上海200433)
中国南海群海绵Agelasmauritiana的化学成分研究
方凤凯,汤 华,刘宝姝,庄春林,孙 鹏,张 文(第二军医大学药学院海洋药物研究中心,上海200433)
目的对采自南中国海海域的群海绵(Agelasmauritiana)的化学成分进行研究。方法采用硅胶色谱柱、Sephadex LH-20凝胶色谱柱、高效液相色谱等色谱方法,对Agelasmauritiana正丁醇萃取物进行分离纯化;应用现代波谱技术对化合物进行化学结构鉴定;用M TT法对化合物进行体外人肺癌细胞株A549细胞生长抑制活性测试。结果共分离得到8个化合物,分别鉴定为:agelasine A(1)、agelasine B(2)、epi-agelasine C(3)、(-)agelasine D(4)、agelasine E(5)、agelasine F(6)、(-)ageloxime D(7)、aurantiamide acetate(8)。体外活性筛选中,这些化合物对A549显示出不同程度的生长抑制活性,化合物1~3的活性与阳性对照阿霉素相近。结论化合物1、2、4、5、6、8为首次从该种海绵中分离得到。首次选用A549对化合物1~7的活性进行评价,化合物2、3的显著生长抑制活性为进一步深入研究提供了依据。
群海绵;二萜生物碱;aurantiamide acetate;结构鉴定;肿瘤生长抑制活性
群海绵属(Agelas)海绵为寻常海绵纲(Demospoginae)、群海绵目(Agelasida)、群绵科(Agelasidae)动物,主要分布在西印度洋、太平洋热带及亚热带水域,约有12种已经得到明确分类学鉴定[1]。其次生代谢产物主要分为3大类:溴吡咯生物碱、萜类生物碱以及鞘糖脂类[1-4],体外生物学活性测试中主要表现为抗污损、抗微生物、细胞毒性以及抑制Na+,K+-ATPase酶促反应等活性[5-11]。
群海绵(Agelasmauritiana)的化学成分研究起始于20世纪70年代,代谢产物主要包括二萜类生物碱、溴吡咯类生物碱、类胡萝卜素等,这些化合物在体外筛选中表现出抗污损、细胞毒性及抗微生物等活性[1,5,7,9]。本研究采用体外细胞毒活性追踪分离方法,对采自中国西沙群海绵A.mauritiana的正丁醇萃取部分的化学成分进行了研究,分离得到8个化合物,包括7个二萜生物碱(1~7)及一个二肽化合物aurantiamide acetate(8);并运用核磁共振、质谱等现代波谱技术结合文献报道对这些化合物的结构进行了化学结构鉴定(图1)。
1 材料和方法
1.1样品 实验样品于2011年8月采自我国南海海域,立即冷冻备用。样本由中国科学院李锦和研究员鉴定为Agelasmauritiana,样本保存在第二军医大学药学院海洋药物研究中心(编号LG-1)。

图1 化合物1~8的结构图
1.2仪器与试剂 Bruker Varian Inova-600核磁共振仪(德国布鲁克仪器有限公司);Thermo LCQ Ion trap质谱仪(美国赛默飞世尔科技公司);XT5显微熔点测定仪(北京科仪电光仪器厂);SGW-1自动旋光仪(上海精密科学仪器有限公司);Agilent 1200高效液相色谱仪[RID检测器,XDB-C18柱(250 mm×30 mm,5μm)];Agilent 1100高效液相色谱仪[RID检测器;YMC Park-C18柱;(250 mm× 10mm,5μm)](美国安捷伦科技有限公司);Sephadex LH-20凝胶由Amersham Pharmacia Biotech生产;TLC薄层板和柱色谱硅胶均由烟台黄务硅胶开发实验厂提供;开放柱色谱所用的溶剂为分析纯;高效液相色谱(HPLC)所用的试剂为色谱纯,均由国药集团上海化学试剂公司生产。
1.3化合物的提取分离 样品湿重质量约为712.2 g,粉碎,丙酮超声提取8次(2 L/次,30 min),合并丙酮提取液,减压浓缩得粗浸膏13.2 g,用1.5 L的蒸馏水混悬,用乙醚萃取5次(1.5 L/次),减压浓缩得乙醚浸膏2.1 g。剩余部分用正丁醇萃取5次(1 L/次),减压浓缩得正丁醇浸膏4.7 g。正丁醇浸膏经大孔吸附树脂梯度洗脱(水∶乙醇=100∶0至纯乙醇)分成9个部分(Fr.1~Fr.9)。