同型半胱氨酸促进血管平滑肌细胞增殖的机制及辛伐他汀的干预作用
席 锐 邹 琛 陈桢玥 吴春芳 陆国平 徐志红
·基础研究·
同型半胱氨酸促进血管平滑肌细胞增殖的机制及辛伐他汀的干预作用
席 锐 邹 琛 陈桢玥 吴春芳 陆国平 徐志红
目的:探讨同型半胱氨酸促进大鼠血管平滑肌细胞(VSMC)增殖的机制及辛伐他汀的干预作用。 方法:贴壁培养的大鼠原代VSMC随机分为3组。(1)对照组:用含10%胎牛血清的DMEM培养基(完全培养基); (2)同型半胱氨酸组:在完全培养基中加入同型半胱氨酸(0.05 mmol/L); (3)辛伐他汀组:在完全培养基中加入同型半胱氨酸(0.05 mmol/L)和辛伐他汀(10 μmol/L)。培养24 h后,光学显微镜下观察各组细胞的形态;采用半定量PCR法检测c-myc 、P27kip1的mRNA水平;用Western blot检测细胞周期蛋白(cyclin )D1、cyclin E、cyclin A和核增殖抗原(PCNA)的蛋白表达水平。 结果:与对照组相比,同型半胱氨酸组c-myc mRNA水平上调,P27kip1mRNA水平下调,下游cyclin D1、cyclin E、cyclin A和PCNA蛋白表达增加。与同型半胱氨酸组相比,辛伐他汀组上述检测指标均得到逆转。 结论:同型半胱氨酸可促进大鼠VSMC c-myc、cyclin D1、cyclin E、cyclin A等增殖相关基因的表达,抑制P27kip1表达。辛伐他汀可逆转上述效应。
同型半胱氨酸;动脉粥样硬化;血管平滑肌细胞
同型半胱氨酸是一种含巯基氨基酸,是体内蛋氨酸分解过程中产生的重要中间产物。临床研究显示,同型半胱氨酸与动脉粥样硬化性疾病(如冠心病、脑血管疾病及外周血管栓塞性疾病等)密切相关,是动脉粥样硬化的独立危险因子[1-11]。同型半胱氨酸的致动脉粥样硬化作用可能与其促进血管平滑肌细胞(VSMC)增殖、导致血管内皮功能障碍以及致炎、促凝和促氧化等作用有关[12-15]。本课题组前期研究证实,同型半胱氨酸可促进大鼠VSMC增殖[16],但具体机制不明。本研究进一步探讨同型半胱氨酸促进VSMC增殖的机制以及辛伐他汀的干预作用。
1 材料与方法
1.1 材料
SD大鼠购自上海中科院动物研究所;兔源细胞周期蛋白(cyclin) D1多克隆抗体以及鼠源核增殖抗原(PCNA)单克隆抗体购自Cell Signaling公司;兔源cyclin A多克隆抗体以及鼠源cyclin E单克隆抗体购自Santa Cruz公司。PCR引物采用Primer 3 软件设计,序列见表1。
1.2 方法
采用组织块贴壁法原代培养SD大鼠的VSMC。取生长状态良好的第4代细胞制成细胞悬液(4×107个/ml),接种于6孔细胞培养板中,每孔1 ml,孵育24 h,换无血清培养24 h后,依次分为3组。(1)对照组:用含10%胎牛血清的DMEM培养基(完全培养基)培养,不加特殊处理;(2)同型半胱氨酸组:完全培养基中加入同型半胱氨酸(0.05 mmol/L);(3)辛伐他汀组:在完全培养基中加入同型半胱氨酸(0.05 mmol/L)和辛伐他汀(10 μmol/L)。各组细胞均设立3个复孔,药物处理时间均为24 h。
采用Trizol法提取细胞总RNA。按逆转录试剂盒说明书合成cDNA。PCR反应条件:94℃变性5 min, 94℃变性45 s,50℃退火1 min,72℃延伸1 min,35个循环,最后再72℃延伸10 min,4℃终止反应。电泳后胶条在凝胶成像仪中摄影,Image Quant软件进行灰度扫描分析。
