(S)-(-)-norlaudanosine和(S)-(-)-O,O-dimethylcoclaurine的合成*
丁 伟,吕 霞,刘世领,朱瑞恒,施小新
(华东理工大学 药学院 制药工程系,上海 200237)
·研究简报·
(S)-(-)-norlaudanosine和(S)-(-)-O,O-dimethylcoclaurine的合成*
丁 伟,吕 霞,刘世领,朱瑞恒,施小新
(华东理工大学 药学院 制药工程系,上海 200237)
N-Ts homoveratrylamine(2)was prepared byN-sulfonylation of 3,4-diemthoxydopamine.(±)-Norlaudanosine(5a)and (±)-O,O-dimethylcoclaurine(5b)were synthesized by Pictet-Spengler reaction of 2with styryl methyl ethers catalyzed by PTS and removal of Ts group.(S)-(-)-5aand (S)-(-)-5bwere obtained by half-equivalent resolution withN-Ac-L-phe.The structures were confirmed by1H NMR,13C NMR,IR,MS and HR-ESI-MS.
Pictet-Spengler reaction;norlaudanosine;O,O-dimethylcoclaurine;synthesis;resolution
苄基四氢异喹啉类化合物在自然界中广泛存在[1-2]。相关研究表明这类结构化合物具有广泛的生理活性[3-5],可方便地转化成其它生物碱,如阿朴啡类生物碱、吗啡类生物碱、小檗碱类生物碱、二苄基四氢异喹啉类生物碱等[6-7]。
norlaudanosine(5a)是合成多种药物分子的关键中间体[3],而O,O-dimethylcoclaurine(5b)是合成去甲乌药碱的前体[8]。目前这两个化合物的单一构型主要通过不对称合成或消旋体拆分制得。但是文献报道的不对称合成方法需要使用价格昂贵的手性催化剂[8-9]或手性辅基[10-11],而传统的全量拆分方法[12-13]需要用到高剂量的拆分剂,造成拆分剂的浪费。
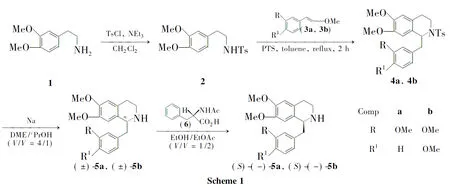
本文以高藜芦胺(1)为起始原料,经N-磺酰化反应制得高藜芦磺酰胺(2);2分别与芳乙烯甲醚(3a和3b)经对甲苯磺酸(PTS)催化的Pictet-Spengler反应后用金属钠脱除Ts基团合成了(±)-5a和(±)-5b;使用半量拆分法,以N-乙酰-L-苯丙氨酸(6)为拆分剂,将(±)-5a或(±)-5b拆分制得(S)-(-)-5a和(S)-(-)-5b(Scheme 1),其结构经1H NMR,13C NMR,IR,MS和HR-ESI-MS确证。并对拆分条件进行了优化,首次报道了(S)-(-)-5b的拆分方法。
1 实验部分
1.1 仪器与试剂
SGW X-4型显微熔点仪(温度未校正);WZZ-1S型自动旋光仪;Bruker ADVANCE 400MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);NICOLET IR 550型红外光谱仪(KBr压片);HP 5989A型质谱仪;Shimadzu LC-20A型高效液相色谱仪[手性柱:Chiralcel OD;流动相:正己烷/异丙醇=80/20(V/V),流速:1.0mL·min-1;UV检测波长:220nm]。
所用试剂均为分析纯。
1.2 合成
(1)2的合成
在反应瓶中依次加入15.00g(27.59mmol),CH2Cl250mL和NEt34.18g(41.39mmol),冰水浴冷却,搅拌下分批(5次)加入对甲苯磺酰氯(TsCl)5.15g(27.04mmol),自然升至室温,反应2h(TLC检测)。加1mol·L-1盐酸20mL,分液,有机层用10% K2CO3溶液(10mL)洗至碱性,无水MgSO4干燥,蒸干得淡黄色固体,用20%甲醇(3×15mL)洗涤,干燥得白色固体28.25g,产率91%,m.p.131℃~132℃;1H NMRδ: 2.40(s,3H),2.68(t,J=6.8Hz,2H),3.16(t,J=6.7Hz,2H),3.78(s,3H),3.83(s,3H),4.39(s,1H),6.54(d,J=1.7Hz,1H),6.60(dd,J=1.7Hz,1H),7.25(d,J=3.7Hz,1H),7.27(s,1H),7.65(d,J=8.2Hz,2H);IRν:3110,2980,1590,1510,1330,1260,810,550cm-1;EI-MSm/z(%): 335[M+,36],184(15),151(100),91(29)。
