无配体铜盐催化的碳氮偶联方法
张保集,张明杰
(1.国家知识产权局专利局专利审查协作江苏中心,江苏 苏州 215000;2.天津大学理学院,天津 300072)
无配体铜盐催化的碳氮偶联方法
张保集1,张明杰2
(1.国家知识产权局专利局专利审查协作江苏中心,江苏 苏州 215000;2.天津大学理学院,天津 300072)
摘要:建立了一种简单、高效的芳卤和含氮杂环偶联方法。以10%(摩尔分数)CuI为催化剂、1.1 eq.NaH为碱时,芳基碘、芳基溴和大部分芳基氯能够高效地和含氮杂环反应,高收率得到各种N-芳基化产物。所用无配体铜盐催化剂体系在空气中稳定且易于重复使用。
关键词:N-芳基化反应;铜盐催化剂;配体;碳氮偶联;芳卤;含氮杂环
亲电性的碳与亲核性的氮、氧、硫等杂原子之间形成的芳基碳碳键、碳杂键一直是有机合成化学中的重要单元。C-N偶联反应是合成各种含有C-N键新型药物和材料极其重要的反应[1-2],广泛应用于医药、农药、日用化工、染料等行业。
形成C-N键的简单方法是过渡金属催化含氮杂环与芳卤发生N-芳基化反应,如金属(铜[3-8]、钯[9-11]、铁[12-15]等)盐催化的Ullmann C-N偶联反应。Taillefer研究组[16-18]、Buchwald研究组[19-28]、Teo研究组[29-30]等致力于研究温和条件下铜盐催化的Ullmann C-N偶联反应。在配体存在下C-N偶联反应效率明显改善,配体类型可分为:N,N-配体、O,O-配体、N,O-配体等,常用配体有:四甲基菲啰啉、L-卟啉[31]、四甲基联二萘胺[32]、环己二胺[33]、羟基亚胺[34]、8-羟基喹啉[35]、二亚胺[36]、脯氨酸等。近年来,也有少量文献报道了无配体存在下的C-N偶联反应,但碱的用量超过2 eq.[37-41]。
金属盐催化的C-N偶联反应存在以下问题[42]:催化剂多为过渡贵金属,毒性较强,污染环境;常规催化剂的催化效率低,投料量较大;催化体系一般需要配体辅助才能发挥较好的催化性能,而配体的制备复杂,成本高;反应所得为复杂混合物,不利于产物的提纯;反应温度高;广试性差;配体对空气、水敏感;反应时间长;收率中等;底物范围窄。因此,开发高效、简单的催化体系(如无配体存在、碱用量少、催化剂能重复利用等)对C-N偶联反应极其重要。
