Study on the Redifferentiation Technology after the Dedifferentiation of Blades of Camellia oleifera Abel.
Dongyuan CAI
Hunan Biological and Electromechanical Polytechnic,Changsha 410127,China
Responsible editor:Nanling WANG Responsible proofreader:Xiaoyan WU
Camellia oleifera Abel.is an im portant and peculiar xylophyta edible oil-bearing tree in southern China,it has a wide application and has great value in develop ment and utilization[1-2].C.oleifera seedlings with good quality and high yield have become the focus of the development of C.oleifera industry.Traditional methods of vegetative propagation seedlings like cuttage and grafting can not meet the increasing demand of C.oleifera production to fine seedlings[1-2].Therefore,establishing tissue culture and rapid propagation system of C.oleifera as soon as possible,which has the merits of strong operability,relatively low cost,short reproductive cycle,fast speed and strong vitality,is the key for indus trialized seedling production of C.oleifera tissue culture[2-3].
Using blades and stems with buds of C.oleifera as explant,calluses can easily be got in initial induction dedifferentiation stage[4-6],but because C.oleifera plants are rich in saponin[1,7],it was difficult to carry out morphological organ differentiation of plants in dedifferentiation stage,thus to get re generation plants[4-5,6,8-9]was not easy.For this,based on obtaining callus and embryos cell by initially inducing dedifferentiation of blades of C.oleifera,this paper further studied the redifferentiation technology of plant morphology,to provide a technical support for volume production of C.oleifera tissue culture seedlings.
Materials and Methods
Experimental materials
The experimental materials were calluses generated after the dedifferentiation of blades of “Xianglin No.1”C.oleifera in Plant Tissue Culture Center of Hunan Biological and Electromechanical Polytechnic.
Experimental methods
Making morphological redifferentiation and proliferation mediaTo screen out the media for morphological organ redifferentiation of calluses generated after the dedifferentiation of blades of C.oleifera,two-factor repeated trials were carried out bychanging the fortified concentrations and qualities of NAA and BA.The experiment design was shown in Table1.
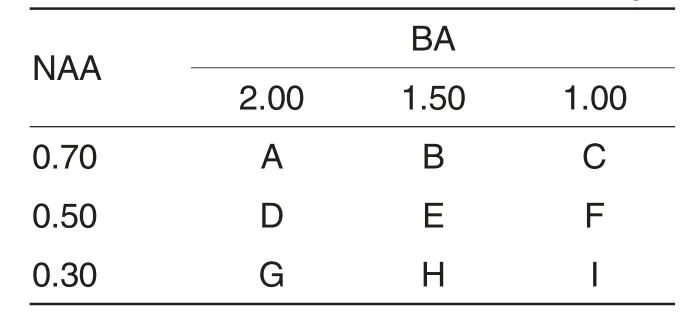
Table1 Two-factor repeated trials on callus redifferentiation after the dedifferentiation of blades of C.oleifera with different fortified concentrations of NAA and BA mg/L
According to 9 media components in Table1,9 150 ml MS media were made,then putting them into 6 culture flasks.The pH values of media was among 5.6-5.8.
InoculationTwo callus lines with different colors and shapes formed after the dedifferentiation of blades of“Xianglin No.1” C.oleifera in primary culture stage were inoculated on the media,namely three inoculation bottles with the features of white-oyster,obvious uneven surface,loose texture and low density,and three inoculation bottles with the features of reseda,unobvious surface undulation and large density.
Conditions of culture after inoculatingThe inoculated materials were cultured in culture room.The temperature of culture room was (25±2)℃.In the first week of culture,the indoor light intensity was 1 000-2 000 lx,the illumination time was 8 -10 h.After that,the illumination time and light intensity should be increased gradually with the redifferentiation of calluses and the occurrence of multiple shoots.
Results and Analyses
Effects of different concentrations of NAA and BA on morphological redifferentiation after the dedifferentiation of blades of C.oleifera
Table2 was the observation and statistics results of calluses generated after the dedifferentiation of blades of C.oleifera in corresponding media for 30 d.Fig.1 - Fig.6 were the process from the formation of calluses of blades of C.oleifera to plant regeneration.From this,we drew that:
(1) Leaves,stems and buds whose calluses with white-light yellow,obvious uneven surface and loose texture had stronger regeneration capacity than that with reseda,unobvious surface undulation and large density.
(2) The optimum medium of the experiment was G medium,namely,MS+NAA (0.3 mg/L)+BA (2.0 mg/L)+saccharose(30 g/L)+agar(7 g/L).From Table2 and Fig.1 - Fig.6,we inferred that the medium can prompt plant regeneration of calluses generated after the dedifferentiation of blades of C.oleifera,to be specific,cytokinin with relatively high concentration and auxin with relatively low concentration can promote the plant regeneration of leaves,stems and buds,etc[10].