Fr.5部分(700 mg)经Sephadex LH-20凝胶色谱柱(CH2Cl2∶MeOH=1∶1)、反相硅胶色谱柱(C18键和硅胶,MeOH∶H2 O=75∶25)、反相高效液相色谱(XDB-C18柱,流动相:35%乙腈-水,流速:18 m l/m in,柱温:35℃)纯化,得到化合物1(19.5 mg,4.3 min)、化合物2(30.5 mg,18.0 min)、化合物3(8.8 mg,20.6 min)、化合物4(18.5 mg,19.3 m in)、化合物5(5.1 mg,23.7 min)、化合物6(5.4 mg,22.5 min)、化合物7(2.0mg,26.3 min),Fr.7部分经Sephadex LH-20凝胶色谱柱(CH2 Cl2∶MeOH=2∶1)、正相硅胶色谱柱(400~600目硅胶,石油醚∶乙酸乙酯=5∶2)、反相高效液相色谱(YMC Park-C18柱,流动相:75%甲醇-水,流速:1.5 m l/min,柱温:35℃)纯化得到化合物8(5.1 mg,23.7 min)。
2 化合物鉴定
2.1化合物1 棕黄色无定形粉末(CHCl3),mp 168.7~176.8℃;[α]20D=-35.2°(c 0.98,CHCl3);ESI-MS(m/z):422.33[M]+;1H NMR(600 MHz,CDCl3,δ):10.44(s,1H),8.44(s,1H),7.15(brs,2H),5.57(brs,J=5.22 Hz,2H),5.41(brs,J=5.58 Hz,2H),5.14(brs,1H),4.05(s,3H),0.85~2.00(m,14H),1.84(brs,3H),1.56(s,3H),0.96(s,3H),0.75(d,J=6.7 Hz,3H),0.68(s,3H);13C NMR(150 MHz,CDCl3,δ);17.45(C-1),24.36(C-2),120.30(C-3),144.37(C-4),40.95(C-5),36.24(C-6),27.38(C-7),36.20(C-8),38.11(C-9),46.35(C-10),33.53(C-11),36.72(C-12),147.73(C-13),115.61(C-14),48.63(C-15),19.86(4-CH3),33.07(5-CH3),16.00(8-CH3),17.92(9-CH3),17.27(13-CH3),156.02(C-2′),149.49(C-4′),109.83(C-5′),152.88(C-6′),141.61(C-8′),31.94(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[8]报道的agelasine A一致,确定化合物1为agelasine A。
2.2化合物2 白色无定形粉末(CHCl3),mp 183.8~184.7℃;[α]20D=-17.1°(c 1.0,
CH3OH);ESI-MS(m/z):422.41[M]+;1H NMR(600 MHz,CDCl3,δ):10.96(s,1H),8.50(s,1H),6.87(brs,2H),5.73(brs,2H),5.42(brs,1H),5.17(brs,1H),4.09(s,1H),1.14~2.02(m,15H),1.87(s,3H),1.57(s,3H),0.98(s,3H),0.77(s,3H),0.71(s,3H);13C NMR(150 MHz,CDCl3,δ):18.57(C-1),27.13(C-2),120.58(C-3),144.76(C-4),38.41(C-5),37.02(C-6),27.67(C-7),35.52(C-8),38.94(C-9),46.66(C-10),33.36(C-11),36.55(C-12),149.82(C-13),116.12(C-14),49.13(C-15),17.89(4-CH3),20.15(5-CH3),18.57(8-CH3),18.21(9-CH3),16.29(13-CH3),156.22(C-2′),152.53(C-4′),110.22(C-5′),147.95(C-6′),142.51(C-8′),32.35(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[8]报道的agelasine B一致,确定化合物2为agelasine B。
