Mechanism analyses of coercivity and remanence enhancement in strontium ferrites
ZAN Fen-lian,MA Yong-qing ,ZHANG Xian,MA Qian,ZHENG Gan-hong,DAI Zhen-xiang
(Anhui Key Laboratory of Information Materials and Devices,School of Physics and Materials Science,Anhui University,Hefei 230039,China)
0 Introduction
M-type strontium hexaferrite(SrFe12O19)was discovered in the 1950s by Philips laboratories[1].As one of ferrous magnetic oxide,it has been intensively investigated during the last years due to its appropriate magnetic properties,chemical stability and low cost compared with rare-earth compounds.It has been recognized that it can be used as permanent magnets,high-density magnetic and magneto-optic recording media,and microwave filters[2-9].
In M-type hexaferrites, the iron ions occupy on five different sites:the octahedral sites,crystallographically known as 2a,12k and 4f2,and the tetrahedral sites 4f1and 2b.In the magnetically ordered state,the 12k,2a and 2b sites(eight Fe3+ions in all)have their spins aligned parallel to each other and to the crystallographic c-axis,whereas spins of 4f1and 4f2sites(four Fe3+ions in all)align in an opposite direction,which leads to the lower saturation magnetization Ms.High-performance permanent magnets for energy-related applications require a large energy product(BH)max.A permanent magnet with a large(BH)maxvalue should exhibit both high remanent magnetization Mrand large coercivity Hc.Both parameters are determined not only by intrinsic properties such as the magnetocrystalline anisotropy Kuand saturation magnetization Ms,but also by structural parameters such as grain sizes and alignment of the granular materials which are sensitive to the preparation conditions.So many reports focus on improving the magnetic properties of SrFe12O19,such as the cationic substitution[10-11],investigation of synthesis method and optimum to the processing conditions,etc.[12-18].In addition,exchange coupling through the interface between hard and soft magnetic phases was found to drastically modify the magnetic properties of nanocomposite combining the high magnetization of a soft-magnetic phase with the high anisotropy of a hard one[19].However,the mechanism for coercivity(Hc)and remanence(Mr)to saturation(Ms)magnetization ratio(Mr/Ms)deserves further investigation which may be useful for improving the magnetic performance of SrFe12O19.
In this work,crystal structure,magnetic properties and exchange-coupling behavior of M-type SrFe12O19prepared by chemical co-precipitation method have been systematically studied by tuning the Fe3+/Sr2+mole ratios in the precursor solution and calcinating temperature.The results show that the lower Fe3+/Sr2+mole ratio(10∶1)greatly reduces the crystallization temperature(about 200 ℃)of single phase SrFe12O19.For the single phase SrFe12O19with Fe3+/Sr2+mole ratio 11∶1 at calcinating temperature of 1 000℃,the optimum magnetic parameters are obtained,the coercivity and saturation magnetization are 4 751 Oe and 62.68 emu·g-1,respectively.For the sample with Fe3+/Sr2+mole ratio 12∶1,soft magnetic γ-Fe2O3and hard magnetic SrFe12O19phases coexist in the sample.The improvement of both coercivity and saturation magnetization has been observed in this sample,which has not been reported before in nanacomposite SrFe12O19ferrites,because the exchange coupling generally leads to the increase of saturation magnetization but the decrease of coercivity in previous reports[19-20].The related mechanism for coercivity and remanence enhancement has been discussed.
1 Experiment
Strontium hexaferrite powders were prepared by the chemical co-precipitation method.The analytically pure ferric nitrate(Fe(NO3)3·9H2O),strontium carbonate(SrCO3),nitric acid(HNO3)and sodium hydroxide(NaOH)were used as starting materials.HNO3was used to dissolve the strontium carbonate and to obtain strontium nitrate solution.First,a series of ferric nitrate(dissolved in distilled water)and strontium carbonate(dissolved in nitric acid)solutions with various Fe3+/Sr2+molar ratios of 12∶1,11∶1 and 10∶1(referred to as A,B and C,respectively)were mixed by gentle heating and stirring for 1 h using a magnetic stirrer.Then,sodium hydroxide as precipitant was slowly poured into the compound solution at room temperature at pH=10.The co-precipitate solution was kept in air for 24 h at room temperature.The coprecipitate was filtered and washed several times using distilled water until the pH value of the solution became neutral,and dried at 90℃ for 24 h.The dried powders were calcined at 500℃ for 5 h,and then sintered at six different temperatures of 600,700,800,900,1 000 and 1 100 ℃ for 2 h in air.The obtained samples are listed in Tab.1.