Effects of G medium on subculture and multiplication effect of C.oleifera
Under the conditions of (25±2) ℃indoor temperature in the culture room,10-12 h illumination time and the strongest illumination intensity of culture room,regeneration buds of C.oleifera were inoculated on G medium for 30-45 d,namely,MS+NAA(0.3 mg/L)+BA (2.0 mg/L)+saccharose(30 g/L)+agar (7 g/L)(Fig.4),then strong tissue culture seedlings without roots were obtained(Fig.5-Fig.6).After this,further multiplication of subculture was carried out on the medium,the multiplication coefficient was 6.50.
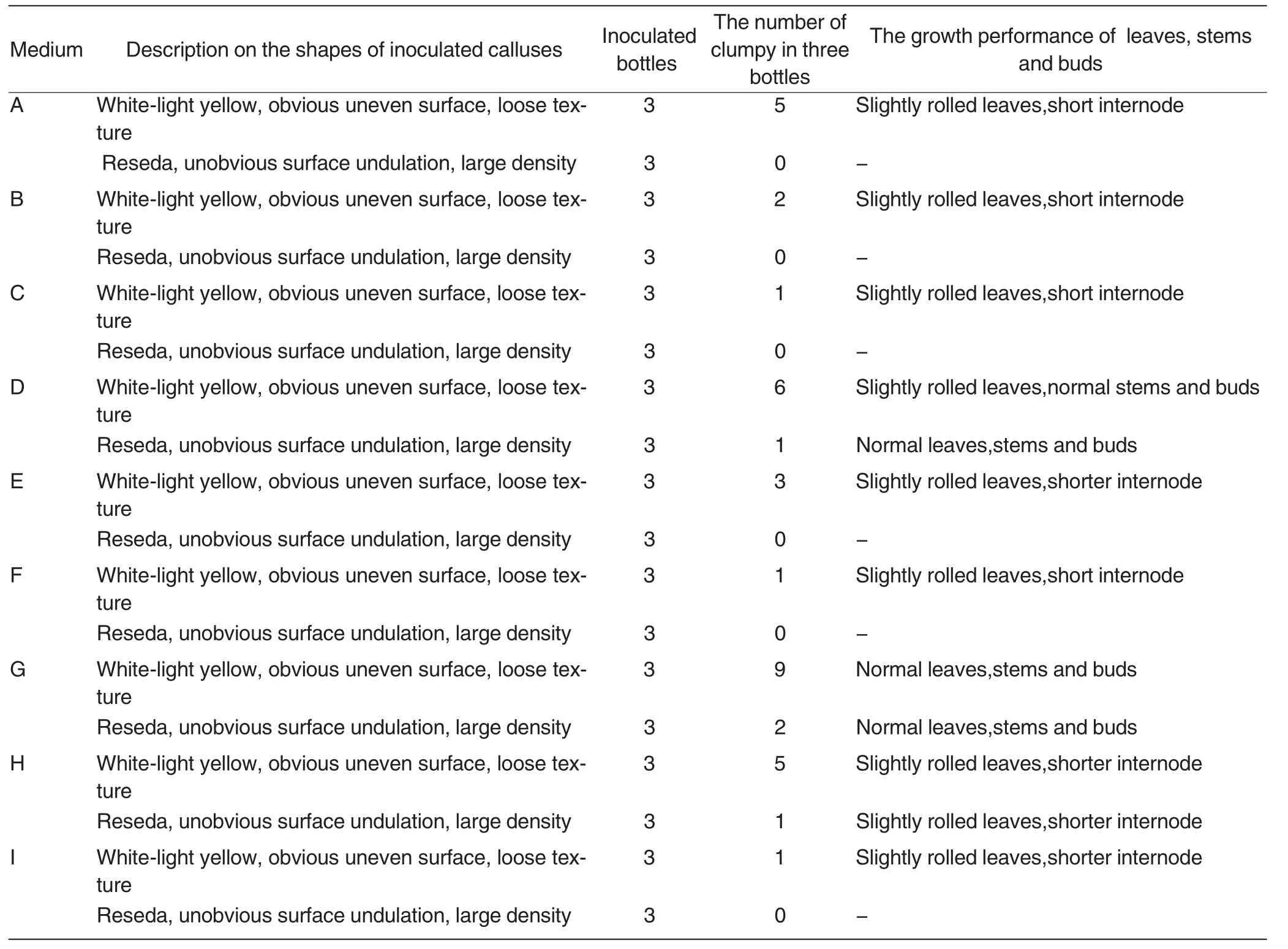
Table2 Comparison on the redifferentiation of C.oleifera on the media with different concentrations of NAA and BA
From above data statistics and experimental results,we drew that in the suitable illumination and temperature,MS+NAA (0.3 mg/L)+BA(2.0 mg/L)was the most suitable condition for multiplication of subculture of C.oleifera.
Conclusions and Discussions
(1) Calluses from blades of C.oleifera generated in initial induction dedifferentiation stage had differences in cell internal structure,shape and color of surface and loose degree of texture; the regeneration capacities of morphological organs of calluses with different structures,colors and shapes had significant differences[2,4,8-9],only calluses with white or yellowish white colors,obvious uneven surface and loose texture,can they have stronger differentiation ability of morphological organs and stronger plant regeneration capacities.