2.3化合物3 白色无定形粉末(CHCl3),mp 123.1~125.3℃;[α]20D=+31.06°(c 0.29,CHCl3);ESI-MS(m/z):422.40[M]+;1H NMR(600 MHz,CDCl3,δ):10.85(s,1H),8.59(s,1H),6.75(brs,2H),5.64(brd,J=6.60 Hz,2H),5.38(brt,J=6.66 Hz,1H),5.31(brs,1H),4.07(s,3H),0.77~2.08(m,13H),2.00(s,3H),1.83(s,3H),0.97(s,3H),0.83(s,3H),0.82(brd,J=3.48 Hz,3H);13C NMR(150MHz,CDCl3,δ):116.24(C-1),23.42(C-2),31.51(C-3),31.47(C-4),43.94(C-5),30.28(C-6),31.28(C-7),43.94(C-8),42.30(C-9),145.70(C-10),29.59(C-11),34.18(C-12),148.22(C-13),115.94(C-14),48.87(C-15),28.04(4α-CH3),27.95(4β-CH3),14.66(8-CH3),23.25(9-CH3),16.61(13-CH3),156.37(C-2′),148.22(C-4′),110.15(C-5′),152.55(C-6′),117.70(C-8′),32.13(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[8,9]报道的epi-agelasine C一致,确定化合物3为epi-agelasine C。
2.4化合物4 白色无定形粉末(CHCl3),mp 188.3~190.2℃;[α]20D=-12.3°(c 1.0,
CH3 OH);ESI-MS(m/z):422.44[M]+;1H NMR(600 MHz,CDCl3,δ):10.36(s,1H),8.40(s,1H),7.08(brs,2H),5.56(brd,J=6.48 Hz,2H),5.40(brt,J=6.48 Hz,1H),4.78(brs,1H),4.43(s,1H),4.06(s,3H),1.86(s,3H),0.69~2.34(m,16H),0.85(s,3H),0.78(s,3H),0.63(s,3H);13C NMR(150MHz,CDCl3,δ):38.53(C-1),19.59(C-2),42.36(C-3),39.92(C-4),56.53(C-5),24.64(C-6),38.53(C-7),148.59(C-8),55.75(C-9),33.80(C-10),39.37(C-11),21.79(C-12),147.44(C-13),116.13(C-14),48.79(C-15),21.80(4α-CH3),33.80(4β-CH3),106.60(8-=CH2),14.72(10-CH3),17.51(13-CH3),156.16(C-2′),149.79(C-4′),110.09(C-5′),151.95(C-6′),116.13(C-8′),32.28(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[8,10]报道的(-)agelasine D一致,确定化合物4为(-)agelasine D。
2.5化合物5 棕黄色无定形粉末(CHCl3),mp 168~172.3℃;[α]20D=-16.88°(c 0.33,CHCl3);ESI-MS(m/z):422.47[M]+:1H NMR(600 MHz,CDCl3,δ):10.12(s,1H),8.53(s,1H),6.72(brs,2H),5.43(brd,J=5.58 Hz,2H),5.07(brs,1H),4.75(brs,1H),4.53(s,1H),4.07(s,3H),0.67~2.17(m,15H),2.12(s,3H),1.59(s,3H),0.90(s,3H),0.86(s,3H);13C NMR(150 MHz,CDCl3,δ):38.35(C-1),23.72(C-2),36.31(C-3),34.90(C-4),53.70(C-5),149.40(C-6),24.84(C-7),32.20(C-8),136.94(C-9),122.58(C-10),26.34(C-11),39.56(C-12),147.73(C-13),115.63(C-14),48.45(C-15),26.06(4α-CH3),28.43(4β-CH3),108.90(6-=CH2),15.87(9-CH3),17.01(13-CH3),156.27(C-2′),149.40(C-4′),109.03(C-5′),152.47(C-6′),139.70(C-8′),31.98(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[8,11]报道的agelasine E一致,确定化合物5为agelasine E。
