香樟木质部挥发性成分的SPME-GC/MS分析
李 权,王晓娴,林金国,江茂生,初雷霞
(1. 福建农林大学材料工程学院,福建 福州 350002; 2. 福建农林大学生命科学学院,福建 福州 350002)
香樟(Cinnamomumcamphora(L.) Presl.)是我国重要的材用和特种经济树种,由于其具有净化有毒空气的能力,已成为南方许多城市园林绿化的首选良木。香樟材的树干、根、枝、叶均具有芳香气味,内含挥发性成分,可提取樟油,包括樟脑、芳樟醇、黄樟素、桉叶油素、α-松油醇等具有抑菌、防虫功效的生物活性成分[1-3]。
近年来,植物提取物的抑菌活性已成为研究热点[4-9]。国外多采用固相微萃取(solid phase microextraction, SPME)技术检测各种植物及其各部位的挥发性成分[10-13],其技术发展较为成熟。长期以来,人们更多地关注利用香樟材做成具有防虫效果的家具[14-15],其芳香气味对人体健康也有一定的益处,但对于香气的具体成分及其功能则了解不多[16-17],国内外分析香樟材挥发成分的报道也较少[18-19],而香樟材挥发性成分的分析研究对香樟材的合理高效利用具有重大意义。因此,本研究采用固相微萃取的高效吸附和富集功能,对香樟木质部的挥发性成分进行提取,再结合气相色谱-质谱(GC/MS)联用技术进行定性和定量分析[20-22],以期为香樟材在医药、食品和化学工业等方面的进一步开发和利用提供科学依据及理论参考。
1 实验部分
1.1 主要材料
香樟材:采集自福州市上街,树龄40~50年,将香樟木质部边材锯切成规格为5 mm×5 mm×10 mm的试件作为实验原料。
1.2 主要仪器与装置
Agilent 7890A GC system 5975C insert MSD型气相色谱-质谱联用仪:美国Agilent公司产品;SPME萃取装置:美国Supelco公司产品,包括手动SPME进样器和萃取柱,萃取柱为50/30 μm DVB/CAR/PDMS三相萃取头。
1.3 实验方法
将香樟试样的表面砂光后放入25 mL SPME气体收集瓶中,拧紧瓶盖,将装有试样的瓶子于40 ℃恒温加热,平衡30 min后,将SPME的萃取头通过聚四氟乙烯隔垫插入到样品瓶中,推出纤维头,顶空吸附15 min后,缩回纤维头,拔出萃取头,再将萃取头直接插入气相色谱-质谱联用仪,启动仪器采集数据,推出纤维头,使其在气相色谱仪进样口温度260 ℃条件下解吸5 min。
1.4 GC/MS分析条件
1.4.1 色谱条件 弱极性DB-5MS弹性石英毛细管柱(30 m×250 μm×0.25 μm),其特点为:苯基亚芳基聚合物实质上等同于(5%苯基)-甲基聚硅氧烷,对活性化合物的识别能力强,具有高灵敏度和质谱图完整性。进样口温度:260 ℃;升温程序:初温60 ℃,保持3 min,以3 ℃/min升至140 ℃,再以5 ℃/min升至210 ℃,保持5 min;载气(He),纯度大于99.999%,流速1.2 mL/min;分流进样,分流比50∶1。
强极性DB-WAXetr弹性石英毛细管柱(60 m×320 μm×0.25 μm),主要分析范围是醇类,混合二甲苯中的杂质,苯乙烯中的杂质,有机酸,溶剂等。进样口温度:250 ℃;升温程序同DB-5MS柱;分流进样,分流比100∶1。
1.4.2 质谱条件 EI离子源,电子能量70 eV,扫描范围m/z45~550,四极杆温度150 ℃,离子源温度230 ℃。
2 结果与讨论
2.1 香樟木质部挥发性成分分析
香樟材由于受品种、加工工艺、地理环境、季节、栽培管理措施等因素影响,其香气的物质构成有一定差异。合理选择色谱柱是建立色谱分析方法的重要环节,本实验采用弱极性和强极性两种色谱柱以得到较好的分离效果。通过对福建本地香樟材木质部挥发性成分进行SPME-GC/MS分析,得到总离子流色谱图,示于图1。用计算机检索并与标准质谱数据库NIST11进行匹配对照解析,结合有关文献,确定各香气物质的化学成分,并采用峰面积归一化法进行相对定量,结果列于表1。从图1可知,采用两种色谱柱分离获得的GC/MS总离子流色谱图的主要成分规律基本相同,但也有部分萃取成分以及所占百分比并不相同。采用DB-5MS色谱柱时,共分离出51个组分,解析出43种挥发性成分,占总峰面积的97.04%,分离效果和所得的峰形较好;色谱柱为DB-WAXetr时,共检测出39个组分,鉴定出34种化合物,占总峰面积的96.04%。采用DB-5MS色谱柱鉴定出的43种成分中,按照保留时间排序,含量较高的有:左旋-α-蒎烯(4.57%)、莰烯(2.14%)、β-蒎烯(2.3%)、双环[3.1.0]-4-甲基-1-异丙基-2-己烯(4.16%)、邻异丙基甲苯(2.15%)、D-柠檬烯(7.49%)、桉叶油醇(13.85%)、樟脑(38.71%)、(R)-4-萜品醇(1.74%)、α-松油醇(2.40%)、黄樟素(2.96%)、α-荜澄茄油烯(4.36%)、1-石竹烯(1.92%)等。