鉴于此,作者建立了一种高效、简单、无需配体、无惰性气体保护、能够循环使用的应用于芳卤和含氮杂环的C-N偶联反应催化剂体系。
1实验方法
所用试剂及溶剂均为分析纯,无需预处理。
具体步骤[43]:将NaH 1.1 mmol、吡唑1.2 mmol、DMSO 2.00 mL依次加入25 mL圆口烧瓶中。室温搅拌30 min,再依次加入CuI 0.1 mmol、溴苯1.0 mmol,加热至一定温度,TLC监测反应进度。反应结束后降温至室温,加入10 mL水稀释,用乙酸乙酯萃取3次。合并萃取有机层,用盐水洗涤促进有机层进一步分离,无水硫酸镁干燥,减压蒸馏,柱层析纯化,得产品。
2结果与讨论
2.1 C-N偶联反应条件的筛选
以溴苯和吡唑为底物,对溶剂、催化剂及NaH用量进行筛选,结果见表1。
(1)溶剂的筛选:从表1中1#~7#实验可以看出,以DMSO作溶剂时,目标产物收率(指分离收率,下同)达92%;以DMF或1,4-二氧六环作溶剂时,收率中等;以四氢呋喃、甲苯、乙腈作溶剂时,没有得到目标产物;无溶剂时,目标产物收率达73%。因此,选择DMSO作为C-N偶联反应溶剂。
(2)铜盐催化剂的筛选:不同价态铜盐催化剂的催化性能差异较大。一价亚铜盐优于二价铜盐,零价铜粉活性最差;无催化剂(16#实验)时,收率较低,仅为8%;反应温度较低(17#实验)时,没有目标产物生成。因此,选择CuI作为C-N偶联反应催化剂。
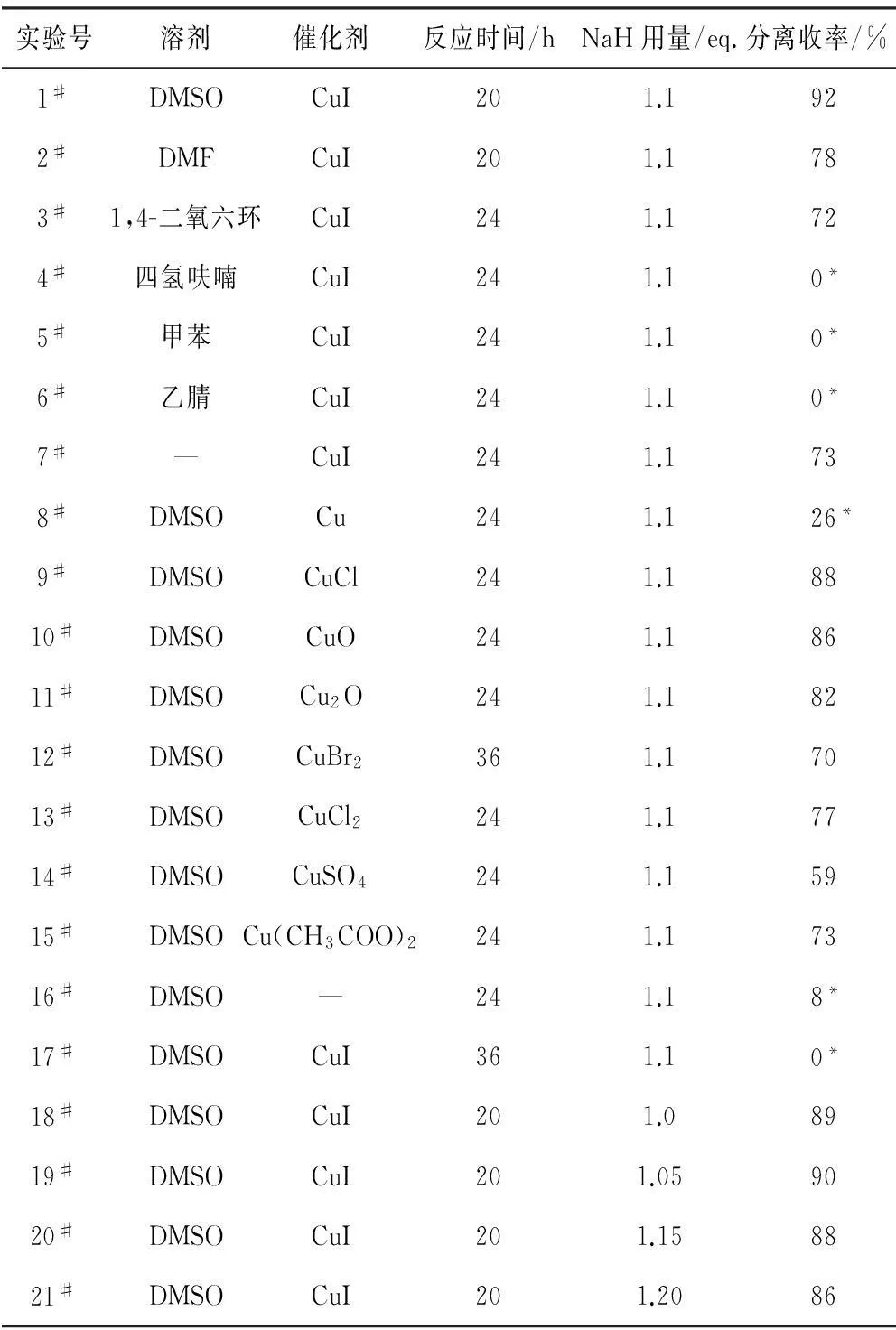
表1 C-N偶联反应条件的筛选Tab.1 Screening on conditions of C-N cross-coupling reaction
反应条件:吡唑1.2 mmol,溴苯1.0 mmol,催化剂0.1 mmol,溶剂2 mL,反应温度120 ℃(17#实验为80 ℃);*为气相收率。