采用Western blot检测cyclin D1、cyclin E、cyclin A和PCNA的蛋白表达水平。
1.3 统计学分析
计量资料以均数±标准差表示,多组间比较用q检验;计数资料以百分数(%)表示,组间比较用χ2检验;所有数据用SPSS 18.0软件进行分析。P<0.05表示差异有统计学意义。
2 结果
2.1 VSMC形态学变化
对照组细胞排列有序;同型半胱氨酸组细胞排列杂乱,呈团状密集样增生;经辛伐他汀干预后细胞密度降低,细胞体积缩小,细胞间连接紧密(见图1)。
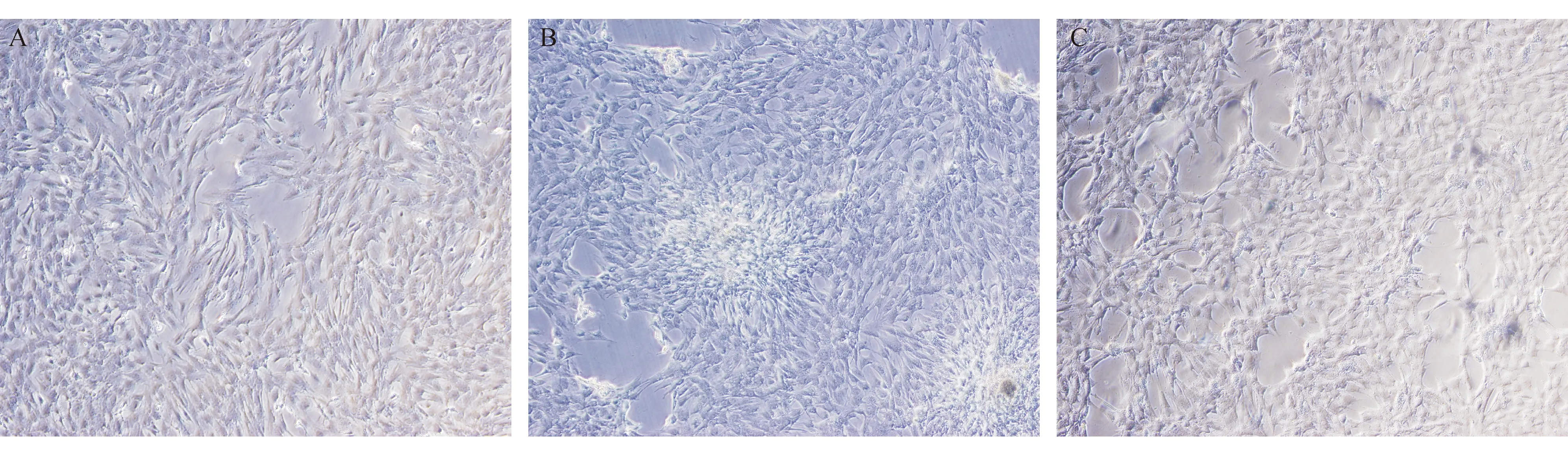
注:A为对照组;B为同型半胱氨酸组;C为辛伐他汀组
2.2 细胞增殖相关基因的表达
与对照组相比,同型半胱氨酸组原癌基因c-myc mRNA水平明显升高,细胞周期蛋白激酶抑制蛋白P27kip1mRNA水平明显下调;与同型半胱氨酸组相比,辛伐他汀组c-myc水平显著降低,P27kip1水平明显上调(P<0.05,见表2)。
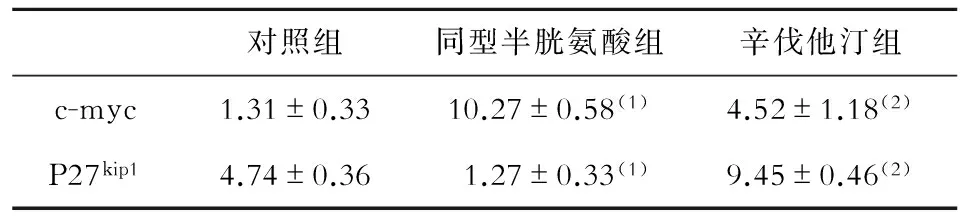
表2 各组c-myc、P27kip1的mRNA相对表达量
与对照组相比,同型半胱氨酸组cyclin D1、cyclin A、cyclin E及PCNA蛋白表达水平明显升高;与同型半胱氨酸组相比,辛伐他汀组上述蛋白表达水平明显降低(P<0.05,见图2、表3)。
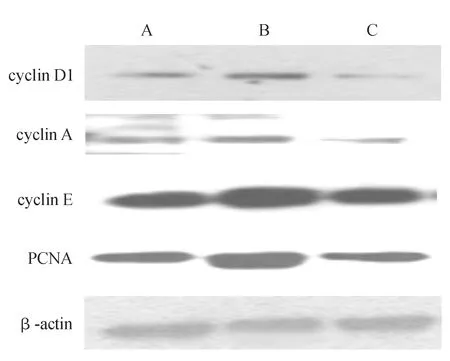
注:A为对照组;B为同型半胱氨酸组;C为辛伐他汀组
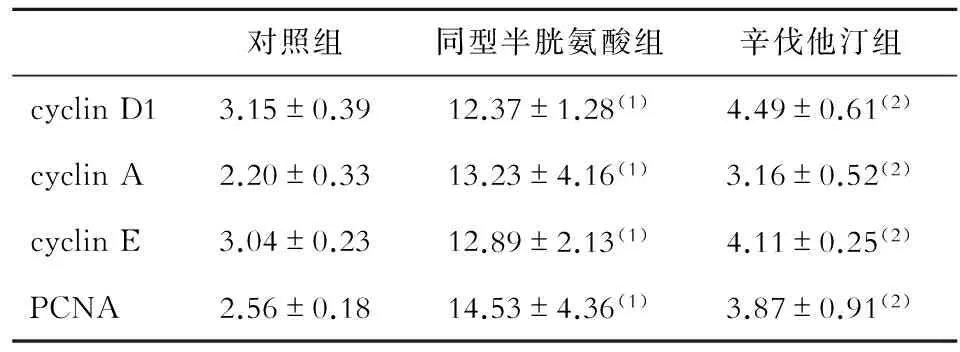
表3 各组cyclin和PCNA蛋白表达水平
3 讨论
同型半胱氨酸促进VSMC增殖的机制尚未阐明,可能与细胞外各种丝裂原激活不同信号传导途径有关。研究发现,同型半胱氨酸可通过兴奋氨基酸受体或氧化还原受体,引起Ca2+内流。一方面激活磷脂酶/三磷酸肌醇传导途径,激活蛋白激酶C,进而激活丝裂素活化蛋白激酶;另一方面可通过钙调蛋白激活钙调蛋白激酶,促进原癌基因c-myc、转录因子c-myb及c-fos的表达,经过丝裂素活化蛋白激酶的催化,促进基因转录,最终导致VSMC增殖[17]。
c-myc是控制细胞生长的基因之一,为myc癌基因家族中关键的一员,其编码产物c-myc蛋白作用于基因调控区,可启动一系列与增殖有关的基因,促进细胞增殖、分化,参与肿瘤发生、转移等病理过程[18-19]。本研究表明,同型半胱氨酸可诱导体外培养的大鼠VSMC c-myc转录增加,说明同型半胱氨酸诱导的VSMC增殖可能与癌基因调控的分子生物学机制有关,但是同型半胱氨酸如何启动c-myc基因转录尚有待进一步研究。
cyclin和细胞周期蛋白依赖性激酶(CDK)是细胞内调节细胞周期的主要物质,cyclin D、cyclin A、cyclin E能诱导细胞进入有丝分裂S期,进而促进细胞增殖[20-21]。P27kip1是细胞周期蛋白依赖性激酶抑制蛋白(CKI)家族中的重要成员之一,可抑制不同的CDK复合物(主要包括cyclin E-CDK2、cyclin D-CDK4、cyclin A等G1期复合物)的活性,使细胞周期不能通过G1-S点而阻断于G1期[22-24]。本研究显示,同型半胱氨酸促进VSMC中 cyclin D1、cyclin E、cyclin A的表达,抑制P27kip1表达,其诱导的VSMC增殖可能与下调P27kip1表达引起cyclin表达升高有关。
