Upgrading Bio-oil by Underpressure Reaction Rectificationover Catalysts Supported by ZrO2-Al2O3
CHENG Yi, DENG Xu-sheng, LIU Liu-jun, LI Rui
(Department of Forest Chemical Processing Engineering,Beijing Forestry University, Beijing 100083, China)
As gradual decrease of the known reserves of fossil fuel feedstock, exploitation of a new kind of sustainable resources comes to be a crisis issue recently. Among the renewable resources, lignocelluloses biomass is particularly suited as an abundant and low-cost feedstock for production of bio-based fuels and energy to substitute fossil resources[1-3]. Hence, the biomass fast pyrolysis has aroused great attention and comes to be an important area in fuel and chemical research[4-5]. Dmitri[6]divided the pyrolysis of biomass into non-catalytic pyrolysis and catalytic pyrolysis process. Non-catalytic pyrolysis process is performed in the absence of air, short residence time (<2 s) and suitable temperature (550 ℃). Bridgwater[7]reported the different modes of pyrolysis and argued that the products of biomass pyrolysis all are improvable and valuable. Among the products, the yield of pyrolysis oil under fast pyrolysis conditions may reach 70 %-80 % with 25 % water contained.
At present, the techniques of upgrading bio-oil are mainly focused on hydrotreating (hydrodeoxygenation, HDO)[8], cracking[9]and esterification[10-12]. Hydrodeoxygenation (HDO) eliminates oxygen in the form of water. For this elimination, catalysts and hydrogen are necessary. Classic hydrotreatment catalysts such as Ni, Co and Pd can be utilized after they are carried on supports or modified[13-15]. The approach can give a product with a high heating value, however, expensive hydrogen and high pressures are needed. Cracking is normally performed over solid acid catalysts such as aluminosilicates and zeolites[16-17]. Content of preferable aromatic in product gives a higher value compared with hydrotreating[18]. But the lower yields and the higher amount of coke formation made the liquid product demand further rening to produce gasoline or diesel hydrocarbons.
Another approach for upgrading bio-oil is esterification. This process may follow the paths that the ester from the acids and acetal from the aldehydes, are obtained by reaction with alcohols.

Water removing is a considerable factor, too. During the process of distillation, the high temperature of the heat source may lead crude oil formatting coke, however, the low temperature could hardly separate water from crude oil totally[20]. Hence, underpressure (10 kPa) reaction rectification was utilized for water removal. The advantage of reaction rectification was compared with 3A molecular sieve dehydration after esterification in this research.
1 Experimental
1.1 Materials and catalysts
The crude bio-oil was got from the pyrolysis reactor which detail information could be found in the literature[21]. The oil employed in this study originated from larch bark and the bio-oil was a dark brown liquid with smoky odor. The water content was measured with the Karl Fischer titration (ZSD-2J) which was produced in Shanghai Anting Instrument Factory. The heat value was measured using an IKA-C200 calorimetric bomb. The dynamic viscosity was determined by NDJ-1B viscometers and the pH value was evaluated by PHS-3C precision pH-meter. Both were manufactured by Shanghai Sanxin Instrument Factory.