Tab.1 A list of the samples with different Fe3+/Sr 2+molar ratio and calcinations temperatures
The crystalline structural analysis was performed by an X-ray diffractometer using Cu Kα radiation source.The morphology of samples was investigated by scanning electron microscopy(SEM).The magnetic properties were measured by quantum design superconducting quantum interference device(SQUID)MPMSsystem(T=300 K,0≤H≤2 T).
2 Results and discussion
2.1 Effects of the Fe3+/Sr 2+ratios on the formation of Sr Fe12O19
To investigate the effect of Fe3+/Sr2+ratios on the formation of SrFe12O19,the XRD spectra of all prepared samples have been measured,and Fig.1 representatively plots the results of single-phase and approaching single-phase SrFe12O19ferrites.As shown in Fig.1,A and B samples calcinated at 900 ℃ show the coexistence of SrFe12O19and a bit of γ-Fe2O3phases,and they exhibit the single phase SrFe12O19after calcinated at 1 000 ℃.However,for the C sample,there appear three phases of SrFe12O19,γ-Fe2O3and SrCO3in the sample calcinated at 700℃,and it exhibits the single phase of SrFe12O19after calcinated at 800℃.Obviously,the Fe3+/Sr2+mole ratio plays a crucial rule in the crystallization temperature of singlephase SrFe12O19,the smaller Fe3+/Sr2+mole ratio is propitious for reducing the crystallization temperature of SrFe12O19,consistent with the results observed in previous reports[21-22].

Fig.1 The XRD spectra of the Sr Fe12 O19 samples prepared with different Fe3+/Sr 2+mole ratios under different calcinations temperature
In the case of the chemical synthesis routes,the deviation of Fe3+/Sr2+mole ratio from 12∶1 may result from the difference of the solubility of the Fe3+and Sr2+cations sources.The optimum Fe3+/Sr2+ratio depends on the use of raw materials as well as the synthesis procedure[23].In the synthesis process of our samples,the solubility of Sr(NO3)2is lower than that of Fe(NO3)3in water which results in the decrease of Sr2+participating in precipitation reaction.And therefore the excess Sr2+or insufficient Fe3+sources facilitates the formation of single-phase SrFe12O19[24].Additionally,the increased diffusion rate of metallic ions in the non-stoichiometric mixture due to induced vacancies permits single-phase SrFe12O19formation at lower temperature[25].
2.2 Effects of calcinations temperature on the magnetic properties
The results in the above section indicate that sample C with 10∶1 Fe3+/Sr2+ratio has the lowest crystallization temperature(800 ℃)for single-phase SrFe12O19.In this section,sample C has been chosen to study the effect of calcinations temperature on the magnetic properties.
The magnetic parameters of the C11-C15samples with Fe3+/Sr2+mole ratio of 10 at the sintering temperatures from 700 to 1 100℃,drawn from the results of magnetic hysteresis loop(not shown here),are shown in Tab.2.