辛伐他汀的非调脂作用包括改善内皮细胞功能、抑制VSMC增殖及维持斑块稳定性,长期应用可减少心血管事件的发生[25]。既往研究发现,辛伐他汀抑制同型半胱氨酸诱导的大鼠VSMC增殖主要是通过降低S期和G2/M期百分率、提高G0/G1期百分率,即诱导G0/G1期静止[23]。本研究显示,经10 μmol/L辛伐他汀干预24 h后,P27kip1表达明显上调,同时cyclin D1、cyclin E、cyclin A和细胞增殖的重要指标PCNA也被辛伐他汀显著抑制。P27kip1和cyclin上述协调性表达的结果,可解释辛伐他汀作用于增殖的VSMC后,细胞周期主要停滞在G0/G1期,S期和G2/M期百分率下降,细胞接近静息水平。
本研究初步探讨了同型半胱氨酸诱导大鼠VSMC增殖的机制及辛伐他汀的作用。P27kip1蛋白表达的变化可能是决定VSMC增殖的关键[22-23]。因此,未来研究的重点是全面认识P27kip1基因的作用,以用于控制细胞增殖的基因治疗。
[1] Varol E. Association between serum homocysteine and arterial stiffness: role of antihypertensive drugs[J]. J Geriatr Cardiol,2014,11(2):175-176.
[2] Loscalzo J, Handy DE. Epigenetic modifications: basic mechanisms and role in cardiovascular disease (2013 Grover Conference series).Pulm Circ,2014,4(2):169-174.
[3] Mangge H, Becker K, Fuchs D,et al. Antioxidants, inflammation and cardiovascular disease[J].World J Cardiol,2014,6(6):462-477.
[4] Chen FP, Wang HM, Chiang FF,et al. The metabolic ayndrome is associated with an increased risk of colorectal polyps independent of plasma homocysteine[J].Ann Nutr Metab,2014,64(2):106-112.
[5] Shenoy V, Mehendale V, Prabhu K,et al. Correlation of serum homocysteine levels with the severity of coronary artery disease[J].Indian J Clin Biochem,2014,29(3):339-344.
[6] Yi X, Zhou Y, Jiang D, et al. Efficacy of folic acid supplementation on endothelial function and plasma homocysteineconcentration in coronary artery disease: A meta-analysis of randomized controlled trials[J].Exp Ther Med,2014,7(5):1100-1110.