1.2 Equipments and procedures
The method of dehydration after esterification was carried out in a three-neck round bottom glassak (250 mL) equipped with magnetic stirrer. The temperature in the reactor was fixed at 80 ℃ using a water bath in atmosphere. Bio-oil (50 mL) andn-butanol (50 mL) were charged into the reactor vessel, then the catalysts (2 %) was added. A typical reaction time was 3 hours. Subsequently, the 3A molecular sieve was used to remove water. The flow diagram of this process is shown in Fig.1.
The reaction rectification experiments were also carried out in a three-neck round bottom glassask (250 mL) following the same charging sequence as the above method. After charging, the flask was connected to a distillation column with a diameter 24 mm, heights 400 mm, and filling with Raschig ring filler. The temperature in the reactor was controlled using a water bath maintained at 60 ℃. The pressure in the set-up was controlled using a water pump and the pressure was reduced to the desired setting less than 10 kPa. A typical reaction time was about 150 min under the reflux ratio is 5. At the end of reaction, the distillate may be separated into water phase and oil phase, and the product were made after mixture oil with the filtered product in bottom. The schematic diagram of the reaction rectification set-up is shown in Fig.2.
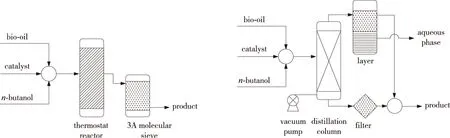
Fig.1 The schematic diagram of dehydration after esterification
2 Result and Discussion
2.1 Directly catalytic esterification
Bio-oil is a complex mixture with more than 400 different chemical compounds at present. It is difcult to evaluate the catalyst performance based on the conversions of individual components[23-24]. However, the paths of ester formation from the acids and acetal formation from the aldehydes give a way that the amount of water produced by the reactions can be employed as an indicator for catalyst performance by means of a Karl Fischer titration. Through the change of water content. The catalyst could be compared. The more water produced, the higher activity performed.

Table 1 The changes of water content of dehydration over 3A molecular sieve after esterification

2.2 Catalytic reaction rectification
The reaction rectification consists of performing the reaction between bio-oil and then-butanol by reaction rectification. To prevent polymerization of the bio-oil at elevated temperatures, the procedure is applied under reduced pressure.n-butanol played two roles in this process, i.e., esterification agent and azeotrope. In atmospheric pressure, the water could azeotroped withn-butanol at 92 ℃. This made the collection seperate into aqueous phase and oil phase. Some low boiling point (<90 ℃) components were dissolved in oil phase. Whereafter, oil-phase were poured into filtered substrate and Karl Fischer titration was used to measure water content.
A formula of esterification-degree (Ed) was given here to characterize the catalytic reaction rectification:
Where,mpis the mass of the product, g;xpis the water content of the product, %;mwis the mass of the water phase, g; thexwis the water content of water phase, %; andmsis the mass of substrate, g;xsis the water content of the substrate, %. This fomula gives the precent of water produced during the reaction. It indicated that the more water generated, the better the catalytic activity. Through the formula, theEdalso proved that the catalyst without acid or base could hardly promot the esterfication(Table 2).

Table 2 The mass and water content in each phases over different catalysts via reaction rectification1)
1)Variable subscript p, w, s denote the product, the water phase and the substrate
2.3 Product properties analysis
The properties of products upgraded were summarized in Table 3. The pH value of the treated bio-oil drops from 2.8 to 1.3 when the acid solid is used as catalyst. While the pH value of upgraded bio-oil catalyzed by the solid base catalyst, it raised to 4.2 under the condition of reaction rectification. This is due to the loss of active groups on the carriers which make catalyst regenerate. The same variation also appeared in the method of dehydration after esterification. The heating value of the product about 26 MJ/kg prepared using distillation is considerably higher than the heating value of the original bio-oil 18.9 MJ/kg. Under the condition of molecular sieve, the value is about 22 MJ/kg. This conversion is the result of the reduced water content of the product and the presence ofn-butanol. The kinematic viscosity of the product after alcohol treatment is positively affected and reduced from 15.8 mm2/s to about 6 mm2/s. This reduction is likely due to the diluting effect ofn-butanol and less to the occurrence of chemical reactions.

Table 3 Properties of crude bio-oil and upgrading bio-oil
3 Conclusions


3.2 Reaction rectification could decrease the water content to 4.8%, 5.4% and 3.8% respectively. This shows that this way is a functional method for removing water;