Tab.2 The magnetic parameters of Sr Fe12O19 with 10∶1 Fe3+/Sr 2+mole ratio

Where Δσ is stress magnitude and Mssaturation magnetization.For C12,C13and C14samples,Mshardly changes.The decrease of Hcwith increasing calcinations temperature may arise from the decrease of stress magnitude,because repeatedly annealing can effectively remove the stress left in sample.For the C15sample,its saturation magnetization obviously decreases.
As reported before[26],at the higher calcinations temperature,part of the trivalent Fe3+with electronic configuration of 3d5ions will reduce to bivalent Fe2+ions with electronic configuration of 3d6.According to the Hund’s rule,the molecular magnetic moment of Fe3+(5 μB)is larger than that of Fe2+(4 μB),which is responsible for the low saturation magnetization of the C15sample.Carefully checking the XRD results(not shown here),we observe that the diffraction intensities of the C15sample at 2 θ=23.28,31.14 and 51.88°are 799,1 701 and 526,respectively,which are much stronger than those of the C14sample(23.2,229 and 132,respectively).It maybe indicates a certain phase transformation[26].Therefore the minimum Hcof C15sample may result from the competing interaction between the stress magnitude and the phase transformation.
The C11sample has the minimum coercivity Hc(957 Oe),saturation magnetization Ms(12.73 emu·g-1)and remnant magnetization Mr(4.92 emu·g-1),respectively,which are due to the presence nonmagnetic impurity of SrCO3and the crystallization of a minute quantity of SrFe12O19as shown in Fig.1.For the singlephase SrFe12O19(samples C12-C15),the coercivity Hcgradually decreases from 4 654 to 1 053 Oe when the calcinations temperature increases from 800 to 1 100℃.According to the technical magnetization theory,the coercivity Hccan be expressed as In addition,the remanence to saturation magnetization ratio Mr/Msfor C12- C14samples exceeds to 0.5,as predicted by Stoner-Wohlfarth model for isotropic nano-crystalline magnetic materials with uniaxial anisotropy,which can be attributed to the intergrain exchange interactions[27-28].The similar phenomenon occurs in the B14sample as discussed below.
2.3 Effects of the Fe3+/Sr 2+ratios on the magnetic properties
From the Fig.1 we can see that all samples become the single-phase SrFe12O19at the calcinations temperature of 1 000℃.So we chose samples A14,B14and C14calcinated at 1 000℃ for studying the effects of Fe3+/Sr2+ratios on the magnetic properties.
Tab.3 shows the magnetic parameters of samples A14,B14and C14with different Fe3+/Sr2+mole ratios,demonstrating that a lower Fe3+/Sr2+mole ratio than stoichiometry can improve magnetic properties of strontium ferrite powder as reported before[21].In reference [21],the authors suggested that a lower Fe3+/Sr2+mole ratio than stoichiometry leads to the production of iron and oxygen vacancies,enhancing the ionic diffusion and improving the magnetic properties.More specifically,we suggest that,for the A14,B14and C14samples,the content of Fe3+vacancies gradually increases,i.e.,the volume concentration of defects(Fe3+vacancies)increases with the Fe3+/Sr2+mole ratio decreasing from 12∶1 to 10∶1.As these three samples are prepared by the same synthetic route and calcinations temperature of 1 000℃,it can be tentatively suggested that these three samples have the equivalent stress.

Tab.3 The magnetic parameters of A14,B14 and C14
Therefore the coercivity Hcmainly results from the contribution of defects(Fe3+vacancies).In this situation,the coercivity Hccan be expressed as

whereβis the volume concentration of defects,and Mssaturation magnetization.The B14and C14samples have the higherβ value than the A14sample,and therefore they have the higher coercivity Hc.Additionally,the decrease of Fe3+/Sr2+mole ratio results in the grain refinement l from58.7 to 50.0 nm(calculated using MDI Jade 5.0 software from the XRD results),leading to the larger grain boundary area and subsequently producing more nuclei of reversed domain.The coercivity Hcdetermined by nuclei of reversed domain is directly proportional to saturation magnetization Ms

which may be the other reason for the higher coercivity of B14and C14samples than that of the A14sample.
2.4 Exchange-coupling behavior observed in A 12-A 15 samples
In section 2.2 and 2.3,we discussed the effect of calcinations temperature and Fe3+/Sr2+mole ratio on the magnetic properties of single-phase SrFe12O19,respectively.In this section,we will discuss the magnetic interactions in the A12-A15samples.
Fig.2 shows the X-ray diffraction patterns of strontium ferrite powders.The A12and A13samples show the diffraction peak of the γ-Fe2O3.The reflection intensity of the γ-Fe2O3decreases with increasing calcinations temperature and disappears after the sample calcinated at 1 000 ℃ which becomes the single-phase SrFe12O19.