(3)碱用量的筛选:随着NaH用量的增加,收率先升高后降低,在NaH用量为1.1 eq.时,收率最高。因此,选择NaH用量为1.1 eq.。
2.2 不同底物的C-N偶联反应效果
选取含取代基团的芳卤作为亲电试剂、含氮杂环为亲核试剂,在优化条件下进行C-N偶联反应,结果见表2。
从表2可看出:(1)由于溴苯、碘苯及其衍生物的活性较高,底物取代基团的电子效应对反应活性影响较小,N-芳基化产物收率适中。(2)氯代芳烃活性较低,底物取代基团的电子效应对反应活性影响较大。这是因为,吸电基团可以显著降低芳环的电子云密度,有利于杂环化合物中氮原子靠近芳环,相反,供电基团不利于二者反应。含有吸电取代基团的氯代芳烃(10#~16#)相对于含有供电取代基团的氯代芳烃(17#~19#)具有更高的活性。(3)甲基取代基团与反应位点的卤元素处于邻位(6#和7#,10#和13#,11#和14#)时,增加了亲电试剂的进攻难度,收率降低。
2.3 催化剂重复利用性能
以溴苯与吡唑为底物进行C-N偶联反应,反应后所得混合物用乙酸乙酯充分洗涤、离心,将滤出物CuI进行真空干燥。将活化后的催化剂再次投入反应体系中,考察催化剂重复使用性能,结果见表3。
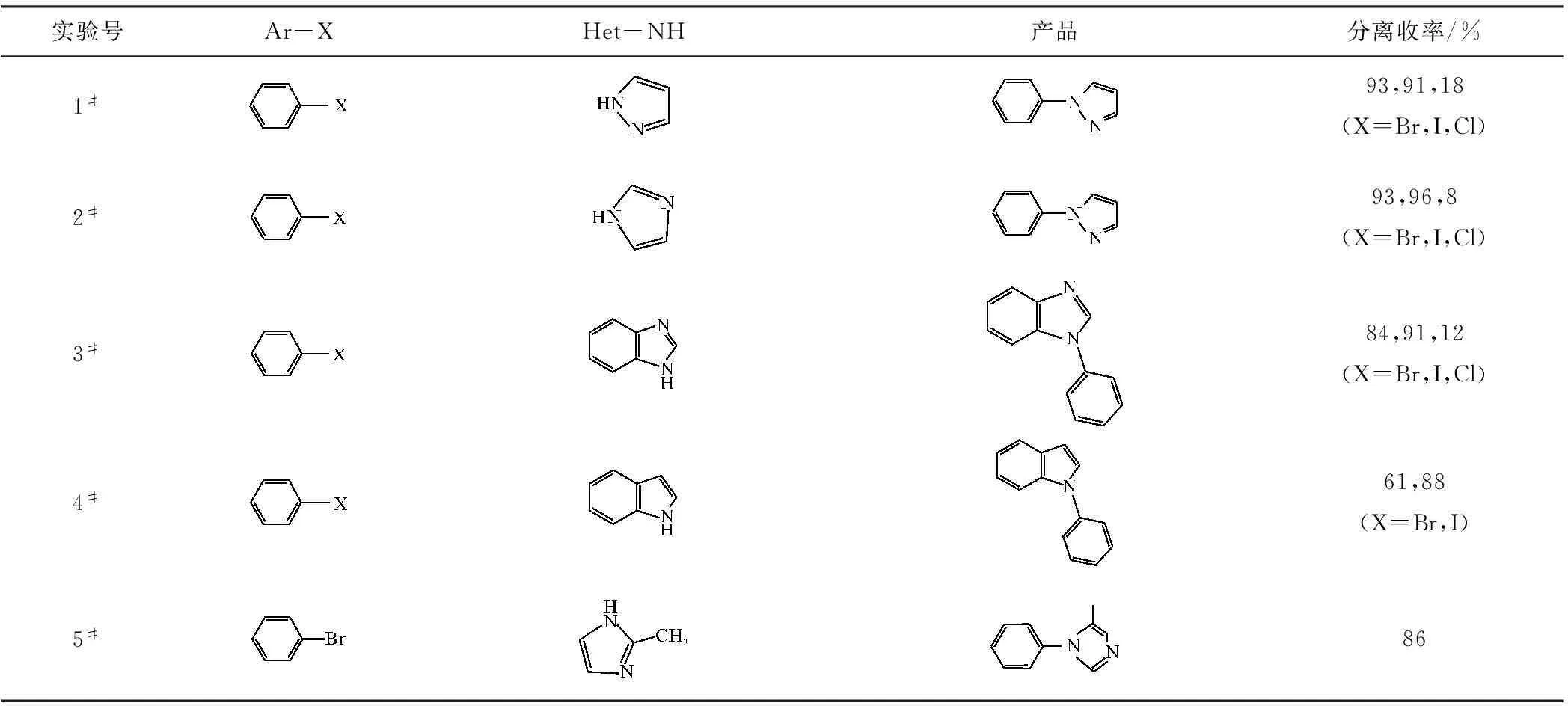
表2 芳卤与含氮杂环C-N偶联反应结果Tab.2 Results of C-N cross-coupling reactions between aryle halides and nitrogen-containing heterocycle
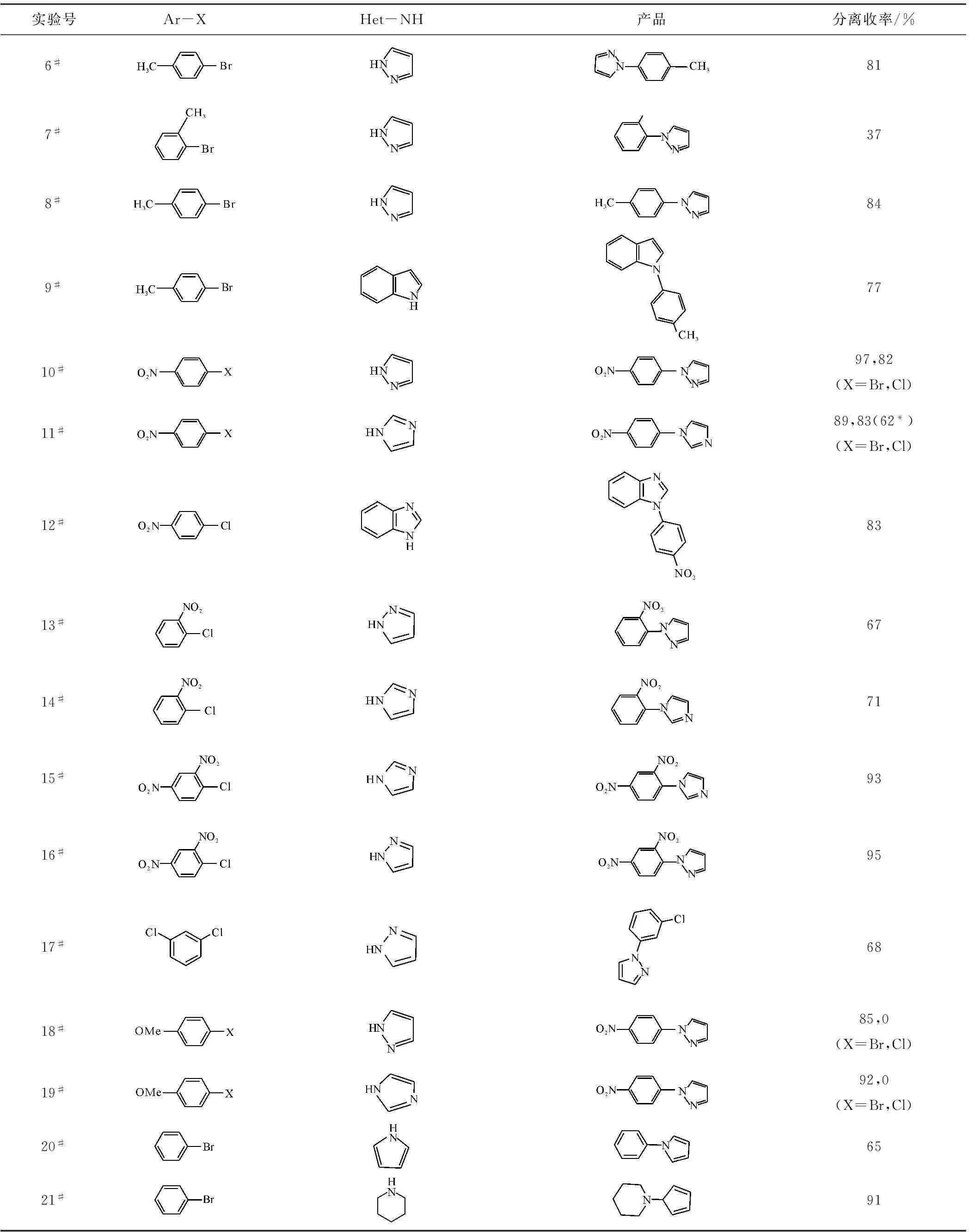
续表2
反应条件:含氮杂环1.2 mmol,NaH 1.1 mmol,芳卤1.0 mmol,CuI 0.1 mmol,DMSO 2 mL,反应温度120 ℃,反应时间24 h;当X=Cl时,1#、2#、3#、18#、19#实验的收率为气相收率;11#实验的催化剂为CuCl。
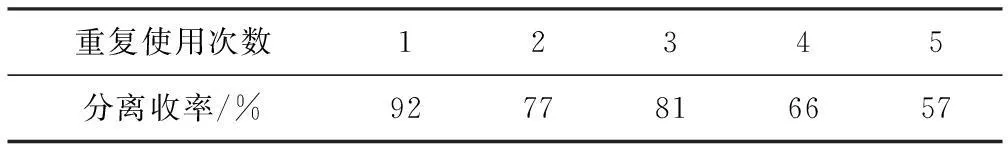
表3 催化剂重复使用性能Tab.3 Recycling performance of catalyst