注:a. DB-WAXetr; b. DB-5MS图1 香樟木质部挥发性成分GC/MS总离子流色谱图Fig.1 Total ion chromatogram of volatile components in Cinnamomum camphora xylem

序号化合物强极性色谱柱DB-WAXetr保留时间/min相对百分含量/%匹配度/%弱极性色谱柱DB-5MS保留时间/min相对百分含量/%匹配度/%1三环[2.2.1.0(2,6)]-1,7,7-三乙基庚烷Tricyclo[2.2.1.0(2,6)]-1,7,7-trimethyl-heptanes5.780.05965.940.03932双环[3.1.0]-2-甲基-5-异丙基-2-己烯 Bicyclo[3.1.0]-2-methyl-5-(1-methylethyl)-hex-2-ene---6.020.51913左旋-α-蒎烯 (1S)-α-Pinene6.006.70976.264.57974双环[3.1.0]-4-甲基-1-异丙基己烷二脱氢衍生物 Bicyclo[3.1.0]hexane-4-methyl-1-(1-methyleth-yl)-didehydro deriv6.051.1394---5双环[2.2.1]-7,7-二甲基-2-亚甲基庚烷 Bicyclo[2.2.1]-7,7-dimethyl-2-methylene-heptane6.640.2097---6莰烯Camphene6.822.71976.772.14967双环[3.1.0]-4-甲基-1-异丙基-2-己烯 Bicyclo[3.1.0]-4-methyl-1-(1-methylethyl)-hex-2-ene8.055.29917.524.16918(1S)-双环[3.1.1]-6,6-二甲基-2-亚甲基庚烷 (1S)-Bicyclo[3.1.1]-6,6-dimethyl-2-methylene-heptane7.722.9997---
续表

序号化合物强极性色谱柱DB-WAXetr保留时间/min相对百分含量/%匹配度/%弱极性色谱柱DB-5MS保留时间/min相对百分含量/%匹配度/%9β-蒎烯 β-Pinene---7.692.3094101-甲基-4-异丙基-1,3-环己二烯1-Methyl-4-(1-methylethyl)-1,3-cyclohexadiene---8.430.049711α-水芹烯 α-Phellandrene9.210.31908.690.478612α-萜品烯 α-Terpinene19.620.27979.100.379713邻异丙基甲苯 1-Methyl-2-(1-methylethyl)-benzene12.692.94979.402.159714D-柠檬烯D-Limonene10.227.85949.627.499415桉叶油醇Eucalyptol10.6115.40949.7613.859416γ-松油烯 γ-Terpinene111.730.309510.730.659517顺式-β-萜品醇 cis-β-Terpineol---11.220.079418香芹孟烯 Carvomentyene13.040.189611.850.449819二环[2.2.1]-1,3,3-三甲基庚-2-酮 Bicyclo[2.2.1]-1,3,3-trimethyl-heptan-2-one---11.970.039520顺式-β-萜品醇cis-β-Terpineol--12.520.089421樟脑 2-Camphanone22.4235.539814.7138.719822龙脑Borneol---15.590.239723R-4-萜品醇 