(2)4的合成(以4a为例)
在反应瓶中依次加入21.00g(2.98mmol)和甲苯15mL,搅拌下依次加入3,4-二甲氧基苯乙烯基甲醚(3a)0.70g(3.58mmol)和PTS 11mg(0.06mmol),回流反应2h。冷却至室温,用1mol·L-1K2CO3溶液(2mL)洗涤,无水MgSO4干燥,浓缩后用乙醇重结晶(加入少量晶种)得N-磺酰化norlaudanosine(4a)1.33g。
用类似的方法合成4b。
4a:白色固体,产率90%,m.p.143℃~145℃;1H NMRδ: 2.34(s,3H),2.34~2.43(m,1H),2.53~2.66(m,1H),3.01(dd,J=13.5Hz,7.2Hz,1H),3.12(dd,J=13.5Hz,5.8Hz,1H),3.28~3.39(m,1H),3.56~3.65(m,1H),3.66(s,3H),3.74(s,3H),3.79(s,3H),3.85(s,3H),5.08(dd,J=6.5Hz,1H),6.20(s,1H),6.43(s,1H),6.51(d,J=1.8Hz,1H),6.56(dd,J=8.1Hz,1.8Hz,1H),6.72(d,J=8.1Hz,1H),7.13(d,J=8.1Hz,2H),7.52(d,J=8.3Hz,2H);13C NMRδ: 21.5,26.9,39.8,43.9,55.7,55.8(2C),55.9,57.5,110.2,110.9,111.2,112.9,122.1,125.6,127.1,127.4,129.4,130.2,137.3,143.0,146.9,147.8(2C),148.6;IRν:2935,1612,1518,1462,1292,1231,1148,1112,1027,976,811,564cm-1;HR-ESI-MSm/z: Calcd for C27H32NO6S{[M+H]+}498.1950,found 498.1950。
N-磺酰化O,O-dimethylcoclaurine(4b): 白色固体,产率92%,m.p.129℃~131℃;1H NMRδ: 2.27(s,3H),2.31~2.42(m,1H),2.48~2.61(m,1H),2.90(dd,J=13.5Hz,7.6Hz,1H),3.05(dd,J=13.5Hz,5.9Hz,1H),3.26~3.37(m,1H),3.55(s,3H),3.56~3.62(m,1H),3.70(s,3H),3.72(s,3H),4.99(dd,J=6.8Hz,1H),6.04(s,1H),6.36(s,1H),6.65~6.75(m,2H),6.82~6.91(m,2H),7.05(d,J=8.1Hz,2H),7.45(d,J=8.3Hz,2H);13C NMRδ: 21.5,26.9,39.8,43.4,55.2,55.7,55.8,57.7,110.2,111.1,113.7,125.4,127.1,127.4,129.8,130.9,137.3,143.0,146.7,147.7,158.4;IRν:2935,1615,1519,1460,1321,1248,1156,1112,813,564cm-1;HR-ESI-MSm/z: Calcd for C26H30NO5S{[M+H]+}468.1845,found 468.1843。
(3)(±)-5的合成[以(±)-5a为例]
在反应瓶中依次加入4a1.00g(2.01mmol)和混合溶剂[V(DME)∶V(iPrOH)=4∶1]20mL,搅拌使其溶解;加入小钠丝0.92g (40mmol),剧烈搅拌下于35℃~ 40℃反应1h(TLC检测)。冰水浴冷却,加入碎冰5g破坏剩余小钠丝,旋蒸除去有机溶剂,用乙酸乙酯(15mL)萃取,有机相用水(10mL)洗涤,洗液用乙酸乙酯(10mL)萃取,合并有机相,用无水MgSO4干燥,旋蒸除溶得无色油状液体,用乙醇10mL溶解,滴加浓盐酸0.3mL,于室温搅拌30min,蒸干溶剂(析出固体),用乙酸乙酯(2mL)洗涤,过滤,滤饼干燥得白色盐酸盐固体0.72g。用1mol·L-1的K2CO3溶液(10mL)中和,用乙酸乙酯(2×10mL)萃取,合并有机相,用无水MgSO4干燥,蒸干得无色油状液体(±)-5a0.64g。
用类似方法合成(±)-5b。