反应条件:吡唑1.2 mmol,溴苯1 mmol,NaH 1.1 mmol,CuI 0.1 mmol,反应温度120 ℃,反应时间24 h,DMSO 2 mL。
从表3可看出,催化剂重复使用5次后,催化性能虽略有下降,但影响不大。
3结论
开发了一种新型的用于C-N偶联反应的无配体铜盐催化剂,在碱用量为1.1 eq.时,芳基碘、芳基溴及大部分芳基氯能够高效地和含氮杂环反应。该催化剂体系高效、简单、能重复使用。
参考文献:
[1]ZHONG C L,HE J T,XUE L Y,et al.A QSAR study on inhibitory activities of 1-phenylbenzimidazoles against the platelet-derived growth factor receptor[J].Bioorg Med Chem,2004,12(15):4009-4015.
[2]GRAUER A,SPATH A,MA D,et al.Metal-catalyzed derivatization of C(alpha)-tetrasubstituted amino acids and their use in the synthesis of cyclic peptides[J].Chem Asian J,2009,4(7):1134-1140.
[3]KIYOMORI A,MARCOUX J F,BUCHWALD S L.An efficient copper-catalyzed coupling of aryl halides with imidazoles[J].Tetrahedron Letters,1999,40(14):2657-2660.
[4]CRISTAU H J,CELLIER P P,SPIMDLER J F,et al.Mild conditions for copper-catalysed N-arylation of pyrazoles[J].Eur J Org Chem,2004,4:695-709.