R-4-Terpineol---16.001.749724α-松油醇 α-Terpineol29.333.288616.672.408625L-葑酮 L-Fenchone17.580.0594---26香茅醇 Citronellol---18.100.069827胡椒酮 Piperitone---19.180.059628黄樟素 Safrole35.031.899820.802.969829(+)-4-蒈烯 (+)-4-Carene8.260.059622.780.089530α-荜澄茄油烯 α-Cubebene19.580.339923.290.249931依兰烯 Ylangene---24.200.119932α-荜澄茄油烯 α-Cubebene20.903.129824.514.369833[3aS-(3aα,3bβ,4β,7α,7aS*)]-八氢-7-甲基-3-亚甲基-4-异丙基-1H-环戊并[1,3]环丙并[1,2]苯[3aS-(3aα,3bβ,4β,7α,7aS*)]-Octahydro-7-methyl-3-methylene-4-(1-methylethyl)-1H-cyclo-penta[1,3]cyclopropa[1,2]benzene 22.000.629825.030.959734[1S-(1α,2β,4β)]-1-甲基-1-乙烯基-2,4-双甲基乙烯基环己烷 [1S-(1α,2β,4β)]-1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane24.840.329125.100.399135β-石竹烯 β-Caryophyllene25.040.749926.301.9299364-萜烯醇 4-Terpineol25.682.0196---37γ-榄香烯 γ-Elemene---26.760.109838α-石竹烯 α-Caryophyllene27.820.699627.770.9497
续表

序号化合物强极性色谱柱DB-WAXetr保留时间/min相对百分含量/%匹配度/%弱极性色谱柱DB-5MS保留时间/min相对百分含量/%匹配度/%394,7-二甲基-1-异丙基-1,2,4a,5,6,8a-六氢化萘 1,2,4a,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene---28.630.099740[s-(E,E)]-1-甲基-5-亚甲基-8-异丙基-1,6-环壬二烯 [s-(E,E)]-1-Methyl-5-methylene-8-(1-methyl-ethyl)- 1,6-cyclodecadiene---28.840.989841[1aS-(1aα,3aα,7aβ,7bα)]-1,1,3a-三甲基-7-亚甲基-1H-环丙并[a]十氢化萘 [1aS-(1aα,3aα,7aβ,7bα)] 7-Methylene-1,1,3a-trimethyl-1H-cyclopropa[a]-decahydro-naphtha-lene29.820.099729.130.089742[1S-(1α,7α,8aα)]-1,8a-二甲基-7-异丙基-1,2,3,4,4a,5,6,8a-八氢化萘 [1S-(1α,7α,8aα)]-1,2,3,5,6,7,8,8a-Octahydro-1,8a-dimethyl-7-(1-methylethenyl)-naphthalene29.620.089829.300.089843[2R-(2α,4aα,8aβ)]-4a,8-二甲基-2-异丙基-1,2,3,4,4a,5,6,8a-八氢化萘 [2R-(2α,4aα,8aβ)]-1,2,3,4,4a,5,6,8a-Octahydro-4a,8-dimethyl-2-(1-methylethenyl)-naphthalene 30.410.0696---44(1α,4aα,8aα)-4,7-二甲基-1-异丙基-1,2,4a,5,6,8a-六氢化萘 (1α,4aα,8aα)- 1,2,4a,5,6,8a-Hexa-hydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene---29.440.099845δ-杜松烯 δ-Cadinene---29.590.069846L-去氢白菖烯 L-Calamenene 