Fig.2 The XRD spectra of the A12- A15samples
The magnetic parameters of A12- A15samples are shown in Tab.4.From Tab.4 we can see that A12and A13samples containing two phases of soft magnetic γ-Fe2O3and hard magnetic SrFe12O19,both the coercivity and the saturation magnetization are higher than those of A14and A15samples just containing single-phase SrFe12O19.In addition,the Mr/Msratio of the A12and A13samples is also larger than that of the A14and A15samples.With increasing the calcinations temperature,the coercivity monotonously decreases.As is wellknown,the coercivity Hcis determined by the effective anisotropy constantthrough[29].For the soft and hard composite system,the effective anisotropy constantcan be expressed by

where fsand fhare the volume fraction,and Ksand Khare anisotropy constant of soft and hard phases,respectively[30].From A12to A15,the volume fraction fsof soft magnetic phase γ-Fe2O3decreases,resulting in the increase of Keff.Khis much larger than Ks,consequently resulting in the increase of Hcdue to.Based on this viewpoint,the single-phase A14and A15samples should have the same Khor Hc,but it is not the case.Therefore,we suggest that the coercivity mechanism here should also be determined by stress anisotropy as given by Eq.(1).

Tab.4 The magnetic parameters of A 12-A 15 samples
Generally,for an assembly of randomly oriented non-interaction crystallites with uniaxial anisotropy polycrystalline,the Mr/Msis 0.5.If the γ-Fe2O3crystallites are exchange-coupled with SrFe12O19,the magnetization direction within each of crystallites is determined by a balance between the magneto-crystalline anisotropy energy which favors alignment of the local magnetization to the local preferred axis,and the exchange interaction which favors mutual alignment of the direction of the magnetization of neighboring grains.The net result of latter effect leads to an increase of Mr/Msjust as the sample calcinated at 800 and 900℃.Besides the intergrain exchange interaction,the“exchange-spring”behavior which results from the reversible rotation of the soft magnetic component for fields not large enough to reverse the hard magnetic phase[31]can also result in the enhancement of remanence magnetization.Thus what is the nature(type and strength)of the intergrain interactions in A12-A15samples?
A usual method to monitor the interactions between the grains is by constructing the δm plots.The δm curves were built using the magnetizing Mr(H)and demagnetizing Md(H)remanent magnetizations.The measuring methods of Mr(H)and Md(H)are identical with those reported in Ref.[20].Mr(H)and Md(H)are normalized by the saturation remanence

and


Any deviations from this law are attributed to interactions between grains.These are monitored usually by plotting the quantity δm which measures the deviations from Eq.(4)according to the following definition[33-35]

The dependence ofδm(H)on the magnetic field H is shown in Fig.3.For the A12and A13samples containing soft magnetic γ-Fe2O3and hard magnetic SrFe12O19phases,theδm values are initially positive(H ≤ 0.6 ×104Oe for the A12sample and H ≤ 0.5 ×104Oe for the A13sample)because the hard phase prevents the demagnetization of the sample due to the presence of magnetizing(exchange-type)interactions.But the values become small negative after reversal,indicating that magnetostatic interactions become dominant.For the A14and A15samples with single-phase SrFe12O19,all the δm values are negative as a result of the cooperative switching of the exchange-coupled grains.The negative peak becomes pronounced for the A15sample.

Fig.3 δm curves for A 12-A 15 samples
Subsequently,we will further discuss the magnetic interactions in A12- A15samples.The derivatives of md(H)and mr(H)with respect to the applied field H are the corresponding irreversible susceptibilities(H)which give a measure of the switching field distributions[34,36],as shown in Fig.4.All the(H)curves exhibit the peaks and corresponding valuesanddrawn from d md/d H and d mr/d H,respectively,are listed in Tab.5.For the A12sample,its/44.05 and/73.13 values are obviously greater than those of A13- A15samples,maybe indicating that the“exchange-spring”appears in the A12sample.