[5]CHOUDARY B M,SRIDHAR C,KANTAM M L,et al.Design and evolution of copper apatite catalysts for N-arylation of heterocycles with chloro- and fluoroarenes[J].J Am Chem Soc,2005,127(28):9948-9949.
[6]KANTAM M L,VENKANNA G T,SRIDHAR C,et al.Copper fluorapatite catalyzed N-arylation of heterocycles with bromo and iodoarenes[J].Tetrahedron Letters,2006,47(23):3897-3899.
[7]LÜ X,WANG Z M,BAO W L.CuI catalyzed C-N bond forming reactions between aryl/heteroaryl bromides and imidazoles in[Bmim]BF4[J].Tetrahedron,2006,62(20):4756-4761.
[8]MAHESWARAN H,KRISHNA G G,PRASANTH K L,et al.Bis(μ-iodo)bis((-)-sparteine)dicopper(Ⅰ):Versatile catalyst for direct N-arylation of diverse nitrogen heterocycles with haloarenes[J].Tetrahedron,2008,64(10):2471-2479.
[9]PARRISH C A,BUCHWALD S L.Use of polymer-supported dialkylphosphinobiphenyl ligands for palladium-catalyzed amination and Suzuki reactions[J].J Org Chem,2001,66(11):3820-3227.