[7] Baszczuk A, Kopczyński Z. Hyperhomocysteinemia in patients with cardiovascular disease[J].Postepy Hig Med Dosw (Online),2014, 68: 579-589.
[8] Cotlarciuc I, Malik R, Holliday EG, et al. Effect of genetic variants associated with plasma homocysteine levels on stroke risk[J].Stroke,2014,45(7):1920-1924.
[9] Wang CY, Chen ZW, Zhang T, et al. Elevated plasma homocysteine level is associated with ischemic stroke in Chinese hypertensive patients[J].Eur J Intern Med,2014,25(6):538-544.
[10] Bertoia ML, Pai JK, Cooke JP, et al. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease[J].Atherosclerosis,2014,235(1):94-101.
[11] Baszczuk A, Musialik K, Kopczyński J, et al. Hyperhom-ocysteinemia, lipid and lipoprotein disturbances in patients with primary hypertension[J].Adv Med Sci,2014,59(1):68-73.
[12] Han XB, Zhang HP, Cao CJ, et al. Aberrant DNA methylation of the PDGF gene in homocysteine mediated VSMC proliferation and its underlying mechanism[J].Mol Med Rep,2014,10(2):947-954.
[13] Kalani A, Kamat PK, Chaturvedi P,et al. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia[J].Life Sci,2014,107(1-2):1-7.
[14] Guo HY, Xu FK, Lv HT,et al. Hyperhomocysteinemia independently causes and promotes atherosclerosis in LDL receptor-deficient mice[J].J Geriatr Cardiol,2014,11(1):74-78.
[15] Wei HJ, Xu JH, Li MH,et al. Hydrogen sulfide induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway[J].Acta Pharmacol Sin,2014,35(6):707-715.