2.6化合物6 棕黄色无定形粉末(CHCl3),mp 117.5~120.3℃;[α]20D=-6.7°(c 2.0,CHCl3);ESI-MS(m/z):422.49[M]+:1H NMR(600 MHz,CDCl3,δ):10.59(s,1H),8.48(s,1H),6.87(brs,2H),5.63(brd,J=5.40 Hz,2H),5.45(brs,1H),5.41(brs,1H),5.01(s,1H),4.06(s,3H),0.90~2.00(m,13H),1.86(s,3H),1.59(s,3H),1.56(s,3H),0.85(d,J=6.42 Hz,3H),0.84(s,3H);13C NMR(150 MHz,CDCl3,δ):25.53(C-1),27.04(C-2),122.42(C-3),139.59(C-4),40.39(C-5),33.22(C-6),34.26(C-7),35.19(C-8),137.15(C-9),124.07(C-10),26.14(C-11),38.30(C-12),146.90(C-13),115.87(C-14),48.65(C-15),19.20(4-CH3),15.86(5-CH3),21.05(6-CH3),16.28(9-CH3),17.36(13-CH3),156.18(C-2′),149.63(C-4′),110.00(C-5′),152.48(C-6′),141.75(C-8′),31.98(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[8,11]报道的agelasine F一致,确定化合物6为agelasine F。
2.7化合物7 棕黄色无定形粉末(CH3 OH),mp 99.5~100.8℃:[α]20D=-7.029°(c 0.84,CHCl3);ESI-MS(m/z):440.60[M+H]+:1H NMR(600 MHz,CH3 OD,δ):8.06(s,1H),7.96(s,1H),5.30(brt,J=6.58 Hz,2H),4.98(brd,J=4.68 Hz,1H),4.49(brs,1H),4.25(brs,1H),4.18(brs,1H),2.92(s,3H),0.81~2.10(m,16H),1.60(s,3H),1.53(s,3H),1.04(s,3H),0.75(d,J=2.82 Hz,3H);13C NMR(150 MHz,CH3 OD,δ):40.70(C-1),19.33(C-2),43.31(C-3),40.70(C-4),56.79(C-5),25.60(C-6),39.41(C-7),149.78(C-8),57.69(C-9),34.49(C-10),40.60(C-11),25.60(C-12),146.90(C-13),145.40/144.90(C-14),45.90/41.57(C-15),16.28/16.08(13-CH3),106.92(8-=CH2),34.07(4α-CH3),20.41(4β-CH3),15.05(10-CH3),157.67/157.32(C-2′),161.48/160.59(C-4′),99.17/97.18(C-5′),161.99/159.65(C-6′),165.91/166.51(C-8′),28.21(9′-NCH3)。MS、1H NMR和13C NMR数据与文献[10]报道的(-)ageloxime D一致,确定化合物7为(-)ageloxime D。
2.8化合物8 白色无定形粉末(CHCl3),mp 178.3~179.4℃;[α]20D=-44.08°(c 0.56,CHCl3);ESI-MS(m/z):445.35[M+H]+;1H NMR(600 MHz,CDCl3,δ);7.03~7.72(m,15H,A r-H),6.82(d,J=8.12 Hz,1H),6.09(d,J=8.45 Hz,1H),4.77(m,1H),4.32(m,1H),3.93(dd,J=11.12 Hz,4.37 Hz,1H),3.82(dd,J=11.06 Hz,4.37 Hz,1H),3.21(dd,J=13.68 Hz,6.82 Hz,1H),3.06(dd,J=13.72 Hz,8.34 Hz,1H),2.75(m,2H),2.02(s,3H);13C NMR(150 MHz,CDCl3,δ):20.93(C-1),170.92(C-2),64.73(C-3),49.59(C-4),170.44(C-6),55.11(C-7),167.27(C-9),38.56(C-10),37.57(C-11),133.79(C-1′),124.19(C-2′,6′),128.76(C-3′,5′,3‴,5‴),132.0(C-4′),136.75(C-1″),128.87(C-2″,6″),129.43(C-3″,5″),127.26(C-4″),136.85(C-1‴),129.26(C-2‴,6‴),126.87(C-4‴)。