Fig.4 (H)curves for A12- A15 samples
Tab.5 The(H)peaks values of A 12 - A 15 samples 104 Oe-1
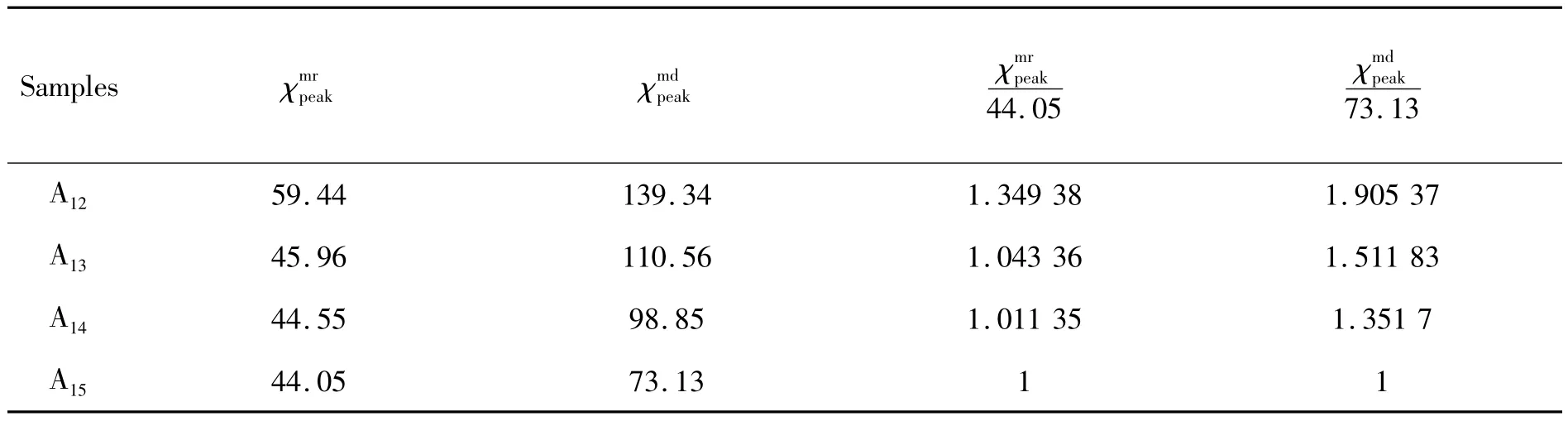
Tab.5 The(H)peaks values of A 12 - A 15 samples 104 Oe-1
Samples χmrpeak χmdpeak χmr peak 44.05 χmd peak 73.13 A1259.44 139.34 1.349 38 1.905 37 A13 45.96 110.56 1.043 36 1.511 83 A14 44.55 98.85 1.011 35 1.351 7 A1544.05 73.13 1 1
3 Conclusion
By tuning the Fe3+/Sr2+mole ratios and calcinations temperature,the crystal structure,magnetic properties and exchange-coupling behavior of M-type strontium hexaferrite prepared by chemical coprecipitation method have been systematically studied.The results show that the lower Fe3+/Sr2+mole ratio(10∶1)greatly reduces the crystallization temperature.
For the single phase SrFe12O19with the Fe3+/Sr2+mole ratio of 10∶1,the increase of calcinations temperature results in the monotonous decrease of coercivity.It can be assigned to decreases of the stress magnitude,and results in the decrease of saturation magnetization,which can be assigned to the certain phase transformation.
For the single phase SrFe12O19calcinated at 1 000℃ with different Fe3+/Sr2+mole ratio,the variation of coercivity may result from the difference of both the volume concentration of deformation(Fe3+vacancies)and nuclei of reversed domain.In addition,the remanence enhancement has been observed in the single phase SrFe12O19samples which can be attributed to the intergrain exchange interactions.
For the samples with Fe3+/Sr2+mole ratio being 12∶1,δm plots indicate exchange type interaction for fields not large enough to switch the hard magnetic phases and magnetostatic interactions for higher fields in the composite ferrites,and complete magnetostatic interactions in the single-phase SrFe12O19ferrite.The results of the irreversible susceptibilitiesχirr(H)drawn from d md/d H and d mr/d H,respectively,indicate that the“exchange-spring”maybe appears in the A12sample.
[1]Thompson G K,Evans B J.The structure-property relationships in M-type hexaferrites:hyperfine interactions and bulk magnetic properties[J].Journal of Applied Physics,1993,73:6295 -6297.
[2]Fu Y P,Lin CH,Pan K Y.Strontium hexaferrite powders prepared by a microwave-induced combustion process and some of their properties[J].Journal of Alloys and Compounds,2003,349:228 -231.
[3]Iqbal M J,Ashiq M N,Pablo H G,et al.Synthesis,physical,magnetic and electrical properties of Al-Gasubstituted co-precipitated nanocrystalline strontium hexaferrite[J].Journal of Magnetism and Magnetic Materials,2008,320:881-886.
[4]Bobzin K,Bolelli G,Bruehl M,et al.Characterisation of plasma-sprayed SrFe12O19coatings for electromagnetic wave absorption[J].Journal of the European Ceramic Society,2011,31:1439 -1449.