31.020.479930.370.819647γ-榄香烯 γ-Elemene33.520.059630.480.089648[1R-(1R*,4R*,6R*,10S*)]-4,12,12-三甲基-4,5-环氧-4,11,11-三甲基-8-亚甲基双环[7.2.0]十一烷4,5-Epoxy-4,11,11-trimethyl-8-methyle-nebicyclo(7.2.0)undecane---31.770.1591491-甲基-1-乙基-2-异丙基-4-(1-甲基乙烯基)环己烷1-Ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-cyclohexane---32.540.069850异黄樟脑 iso-Safrole33.330.0989---51(2R-cis)-α,α,4a,8-四甲基-1,2,3,4,4a,5,6,7-八氢化萘-2-醇 (2R-cis)-Octahydro-α, α,4a,8-tetra-methyl-2,3,4,4a,5,6,7-naphthalenemethan-2-ol39.150.1998---52α-松油醇 α-Terpineol40.630.0992---
注:对所检测到的物质在NIST数据库中进行匹配鉴定
2.2 香樟木质部挥发性成分分类分析
采用DB-5MS色谱柱分离的香樟木质部挥发性成分中各类化合物的相对百分含量示于图2。由表1和图2可以看出,其挥发性成分主要包括:烃类32种、醇类7种、酮类3种、酯类1种。烃类中主要以萜类为主,包括α-蒎烯、莰烯、β-蒎烯等,这些物质具有消炎镇痛、抑菌驱虫等功效。莰烯是合成香料、农药等重要的化工原料。β-蒎烯是国家标准(GB 2760&2011)中规定的可使用的香料,同时还是配制肉豆蔻和柠檬等柑橘类香精,以及人工合成β-蒎烯树脂、樟脑、冰片、维生素E等的重要原料。桉叶油醇[23-24]是配制薰衣草油和穗薰衣草油等精油的香料,还可配制口腔剂香精,以及药皂、喷雾剂等,同时还具有抗菌和抗肿瘤的生物活性,在医药方面常用于解热、消炎、防腐、平喘及镇痛等。α-松油醇[25-26]被广泛用于各种用途的香精配方,且具有抑菌活性,α-蒎烯具有一定的抗真菌效果以及镇咳和祛痰的作用。樟脑[27-28]在整个香樟木质部挥发性成分中的比例高达35%以上,樟脑对多种真菌的生长具有强烈的抑制作用,是一种刺激性化学药品,能够治疗与炎症相关的疾病,比如类风湿性关节炎、扭伤、支气管炎等,中国的传统中药老虎油主要由樟脑和薄荷脑制成,对于缓解支气管炎效果显著。Lee等[29]研究发现,香樟的乙酸乙酯以及正丁醇提取物具有强烈的抗氧化活性。采用DB-5MS还检测出(R)-4-萜品醇[30],该化合物可用于配制香精、高级溶剂及除臭剂,是调配铃兰、紫丁香型香精的主剂,耐碱性强,适用于皂用香精。
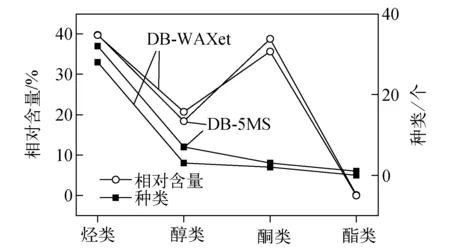
图2 不同色谱柱检测香樟挥发性成分的相对百分含量及种类Fig.2 Relative contents and volatile components of Cinnamomum camphora xylem by different chromatographic column
3 结论
采用SPME-GC/MS技术分析了香樟木质部挥发性成分,得出以下结论:
1)采用DB-5MS色谱柱时,分离出51个组分,共解析出43种挥发性成分,占总峰面积的97.04%,其峰形及分离效果比采用DB-WAXetr色谱柱的好。
2)采用DB-5MS色谱柱分离的香樟木质部挥发性成分主要包括:烃类32种、醇类7种、酮类3种、酯类1种,其中烃类以萜类为主。鉴定出香樟木质部挥发性成分中含有左旋-α-蒎烯(4.57%)、D-柠檬烯(7.49%)、桉叶油醇(13.85%)、樟脑(38.71%)、α-松油醇(2.4%)等大量功能性物质,具有驱虫、抑菌等多种生物活性,同样还是合成香精香料、生物医药、化工产品的重要原料。
[1] MIYAZAWA M, HASHIMOTO Y, TANIGUC- HI Y, et al. Headspace constituents of the tree remain ofCinnamomumcamphora[J]. Natural Product Letters, 2001, 15(1): 63-69.