(2)Calluses generated after the dedifferentiation of blades of C.oleifera can successfully carry out redifferentiation of morphological organs under relatively low concentration of NAA(0.3 mg/L)and relatively high concentration of BA (2.0 mg/L).The conclusion was in accordance with the research results of Tang Guotao et al.[3],Zhang Zhijun[6],Wang Duan[8]and Huang Liya et al.[9]who insisted that suitable volume of auxin addition and appropriately improving volume of cytokinin addition can benefit to the redifferentiation of morphological organs of tissue culture seedlings.
(3)C.oleifera is a perennial evergreen woody plant,some components in the plant can easily be oxidized in tissue culture process,thus making media get darker and darker,finally browning phenomenon occurred[1,3,5,11].If observing the change of media in tissue culture process,we should transplant timely.Timely transplant is one of the effective measures to prevent browning[11].
(4)After culturing in the medium of MS+NAA (0.3 mg/L)+BA (2.0 mg/L)+saccharose (30 g/L)+agar (7 g/L)for a period of time,regeneration buds without roots were obtained; if we wanted to obtain whole regeneration plants of C.oleifera with roots,rooting experiments and study should be further done.
[1]ZHUANG RL(庄瑞林).Camellia oleifera of China (中国油茶)[M].Beijing:China Forestry Publishing House(北京:中国林业出版社),1988.
[2]SONG XC (宋晓琛),YU F (于 芬),DAI XY (戴小英),et al.Rooting mechanism with the method of anatomy for tissue culture in Camellia oleifera(油茶组培苗生根的解剖学机理研究) [J].Chinese Agricultural Science Bulletin (中国农学通报),2013,29(34):7-11.
[3]TANG GT(唐国涛),ZHANG HY(张汉永),HUANG JR(黄锦荣),et al.A preliminary study on tissue culture technology of Camellia oleifera(油茶组织培养繁殖技术初步研究)[J].Forestry Science and Technology of Guangdong Province(广东林业科技),2014,30(3):25-29.
[4]YUAN DY (袁德义),FAN XM (范晓明),TAN XF (谭晓风),et al.Culture in vitro and rapid propagation techniques of buds and leafs in Camellia oleifera(油茶带芽茎段及叶片离体培养再生体系的建立)[J].Journal of Nanjing Forestry University(Natural Sciences)(南京林业大学学报(自然科学版)),2013,37(5):35-39.
[5]FAN XM(范晓明).Study on technology about the tissue culture of Camellia oleifera (油茶组织培养技术研究) [D].Changsha:Central South University of Forestry and Technology(长沙:中南林业科技大学),2011:6.
[6]ZHANG ZJ(张智俊),LUO SP(罗淑萍),LI YL (李亚玲),et al.Plant regeneration through somatic embryogenesis formation from cotyledons of Camellia oleifera clone (油茶优良无性系子叶体细胞胚植株再生)[J].Chinese Bulletin of Botany (植物学通报(增刊)),2005,22(B08):43-49.
[7]SHU QL(束庆龙),ZHANG LF(张良富).The planting of Camellia oleifera and diseases and insect pests prevention and control in China(中国油茶栽培与病虫 害 防 治)[M].Beijing:China Forestry Publishing House (北京:中国林业出版社),2009:1-30.
[8]WANG D (王 端),CHEN YZ (陈永忠),WANG XN(王湘南),et al.Study on leaf callus induction of Camellia oleifera superior clone(油茶优良无性系叶片愈伤组织诱导研究)[J].Economic Forest Researches(经济林研究),2009,27(2):35-39.
[9]HUANG LY(黄莉雅),ZHANG RQ(张日清),MA JL (马锦林),et al.Screening of induction conditions of callus and bud in Camellia oleifera(油茶愈伤组织和芽诱导培养条件的筛选)[J].Economic Forest Researches(经济林研究),2010,28(1):30-34.
[10]WU YM(吴幼媚),WANG PL(王鹏良),YAO SC (姚绍嫦),et al.Morphology and anatomy of organogenesis from tissue culture plantlets of Camellia oleifera (油茶组培苗器官发生的形态学与解剖学研究)[J].Guihaia (广西植物),2013,33(6):745-750.
[11]WANG ZL(王振龙),DU GP(杜广平),LI JY (李菊艳).Plant Tissue Culture Curriculum (植物组织培养教程)[M].Beijing:China Agricultural University Press (北京:中国农业大学出版社),2011.
 Agricultural Science & Technology2015年5期
Agricultural Science & Technology2015年5期
- Agricultural Science & Technology的其它文章
- Effects of Exogenous Glycine Betaine on Oxidation Metabolism in Cucumbers during Low-temperature Storage
- A Preliminary Study on Genetic Variation of gE Gene of an Epidemic Pseudorabies Virus Strain and Its Pathogenicity to Piglets
- Development and Application of a Quantitative Competitive PCR Assay for Detecting Mycoplasma hyopneumoniae
- A New Rapid and Batch-oriented Crushing Method for DNA Extraction from Maize Leaves
- Effects of Reducing Application Amount of Base Fertilizer and Increasing Application Time of Leaf Fertilizer on Corn Yield
- Screening,Identification and Fermentation Property of a Yeast Strain R6 Accumulating Alpha-ketoglutaric Acid