[5]Pullar R C,Bdikin I K,Bhattacharya A K.Magnetic properties of randomly oriented BaM,SrM,Co2Y,Co2Z and Co2W hexagonal ferrite fibres[J].Journal of the European Ceramic Society,2012,32:905 -913.
[6]Thakur A,Singh R R,Barman P B.Crystallization kinetics of strontium hexaferrite:correlation to structural,morphological,dielectric and magnetic properties[J].Electronic Materials Letters,2012,8(6):595 - 603.
[7]Bsoul I,Mahmood SH,Lehlooh A F,et al.Structural and magnetic properties ofTixRuxO19[J].Journal of Alloys and Compounds,2013,551:490 -495.
[8]Rai B K,Mishra S R,Nguyen V V,et al.Synthesis and characterization of high coercivity rare-earth ion dopedRE0.1Fe10Al2O19(RE:Y,La,Ce,Pr,Nd,Sm,and Gd)[J].Journal of Alloys and Compounds,2013,550:198-203.
[9]Yasukawa Y,Liu X X,Morisako A.Observation of magnetic/electric domains and control of electric polarization by magnetic field in BiFeO3/SrFe12O19bilayers[J].Journal of Magnetism and Magnetic Materials,2013,327:95 -102.
[10]Song F Z,Shen X Q,Xiang J,et al.Characterization and magnetic properties of BaxSr1-xFe12O19(x=0 -1)ferrite hollow fibers via gel-precursor transformation process[J].Journal of Alloys and Compounds,2010,507:297 - 301.
[11]Rezlescu N,Doroftei C,Rezlescu E,et al.The influence of heat-treatment on microstructure and magnetic properties of rare-earth substituted SrFe12O19[J].Journal of Alloys and Compounds,2008,451:492 -496.
[12]Ataie A,Heshmati-Manesh S.Synthesis of ultra-fine particles of strontium hexaferrite by a modified co-precipitation method[J].Journal of the European Ceramic Society,2001,21:1951 -1955.
[13]Zi Z F,Sun Y P,Zhu X B,et al.Structural and magnetic properties of SrFe12O19hexaferrite synthesized by a modified chemical co-precipitation method[J].Journal of Magnetism and Magnetic Materials,2008,320:2746 -2751.
[14]Wang J F,Ponton C B,Harris I R.A study of Pr-substituted strontium hexaferrite by hydrothermal synthesis[J].Journal of Alloys and Compounds,2005,403:104 -109.
[15]Brito P CA,Gomes R F,Duque J G S,et al.SrFe12O19prepared by the proteic sol-gel process[J].Physica B,2006,384:91 -93.
[16]Ding J,Miao W F,McCormick P G,et al.High-coercivity ferrite magnets prepared by mechanical alloying[J].Journal of Alloys and Compounds,1998,281:32 -36.
[17]Guo Z B,Ding W P,Zhong W,et al.Preparation and magnetic properties of SrFe12O19particles prepared by the salt-melt method[J].Journal of Magnetism and Magnetic Materials,1997,175:333 -336.
[18]Nikkhah-Moshaie R,Ataie A,Ebrahimi SA.Processing of nano-structured barium hexaferrite by self-propagating high temperature synthesis(SHS)using nitrate precursor[J].Journal of Alloys and Compounds,2007,429:324 -328.
[19]Liu X S,Zhong W,Gu B X,et al.Exchange-coupling interaction in nanocomposite SrFe12O19/γ - Fe2O3permanent ferrites[J].Journal of Applied Physics,2002,92:1028 -1032.