[2] LIU H, MISHRA A K, HE B, et al. Composition and antifungal activity of essential oils from Artemisia princeps andCinnamomumcamphora[J]. International Pest Control, 2001, 43(2): 72-74.
[3] WENLI P, SHANGLIAN Z. Extraction and determination of volatile constituents of wood fromCinnamomumcamphoraby Agilent-GC/MS [C]. Industrial and Information Systems (IIS), 2010 2nd International Conference on. IEEE, 2010, 1: 557-560.
[4] BEZERRA D P, BRITTO A C S, OLIVEIRA A C A, et al. 998 Assessment of antitumor properties of the essential oil from the leaves ofGuatteriaFriesiana[J]. European Journal of Cancer, 2012, 48(5): S241.
[5] ZEMA D A, ANDILORO S, BOMBINO G, et al. Depuration in aerated ponds of citrus processing wastewater with a high concentration of essential oils [J]. Environmental Technology, 2012, 33(11): 1 255-1 260.
[6] JOHANN S, CISALPINO P S, WATANABE G A, et al. Antifungal activity of extracts of some plants used in Brazilian traditional medicine against the pathogenic fungus Paracoccidioides brasiliensis [J]. Pharmaceutical Biology, 2010, 48(4): 388-396.
[7] BUKVICKI D, GOTTARDI D, VELJIC M, et al. Identification of volatile components of liverwort (Porellacordaeana) extracts using GC/MS-SPME and their antimicrobial activity [J]. Molecules, 2012, 17(6): 6 982-6 995.
[8] KORUKLUOGLU M, GURBUZ O, SAHAN Y, et al. Chemical characterization and antifungal activity ofOriganumonitesL. essential oils and extracts [J]. Journal of Food Safety, 2009, 29(1): 144-161.
[9] 何 莲, 张 宏, 李 琪, 等. 枇杷花系统溶剂提取物抑菌作用研究[J]. 食品科学, 2007, 28(12): 109-112. HE Lian, ZHANG Hong, LI Qi, et al. Study on bacteriostasis of extracts from flowers ofEriobotryajaponica(Thunb.) Lindl. by systematic solvents [J]. Food Science, 2007, 28(12): 109-112(in Chinese).
[11] NEZHADALI A, PARSA M. Study of the volatile compounds inArtemisiaabsinthiumfrom Iran using HS/SPME/GC/MS [J]. Advances in Applied Science Research, 2010, 1(3): 174-179.
[12] DENG C, WANG A, SHEN S, et al. Rapid analysis of essential oil fromFructusAmomiby pressurized hot water extraction followed by soliD-phase microextraction and gas chromatography-mass spectrometry [J]. Journal of Pharmaceutical and Biomedical Analysis, 2005, 38(2): 326-331.
[13] ZHOU T, YANG B, ZHANG H, et al. Identification of volatile compounds inChrysanthemummorifoliumby microwave distillation solid-phase microextraction coupled with GC/MS[J]. Journal of AOAC International, 2009, 92(3): 855-861.
[14] MISHRA A K, DWIVEDI S K, KISHORE N, et al. Fungistatic properties of essential oil ofCinnamomumcamphora[J]. Pharmaceutical Biology, 1991, 29(4): 259-262.
[15] ROSZAINI K, AZAH M A N, MAILINA J, et al. Toxicity and antitermite activity of the essential oils fromCinnamomumcamphora,Cymbopogonnardus,MelaleucacajuputiandDipterocarpussp.againstCoptotermescurvignathus[J]. Wood Science and Technology, 2013, 47(6): 1 273-1 284.