3.4 According to the high catalytic activities and low water content, the products were characterized to be a promising fuel.
:
[1]WILD P J,REITH H,HEERES E. Biomass pyrolysis for chemicals[J].Biofuels,2011,2(2):185-208.
[2]蒋剑春.生物质能源应用研究现状与发展前景[J].林产化学与工业,2002,22(2):75-80.
[3]蒋剑春,应浩.中国林业生物质能源转化技术产业化趋势[J].林产化学与工业,2005,25(10):5-9.
[4]BRIDGWATER A V,PEACOCKE G V C.Fast pyrolysis processes for biomass[J].Renewable and Sustainable Energy Reviews,2000,4(1):1-73.
[5]路冉冉,商辉,李军.生物质热解液化制备生物油技术研究进展[J].生物质化学工程,2010,44(3):54-59.
[6]BULUSHEV D A,ROSS J R H.Catalysis for conversion of biomass to fuels via pyrolysis and gasification:A review[J].Catalysis Today,2011,171(1):1-13.
[7]BRIDGWATER T.Biomass pyrolysis[J].Biomass & Bioenergy,2007,31 (4):7-18.
[8]MORTENSEN P M,GRUNWALDT J D,JENSEN P A,et al.A review of catalytic upgrading of bio-oil to engine fuels[J].Applied Catalysis(A):General,2011,407(1):1-19.
[10]XU Jun-ming,JIANG Jian-chun,SUN Yun-juan,et al.Bio-oil upgrading by means of ethyl ester production in reaction rectification to remove water and to improve storage and fuel characteristics[J].Biomass & Bioenergy,2008,32(11):1056-1061.
[11]XU Jun-ming,JIANG Jian-chun,DAI Wei-di,et al.Bio-oil upgrading by means of ozone oxidation and esterification to remove water and to improve fuel characteristics[J].Energy & Fuels,2011,25(4):1798-1801.
[12]ZHANG Qi,CHANG Jie,WANG Tie-jun,et al.Upgrading bio-oil over different solid catalysts[J].Energy & Fuels,2006,20(6):2717-2720.
[13]ZHANG Xing-hua,WANG Tie-jun,MA Long-long,et al.Hydrotreatment of bio-oil over Ni-based catalyst[J].Bioresource Technology,2013,127(1):306-311
[14]DO P T M,FOSTER A J,CHEN J,et al.Bimetallic effects in the hydrodeoxygenation of meta-cresol onγ-Al2O3supported Pt-Ni and Pt-Co catalysts[J].Green Chemistry,2012,14(5):1388-1397.
[15]GUNAWAN R,LI X,LIEVENS C,et al.Upgrading of bio-oil into advanced biofuels and chemicals(Ⅰ).Transformation of GC-detectable light species during the hydrotreatment of bio-oil using Pd/C catalyst[J].Fuel,2013,111(9):709-717
[16]HEW K L,TAMIDI A M,YUSUP S,et al.Catalytic cracking of bio-oil to organic liquid product (OLP)[J].Bioresource Technology,2010,101(22):8855-8858.
[17]PENG Jun,CHEN Ping,LOU Hui,et al.Catalytic upgrading of bio-oil by HZSM-5 in sub-and super-critical ethanol[J].Bioresource Technology,2009,100(13):3415-3418.
[18]XU Jun-ming,JIANG Jian-chun,CHEN Jie,et al.Biofuel production from catalytic cracking of woody oils[J].Bioresource Technology,2010,101(14):5586-5591.
[19]TANG Zhe,LU Qiang,ZHAGN Ying,et al.One step bio-oil upgrading through hydrotreatment,esterification,and cracking[J].Industrial & Engineering Chemistry Research,2009,48(15):6923-6929.
[20]XU Jun-ming,JIANG Jian-chun,SUN Yun-juan,et al.A novel method of upgrading bio-oil by reactive rectification[J].Journal of Fuel Chemistry and Technology,2008,36(4):421-425.
[21]LI Rui,DENG Xu-sheng,GOU Jin-sheng,et al.Vacuum Paddle Fast Pyrolysis Reactor Design and Internal Heat Transfer Investigation[C].International Conference on Materials and Manufacturing Research.2012,704:468-474.
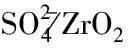
[23]CZERNIK S,BRIDGWATER A V.Overview of applications of biomass fast pyrolysis oil[J].Energy & Fuels,2004,18(2):590-598.
[24]DE WILD P,DEN UIL H,REITH H,et al.Bioenergy(Ⅱ):Biomass valorisation by a hybrid thermochemical fractionation approach[J].International Journal of Chemical Reactor Engineering,2009,7:A51.