(±)-5a:产率92%;1H NMRδ: 1.85(br s,1H),2.64~2.80(m,2H),2.81~2.97(m,2H),3.09~3.28(m,2H),3.84(s,3H),3.86(s,6H),3.88(s,3H),4.13(dd,J=9.1Hz,4.1Hz,1H),6.60(s,1H),6.67(s,1H),6.76(s,1H),6.78~6.86(m,2H);13C NMRδ: 29.5,40.9,42.2,55.81,55.84,55.9,56.0,56.8,109.3,111.2,111.8,112.3,121.4,127.4,130.3,131.4,147.0,147.4,147.6,148.9;IRν:3598,3331,2933,2831,1734,1608,1514,1463,1216,1113,1028,803cm-1;EI-MSm/z: 343[M+,1],206(1),192(100),176(7),151(3)。
(±)-5b: 无色油状液体,产率91%;1H NMRδ: 1.95(s,1H),2.62~2.80(m,2H),2.80~2.95(m,2H),3.10~3.25(m,2H),3.79(s,3H),3.81(s,1H),3.85(s,3H),4.10(dd,J=9.4Hz,4.1Hz,1H),6.59(s,1H),6.63(s,1H),6.82~6.89(m,2H),7.13~7.20(m,2H);13C NMRδ: 29.5,40.7,41.8,55.2,55.8,55.9,56.9,109.4,111.8,114.0,127.3,130.3,130.6,131.0,146.9,147.4,158.2;IRν:3330,2935,2829,1610,1469,1300,1245,1179cm-1;HR-ESI-MSm/z: Calcd for C19H23NO3{[M+H]+}314.1756,found 314.1743。
(4)(S)-(-)-5的合成[以(S)-(-)-5a为例]
2 结果与讨论
2.1 Pictet-Spengler反应条件优化
实验中我们首先尝试了1和3的Pictet-Spengler反应,发现反应需过量酸催化且收率很低(20%~30%);之后参考文献[15]方法尝试了高藜芦羧酰胺与3的Pictet-Spengler反应,发现反应仍需过量三氟乙酸催化,收率中等(62%);最后改用高藜芦磺酰胺2与3进行Pictet-Spengler反应,收率很高,且只需2mol%PTS催化。
对于2与3的Pictet-Spengler反应,若选用乙酸或苯甲酸等弱酸作催化剂,则反应几乎不能进行;若用较强的Lewis酸,如三氟化硼乙醚、四氯化钛、氯化铁、氯化铝等作催化剂,则原料3容易分解,反应较乱;而选用中强酸PTS作催化剂,收率较高。
2.2 拆分条件优化
对于(±)-5a的手性拆分,王保成[12]用1.06eq.N-乙酰-D-苯丙氨酸为拆分剂,以97%的纯度和32%的产率制得(R)-(+)-5a;Hill等[13]用1.00eq.N-乙酰-L-亮氨酸作拆分剂,以97%的纯度和40%的产率制得(R)-(+)-5a。本文用0.50eq.6为拆分剂,以99%的纯度和34%的产率制得(S)-(-)-5a,相比文献方法,减少拆分剂的用量,避免了浪费。
考察了以6为拆分剂时不同溶剂对拆分效果的影响,结果见表1。从表1可以看出,用AcOEt作溶剂时,产率较高,但ee值不高;而用EtOH作溶剂时,产率不高,但ee值很高;而用EtOH/AcOEt(V/V=1/2)混合溶剂作溶剂时,产率和ee值均较高。
(±)-5b的手性拆分未见文献报道,本文首次通过拆分制得(S)-(-)-5b。其最佳拆分条件与(±)-5a一致。
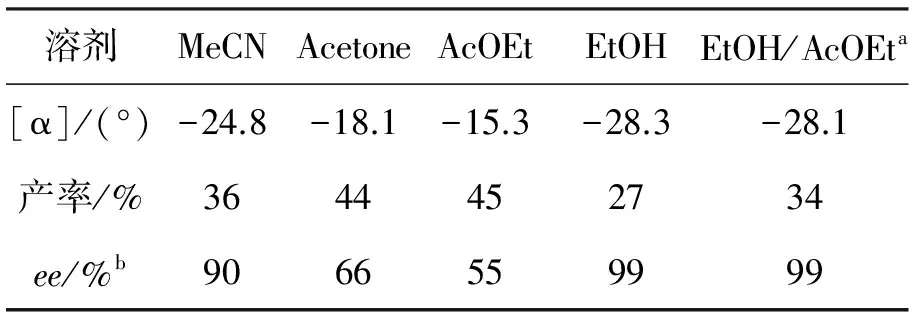
表1 溶剂对拆分(±)-5a的影响Table1 Effect of solvents on resolution of (±)-5a
aV/V=1/2;b由手性柱Chiralcel OD测定
3 结论
首次通过Pictet-Spengler反应合成了(S)-(-)-norlaudanosine和(S)-(-)-O,O-dimethylcoclaurine。在文献方法的基础上对(±)-norlaudanosine的拆分进行了改进,并且首次对(±)-O,O-dimethylcoclaurine的手性拆分进行了研究。
[1] Hesse M.Thieme Pocket Textbook of Organic Chemistry[M].Thieme:Stuttgart,1978.