[10]URGAONKAR S,NAGARAJAN M,VERKADE J G.P(i-BuNCH2CH2)3N:An effective ligand in the palladium-catalyzed amination of aryl bromides and iodides[J].J Org Chem,2003,68(2):452-459.
[11] XIE X,ZHANG T Y,ZHANG Z.Synthesis of bulky and electron-rich MOP-type ligands and their applications in palladium-catalyzed C-N bond formation[J].J Org Chem,2006,71(17):6522-6529.
[12]CORREA A,BOLM C.Iron-catalyzed N-arylation of nitrogen nucleophiles[J].Angew Chem,2007,119(46):9018-9021.
[13]GUO D L,HUANG H,XU J Y,et al.Efficient iron-catalyzed N-arylation of aryl halides with amines[J].Org Lett,2008,10(20):4513-4516.
[14]SWAPNA K,KUMAR A V,REDDY V P,et al.Recyclable heterogeneous iron catalyst for C-N cross-coupling under ligand-free conditions[J].J Org Chem,2009,74(19):7514-7517.
[15]HANG W L,CHAN A S C,KWONG F Y.Iron complex-catalyzed N-arylation of pyrazoles under aqueous medium[J].Tetrahedron Letters,2009,50(42):5868-5871.
[16]CRISTAU H J,CELLIER P P,SPINDLER J F,et al.Highly efficient and mild copper-catalyzed N- and C-arylations with aryl bromides and iodides[J].Chemistry,2004,10(22):5607-5622.
[17]OSHOVSKY G V,OUALI A,NING X,et al.Thiazolyl phosphine ligands for copper-catalyzed arylation and vinylation of nucleophiles in organic and aqueous media[J].Organometallics,2008,27(21):5733-5736.
[18]MONNIER F,TAILLEFER M.Catalytic C-C,C-N,and C-O Ullmann-type coupling reactions[J].Angew Chem Int Ed,2009,48(38):6954-6971.
[19] WAGAW S,BUCHWALD S L.The synthesis of aminopyridines:A method employing palladium-catalyzed carbon-nitrogen bond formation[J].Org Chem,1996,61(21):7240-7241.
[20]KLAPARS A,ANTILLA J C,HUANG X,et al.A general and efficient copper catalyst for the amidation of aryl halides and the N-arylation of nitrogen heterocycles[J].J Am Chem Soc,2001,123(31):7727-7729.
[21]KLAPARS A,HUANG X,BUCHWALD S L.A general and efficient copper catalyst for the amidation of aryl halides[J].J Am Chem Soc,2002,124(25):7421-7428.
[22]ANTILLA J C,KLAPARS A,BUCHWALD S L.The copper-catalyzed N-arylation of indoles[J].J Am Chem Soc,2002,124(39):11684-11688.
[23]KWONG F Y,KLAPARS A,BUCHWALD S L.Copper-catalyzed coupling of alkylamines and aryl iodides:An efficient system even in an air atmosphere[J].Org Lett,2002,4(4):581-584.
[24] JOB G E,BUCHWALD S L.Copper-catalyzed arylation of beta-amino alcohols[J].Org Lett,2002,4(21):3703-3706.
[25] KWONG F Y,BUCHWALD S L.Mild and efficient copper-catalyzed amination of aryl bromides with primary alkylamines[J].Org Lett,2003,5(6):793-796.
[26] ENGUEHARD C,ALLOUCHI H,GUEIFFIER A,et al.Easy access to novel substituted 6-aminoimidazo[1,2-a]pyridines using palladium- and copper-catalyzed aminations[J].J Org Chem,2003,68(11):4367.
[27]ANTILLA J C,BASKIN J M,NARDER TE,et al.Copper-diamine-catalyzed N-arylation of pyrroles,pyrazoles,indazoles,imidazoles,and triazoles[J].J Org Chem,2004,69(17):5578-5587.
[28]ALTMAN R A,KOVAL E D,BUCHWALD S L.Copper-catalyzed N-arylation of imidazoles and benzimidazoles[J].J Org Chem,2007,72(16):6190-6199.