MS、1H NMR和13C NMR数据与文献[12,13]报道的一致,确定化合物8为aurantiamide acetate。
3 细胞毒性实验
实验选择人肺癌细胞株A549作为测试细胞株,采用四氮唑盐还原法(M TT法)进行活性检测,以阿霉素作为阳性对照药物[14]。受试化合物对A549细胞生长抑制活性见表1。
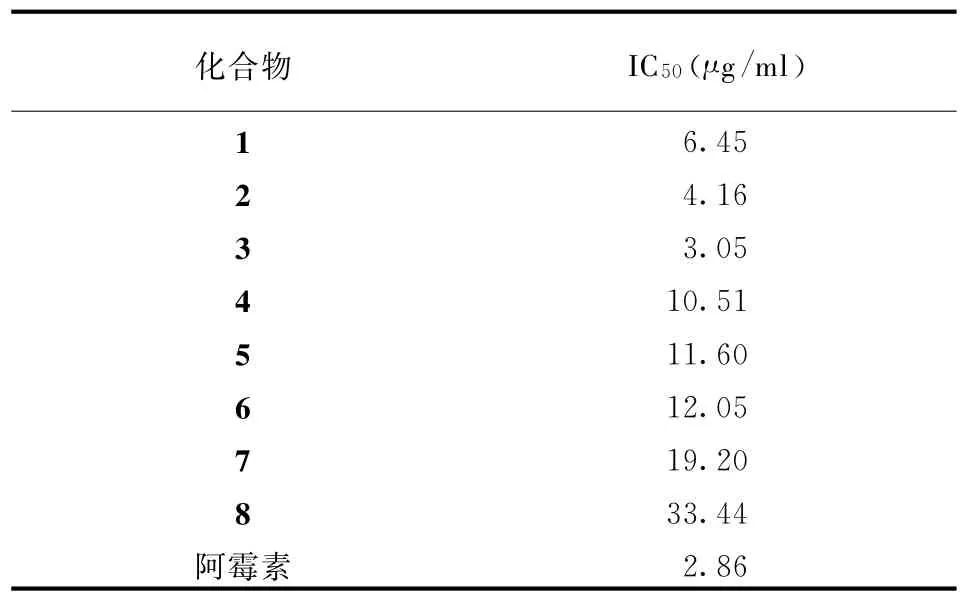
表1 化合物1~8对A549细胞株的生长抑制活性
4 讨论
从群海绵中共分离得到两类化合物,包括7个二萜生物碱(1~7)及一个二肽aurantiam ide acetate(8)。除化合物3和7以外,其余化合物均为首次从该种海绵中分离得到。二肽aurantiamide acetate(8)曾从多种中草药植物中分离得到,在海绵中,仅在Sigmadocia cym iform ls Esper中曾有报道[12,13,15]。本次报道是第二次从海绵中发现该化合物。有意思的是化合物(-)ageloxime D(7)在氘代甲醇中由于次黄嘌呤上肟基与邻位氮原子间质子转移产生的结构互变,导致其13C NMR谱图中部分碳信号会裂分成两组不同的信号[16]。
在体外细胞毒性测试中,这些化合物对人肺腺癌细胞株A549显示出不同程度的生长抑制活性,化合物2、3的活性与阳性对照阿霉素相近(表1)。这是首次选用A549细胞株对化合物1~7进行生物活性测试。这些化合物对肿瘤细胞株A549显著的生长抑制活性为其进一步深入研究提供了依据。【参考文献】
[1] Gordaliza M.Terpenyl-purines from the sea[J].Mar Drugs,2009,7(4):833-849.
[2] Yasuda T,A raki A,Kubota T,et al.Bromopyrrole alkaloids from marine sponges of the genus Agelas[J].J Nat Prod,2009,72(3):488-491.
[3] Putra MY,Jasw ir I.The alkaloids from Indonesian marine sponges[J].Oceanography,2014,2(2):1-10.
[4] Costantino V,Fattorusso E,M angoni A.Glycolipids from sponges,I.Glycosyl ceram ide composition of the marine sponge Agelas clathrodes[J].Eur JO rg Chem,1995,(8):1471-1475.
[5] Tsukamoto S,Kato H,Hirota H,et al.Mauritiam ine,a new antifouling oroidin dimer from themarine sponge Agelas mauritiana[J].JNat Prod,1996,59(5):501-503.
[6] Kondracki1 M LB,Kornprobst JM.M arine pharmacology:potentialities in the treatment of infectious diseases,osteoporosis and alzheimer′s disease[J].Adv Biochem Eng/Biotechnol,2005,97(2):105-131.
[7] Tanaka Y,Soejima T,Katayama T.Biochem ical studies of the carotenoids in porifera distribution of the carotenoids in porifera[J].Bull Jpn Soc Sci Fish,1978,44(11):1283-1285.