[16] 徐志红,陆国平,吴春芳.同型半胱氨酸对血管平滑肌细胞增殖与胶原合成变化的影响[J]. 中华老年心脑血管病杂志,2007,9(1):45-48.
[17] Fernandez-Sanchez ME, Gonatopoulos-Pournatzis T, Preston G,et al. S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation[J].Mol Cell Biol, 2009,29(23):6182-6191.
[18] Jiang W, Zhang Y, Meng F,et al. Identification of active transcription factor and miRNA regulatory pathways in Alzheimer′s disease[J].Bioinformatics, 2013,29(20):2596-2602.
[19] Halaby MJ, Hakem R, Hakem A. Pirh2: an E3 ligase with central roles in the regulation of cell cycle, DNA damage response, and differentiation[J].Cell Cycle, 2013,12(17):2733-2737.
[20] Hindley C, Philpott A. The cell cycle and pluripotency. Biochem J, 2013 ,451(2):135-143.
[21] Hu XT, Zuckerman KS. Role of cell cycle regulatory molecules in retinoic acid- and vitamin D3-induced differentiation of acute myeloid leukaemia cells[J].Cell Prolif, 2014,47(3):200-210.
[22] Cerqueira A, Martín A, Symonds CE, et al. Genetic characterization of the role of the Cip/Kip family of proteins as cyclin-dependent kinaseinhibitors and assembly factors[J].Mol Cell Biol,2014,34(8):1452-1459.
[23] Tane S, Ikenishi A, Okayama H. CDK inhibitors, p21(Cip1) and p27(Kip1), participate in cell cycle exit of mammalian cardiomyocytes[J].Biochem Biophys Res Commun, 2014,443(3):1105-1109.
[24] Al-Ghoul WM, Kim MS, Fazal N, et al. Evidence for simvastatin anti-inflammatory actions based on quantitative analyses of NETosis and other inflammation/oxidation markers[J]. Results Immunol, 2014, 4:14-22.
[25] 葛 进,赵 姜.阿托伐他汀对冠心病患者血清ghrelin水平的影响[J].国际心血管病杂志,2013,40(5):326-328.
(收稿:2014-07-21 修回:2014-12-03)
(本文编辑:丁媛媛)
Homocysteine on vascular smooth muscle cell proliferation and effects of simvastatin
XIRui1,ZOUChen1,CHENZhenyue1,WUChunfang1,LUGuoping1,XUZhihong2.
1DepartmentofCardiology; 2DepartmentofGeriatrics,RuijinHospital,ShanghaiJiaotongUniversity,Shanghai200025,China
Objective:To explore the mechanism of homocysteine promoting the proliferation of rat vascular smooth muscle cells (VSMC) and intervention effects of simvastatin. Methods:Primary rat VSMC were divided into 3 groups. Cells in control group were cultured in DMEM with 10% fetal bovine serum. Cells in homocysteine group were treated with 0.05 mmol/L homocysteine,and cells in simvastatin group were treated with 0.05 mmol/L homocysteine and 10 μmol/L simvastatin. Morphology of cells was observed under optical microscope. The mRNA levels of c-myc和P27kip1were analyzed by semi-quantitative PCR, and the protein levels of cyclin D1, cyclin E, cyclin A and PCNA were evaluated by Western blot. Results:Compared with control group, the mRNA level of c-myc and protein levels of cyclin D1, cyclin E, cyclin A and PCNA in homocysteine group were significantly increased, while the mRNA level of P27kip1was decreased. After treatment with simvastatin, P27kip1was upregulated compared with homocysteine group accompanied by the inhibition of c-myc, cyclin D1, cyclin E, cyclin A and PCNA. Conclusion:Homocysteine promotes the expression of proliferation associated genes such as c-myc, cyclin D1, cyclin E, cyclin A and PCNA and inhibits P27kip1expression, which can be reversed by simvastatin.
Homocysteine; Atherosclerosis; Smooth muscle cells
上海市科委重大基础研究项目(054119634)
200025 上海交通大学附属瑞金医院心脏科(席 锐,邹 琛,陈桢玥,吴春芳,陆国平);老年病科(徐志红)
徐志红,Email:zhihxu@163.com
10.3969/j.issn.1673-6583.2015.01.014