[29]TEO Y C,YONG F F,POH C Y,et al.Manganese-catalyzed cross-coupling reactions of nitrogen nucleophiles with aryl halides in water[J].Chem Commun,2009,(41):6258-6260.
[30]YONG F F,TEO Y C,TAY S H,et al.A ligand-free copper(Ⅰ) oxide catalyzed strategy for the N-arylation of azoles in water[J].Tetrahedron Letters,2011,52(11):1161-1164.
[31]CHOUHAN G,WANG D,ALPER H.Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles[J].Chem Commun,2007,(45):4809-4811.
[32]RAO R K,NAIDU A B,JASEER E A,et al.An efficient,mild,and selective Ullmann-type N-arylation of indoles catalyzed by copper(Ⅰ) complex[J].Tetrahedron,2009,65(23):4619-4624.
[33]ANTILLA J C,KLAPARS A,BUCHWALD S L.The copper-catalyzed N-arylation of indoles[J].J Am Chem Soc,2002,124(39):11684-11688.
[34]MA H C,JIANG X Z.N-Hydroxyimides as efficient ligands for the copper-catalyzed N-arylation of pyrrole,imidazole,and indole[J].J Org Chem,2007,72(23):8943-8946.
[35]PERIASAMY M,VAIRAPRAKASH P,DALAI M.New diimine-copper complexes:An efficient and simple catalyst system for Buchwald N-arylation of indole[J].Organometallics,2008,27(8):1963-1966.
[36]LIU L B,FROHN M,XI N,et al.A soluble base for the copper-catalyzed imidazole N-arylations with aryl halides[J].J Org Chem,2005,70(24):10135-10138.
[37]KANTAM M L,YADAV J,LAHA S,et al.N-Arylation of heterocycles with activated chloro- and fluoroarenes using nanocrystalline copper(Ⅱ) oxide[J].Adv Synth Catal,2007,349(11-12):1938-1942.
[38]CORREA A,BOLM C.Ligand-free copper-catalyzed N-arylation of nitrogen nucleophiles[J].Adv Synth Catal,2007,349(17-18):2673-2676.
[39]SREEDHAR B,ARUNDHATHI R,LINGA R,et al.CuI Nanoparticles for C-N and C-O cross coupling of heterocyclic amines and phenols with chlorobenzenes[J].J Org Chem,2009,74(20):7951-7954.
[40]COLACINO E,VILLEBRUN L,MARTINEZ J,et al.PEG3400-Cu2O-Cs2CO3:An efficient and recyclable microwave-enhanced catalytic system for ligand-free Ullmann arylation of indole and benzimidazole[J].Tetrahedron,2010,66(21):3730-3735.
[41]GUO D L,HUANG H,ZHOU Y,et al.Ligand-free iron/copper cocatalyzed N-arylations of aryl halides with amines under microwave irradiation[J].Green Chem,2010,12:276-281.
[42]蔡振亚.室温、无配体条件下纳米CuI催化C-N偶联反应的研究[D].合肥:合肥工业大学,2012.
[43]张保集.取代吡唑硼酸合成和碳氮偶联反应研究[D].天津:天津大学,2012.
10.3969/j.issn.1672-5425.2015.12.013
C-N Cross-Coupling Reaction Catalyzed by Ligand-Free Copper Salt
ZHANG Bao-ji1,ZHANG Ming-jie2
(1.Patent Examination Cooperation Jiangsu Center of the Patent Office,SIPO,Suzhou 215000,China;
2.School of Science,Tianjin University,Tianjin 300072,China)
Abstract:A simple and high-efficient C-N cross-coupling method of aryl halides with nitrogen-containing heterocycles was reported.Using 10%(molar fraction) CuI as catalyst and 1.1 eq.NaH as base,aryl iodides,aryl bromides and many aryl chlorides could efficiently react with nitrogen-containing heterocycles,and high yield of N-arylated products were obtained.The ligand-free copper salt catalyst system was stable in air and could be reused.
Keywords:benzene;degradation;domestication;screening;identification;biological filter tower;operation co-nditionsdoi:
中图分类号:TQ 251O 626
文献标识码:A
文章编号:1672-5425(2015)12-0054-05
作者简介:张保集(1985-),男,山东人,硕士,研究方向:有机合成化学,E-mail:zhangbaoji1126@163.com;通讯作者:张明杰,教授。
收稿日期:2015-09-11