[8] Wu HM,Nakamura H,Kobayashi J,et al.Structures of agelasines,diterpenes having a 9-methy ladeninium chromophore isolated from the Okinawan marine sponge Agelas nakamurai Hoshino[J].Bull Chem Soc Jpn,1986,59(8):2495-2504.
[9] Hattori T,Adachi K,Shizuri Y.New agelasine compound from themarine sponge Agelasmauritiana as an antifouling substance againstmacroalgae[J].JNat Prod,1997,60(4):411-413.
[10] Hertiani T,Ebel RE,O rtlepp S,et al.From anti-fouling to biofilm inhibition:new cytotoxic secondary metabolites from tw o Indonesian Agelas sponges[J].Bioorg M ed Chem,2010,18(3):1297-1311.
[11] Wu HM,Nakamura H,Kobayashi J,et al.Agelasine E and F,novel monocyclic diterpenoids w ith a 9-methy ladeninium unit possessing inhibitory effects on Na,K-ATPase isolated from the Okinaw an sea sponge Agelas nakamurai Hoshino[J].Tetrahedron Lett,1984,25(34):3719-3722.
[12] Wang S,Jiang Y,Zeng KW,et al.Antineuroinflammatory constituents from A rtem isia argyi[J].J Chin Pharm Sci,2013,22(4):377-380.
[13] Dosumu OO,Onocha P,Ekundayo O,etal.Isolation of aurantiamides from Gomphrena celosioides C.Mart[J].J Pharm Res,2014,13(1):143-147.
[14] Stone V,Shaw J,Brown DM,et al.The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function[J].Toxicol Vitro,1998,12(6):649-659.
[15] 王明焱,陆伟刚,曾陇梅,等.莳萝曲网海绵化学成分的研究[J].应用化学,2002,19(1):1-3.
[16] Hertiani T.Isolation and structure elucidation of bioactive secondary metabolites from Indonesian marine sponges[D].Düsseldorf:Gedrucktmit Unterstützung des Deutschen Akadem ischen Austauschdienstes,2007:149-150.
Study on chem ical constitunts of sponge Agelasmauritiana from the South China Sea
FANG Fengkai,TANG Hua,LIU Baoshu,ZHUANG Chunlin,SUN Peng,ZHANG Wen(Research Center for Marine Drugs,School of Pharmacy,Second M ilitary Medical University,Shanghai200433,China)
ObjectiveTo investigate the chem ical constituents of marine sponge Agelasmauritiana collected from the South China Sea.MethodsThe n-butanol extract ofmarine sponge Agelasmauritiana was separated and purified by repeated column chromatography on silica gel,Sephadex LH-20,and reversed-phase high-performance liquid chromatography(RPHPLC).The chemical structures of those obtained compoundswere determined on the basis of spectroscopic analysis and comparison with reported data.The tumor cell grow th inhibitory activity of these compounds towards human lung carcinoma cells A549 was tested.ResultsEight compounds were isolated,including agelasine A(1),agelasine B(2),epi-agelasine C(3),(-)agelasine D(4),agelasine E(5),agelasine F(6),(-)ageloxime D(7)and aurantiam ide acetate(8).These compounds displayed different level of tumor cell grow th inhibitory activity towards cell A 549 in vitro.Compounds1-3 showed significantactivity towards cell A 549,being similar to thatof the positive controlof adriamycin.ConclusionCompounds1,2,4,5,6,8 were isolated for the first time from the sponge Agelasmauritiana.Cell A549 was selected for the first time for the activity evaluation of compounds 1~7.Significant inhibition activity of compounds 2、3may hold as a basis for further research.
Agelasmauritiana;diterpene alkaloid;aurantiamide acetate;structure identification;tumor cell grow th inhibitory activity
R284
A
1006-0111(2015)03-0242-05
10.3969/j.issn.1006-0111.2015.03.013
2015-03-05
2015-04-16
[本文编辑] 顾文华
科技部863项目(2013AA092902),科技部国际合作项目(2014DFG32640),上海市卫生系统优秀学科带头人计划(XBR2013111),上海市优秀学科带头人计划(15XD1504600)
方凤凯,硕士研究生.Tel:(021)81871259;E-mail:longineslovers@163.com
张 文,博士,教授,博士生导师.研究方向:海洋活性物质的发现及关键技术研究.Tel:(021)81871257;E-mail:w enzhang1968@163.com