[2] Liscombe D K,MacLeod B P,Loukanina N,etal.Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms[J].Phytochemistry,2005,66:1374-1393.
[3] Khorana N,Markmee S,Ingkaninan K,etal.Evaluation of a new lead for acetylcholinesterase inhibition[J].Med Chem Res,2009,18:231-241.
[4] Li Y,Zhang H B,Huang W L,etal.Design and synthesis of tetrahydroisoquinoline derivatives as potential multidrug resistance reversal agents in cancer[J].Bioorg Med Chem Lett,2008,18:3652-3655.
[5] Zou Z H,Lan X B,Qian H,etal.Synthesis and evaluation of furoxan-based nitric oxide-releasing derivatives of tetrahydroisoquinoline as anticancer and multidrug resistance reversal agents[J].Bioorg Med Chem Lett,2011,21:5934-5938.
[6] Kitamura M,Hsiao Y,Otha M,etal.General asymmetric synthesis of isoquinoline alkaloids.Enantioselective hydrogenation of enamides catalyzed by BINAP-ruthenium(Ⅱ)complexes[J].J Org Chem,1994,59:297-310.
[7] Huang W J,Singh O V,Chen C H,etal.Synthesis of (±)-Glaucine and (±)-Neospirodienone via an one-pot Bischler-Napieralski reaction and oxidative coupling by a hypervalent iodine reagent[J].Helvetica Chimica Acta,2004,87:167-174.
[8] Poy M K,Lee D K,Kim D H,etal.Enantioselective synthesis of (R)-(+)- and (S)-(-)-higenamine and their analogues with effects on platelet aggregation and experimental animal model of disseminated intravascular coagulation[J].Bioorg Med Chem Lett,2008,18:4110-4114.
[9] Werner F,Blank N,Opatz T.Synthesis of (-)-(S)-norlaudanosine,(+)-(R)-O,O-dimethylcoclaurine,and (+)-(R)-salsolidine by alkylation of anα-aminonitrile[J].Eur J Org Chem,2007,3911-3915.
[10] Pedrosa R,Andres C,Iglesias J M.A novel straightforward synthesis of enantiopure tetrahydroisoquinoline alkaloids[J].J Org Chem,2001,66:243-250.
[11] Munchhof M J,Meyers A I.A novel asymmetric route to the 1,3-disubstituted tetrahydroisoquinoline,(-)-argemonine[J].J Org Chem,1996,61:4607-4610.
[12] 王保成.四氢罂粟碱的拆分研究[J].化工时刊,2009,23:28-29.
[13] Hill D A,Turner G L.Neuromuscular blocking agents[P].US 5453510,1995.
[14] Yamazaki N,Suzuki H,Aoyagi S,etal.Lewis acid-mediated nucleophilic alkylations on chiral [6,3a,4]oxadiazaindano[5,4-a]isoquinolines.Asymmetriy synthesis of 1-alkyl substituted tetrahydroisoquinolines[J].Tetrahedron Lett,1996,37:6161-6164.
[15] Comins D L,Thakker P M,Baevsky M F.Chiral auxiliary mediated pictet-spengler reactions:Asymmetric syntheses of (-)-laudanosine,(+)-glaucine and (-)-xylopinine[J].Tetrahedron,1997,53:16327-16340.
2014-02-27
国家自然科学基金资助项目(20972048);上海市教育发展基金资助项目(03SG27)
丁伟(1986-),男,汉族,浙江绍兴人,博士研究生,主要从事药物合成的研究。
施小新,教授,博士生导师,Tel.021-64252052,E-mail: xxshi@ecust.edu.cn
以高藜芦胺为起始原料,经N-磺酰化反应制得高藜芦磺酰胺(2);2分别与芳乙烯甲醚经对甲苯磺酸催化的Pictet-Spengler反应后用金属钠脱除Ts基团合成了(±)-norlaudanosine(5a)和(±)-O,O-dimethylcoclaurine(5b);使用半量拆分法,以N-乙酰-L-苯丙氨酸为拆分剂,制得(S)-(-)-5a和(S)-(-)-5b,其结构经1H NMR,13C NMR,IR,MS和HR-ESI-MS确证。
Pictet-Spengler反应;norlaudanosine;O,O-dimethylcoclaurine;合成;拆分
O625.3;O626.3
A
1005-1511(2014)05-0679-04
Synthesisof(S)-(-)-norlaudanosineand(S)-(-)-O,O-dimethylcoclaurine
DING Wei, LU Xia, LIU Shi-ling, ZHU Rui-heng, SHI Xiao-xin
(Department of Pharmaceutical Engineering,School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China)