[16] YONG Y, TAO W Y. Head-space solid phase micro- extraction followed by GC/MS analysis of the volatile components in seeds ofCinnamonumcamphora[J]. American Journal of Biochemistry and Biotechnology, 2005, 1(3): 173-175.
[17] SINGH P, SRIVASTAVA B, KUMAR A, et al. Fungal contamination of raw materials of some herbal drugs and recommendation ofCinnamomumcamphoraoil as herbal fungitoxicant [J]. Microbial Ecology, 2008, 56(3): 555-560.
[18] ESTÉVEZ M, VENTANAS S, RAMREZ R, et al. Analysis of volatiles in porcine liver ptés with added sage and rosemary essential oils by using SPME-GC-MS [J]. Journal of Agricultural and Food Chemistry, 2004, 52(16): 5 168-5 174.
[19] PANDEY A K, BORA H R, DEKA S C, et al. Composition of the essential oil of the bark ofCinnamomumcamphora[J]. Journal of Medicinal and Aromatic Plant Sciences, 1997, 19(2): 408-409.
[20] DONG L, WANG J, DENG C, et al. Gas chromatography-mass spectrometry following pressurized hot water extraction and solid-phase microextraction for quantification of eucalyptol, camphor, and borneol inChrysanthemumflowers [J]. Journal of Separation Science, 2007, 30(1): 86-89.
[21] DENG C, MAO Y, YAO N, et al. Development of microwave-assisted extraction followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry for quantification of camphor and borneol inFlosChrysanthemiIndici[J]. Analytica Chimica Acta, 2006, 575(1): 120-125.
[22] LI L, ZHAO J. Determination of the volatile com- position ofRhodobryumgiganteum(Schwaegr.) Par.(Bryaceae) using solid-phase microextraction and gas chromatography/mass spectrometry (GC/MS) [J]. Molecules, 2009, 14(6): 2 195-2 201.
[23] MOTEKI H, HIBASAMI H, YAMADA Y, et al. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line [J]. Oncology Reports, 2002, 9(4):757-760.
[24] SANTOS F A, RAO V S N. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils [J]. Phytotherapy Research, 2000, 14(4): 240-244.
[25] ALVAREZ-CASTELLANOS P P, BISHOP C D, PASCUAL-VILLALOBOS M J. Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthmumcoronarium) against agricultural pathogens[J]. Phytochemistry, 2001, 57(1):99-102.
[26] de SOUSA D P, QUINTANS Jr L, de ALMEIDA R N. Evolution of the anticonvulsant activity ofα-terpineol[J]. Pharmaceutical Biology, 2007, 45(1): 69-70.
[27] 马桢红, 陈淑玉. 樟脑油精药效及其安全性评价[J]. 中国媒介生物学及控制杂志, 2001, 12(1): 58-60. MA Zhenhong, CHEN Shuyu. Laboratory evaluation of efficacy and safety of camphor oil spray [J]. Chinese Journal of Vector Biology and Control, 2001, 12(1): 58-60(in Chinese).
[28] SCHATTNER P, RANDERSON D. Tiger balm as a treatment of tension headache. A clinical trial in general practice [J]. Australian Family Physician, 1996, 25(2):216-218.
[29] LEE H J, HYUN E A, YOON W J. In vitro anti-inflammatory and anti-oxidative effects ofCinnamomumcamphoraextracts [J]. Journal of Ethnopharmacology, 2006, 103(2):208-216.
[30] 郭林林, 张党权, 谷振军. 樟树根材苯/醇提取物的Py-GC/MS分析[J]. 中南林业科技大学学报, 2011, 31(1): 142-147. GUO Linlin, ZHANG Dangquan, GU Zhenjun. Analysis of chemical components of benzene/ethanol extractives from root wood ofCinnamomumcamphoraby Py-GC/MS[J]. Central South University of Forestry & Technology, 2011, 31(1): 142-147(in Chinese).