[20]Soares J M,Cabral F A O,Araújo J H D,et al.Exchange-spring behavior in nanopowders of CoFe2O4- CoFe2[J].Applied Physics Letters,2011,98:072502.
[21]Fu Y P,Lin C H.Fe/Sr ratio effect on magnetic properties of strontium ferrite powders synthesized by microwaveinduced combustion process[J].Journal of Alloys and Compounds,2005,386:222 -227.
[22]Hessien M M,Rashad M M,El-Barawy K.Controlling the composition and magnetic properties of strontium hexaferrite synthesized by co-precipitation method[J].Journal of Magnetism and Magnetic Materials,2008,320:336-343.
[23]Mali A,Ataie A,Mali A,et al.Influence of Fe/Ba molar ratio on the characteristics of Ba-hexaferrite particles prepared by sol-gel combustion method[J].Journal of Alloys and Compounds,2005,399:245 -250.
[24]Liua X Y,Wanga J,Ganb L M,et al.An ultrafine barium ferrite powder of high coercivity from water-in-oil microemulsion[J].Journal of Magnetism and Magnetic Materials,1998,184:344 -354.
[25]Ranea M V,Bahadura D,Kulkarnib SD,et al.Magnetic properties of Ni/Zr substituted barium ferrite[J].Journal of Magnetism and Magnetic Materials,1999,195:256 -260.
[26]Seifert D,Töpfer J,Langenhorst F,et al.Synthesis and magnetic properties of La-substituted M-type Sr hexaferrites[J].Journal of Magnetism and Magnetic Materials,2009,321:4045 - 4051.
[27]Hadjipanayis G C,Gong W.Magnetic hysteresis in melt-spun Nd-Fe-Al-B-Si alloys with high remanence[J].Journal of Applied Physics,1988,64:5559 -5561.
[28]Schrefl T,Fidler J,Kronmiiller H.Remanence and coercivity in isotropic nanocrystalline permanent magnets[J].Physical Review B,1994,49:6100 -6114.
[29]Sun T,Borrasso A,Liu B,et al.Synthesis and characterization of nanocrystalline zinc manganese ferrite[J].Journal of the American Ceramic Society,2011,94(5):1490 -1495.
[30]Skomski R,Coey JM D.Giant energy product in nanostructured two-phase magnets[J].Physical Review B,1993,48(21):15812-15815.
[31]Kneller E F,Hawig R.The exchange-spring magnet:a new material principle for permanent magnets[J].IEEE Transaction on Magnetics,1991,27:3588 -3560.
[32]Wohlfarth E P.Relations between different modes of acquisition of the remanent magnetization of ferromagnetic particles[J].Journal of Applied Physics,1958,29:595 -596.
[33]Zeng H,Sun S H.Exchange-coupled FePt nanoparticle assembly[J].Applied Physics Letters,2002,80:2583 -2585.
[34]O'Grady K,EI-Hilo M,Chantrell R W.The characterisation of interaction effects in fine particle systems[J].IEEE Transaction on Magnetics,1993,29:2608 -2613.
[35]Che R X,Gao H,Zhao H B.Preparation of permanent magnetic nanocomposite by sol-gel method and the magnetic properties[J].Journal of Functional Materials,2006,37:146 -149.
[36]Soares J M,Machado F L A.Fe interparticle interactions in Fexgranular alloys(2 < x<50)[J].Physical Review B,2005,72:184405.

