5-羟色胺在胆管上皮细胞与门管成纤维细胞自分泌/旁分泌中的作用
陈莉萍,王雅文,樊文梅,肖 漓,石炳毅解放军第309医院 器官移植研究所ICU,北京 0009;国家食品药品监督管理总局医疗器械技术评审中心,北京 00044
基础研究
5-羟色胺在胆管上皮细胞与门管成纤维细胞自分泌/旁分泌中的作用
陈莉萍1,王雅文2,樊文梅1,肖 漓1,石炳毅11解放军第309医院 器官移植研究所ICU,北京 100091;2国家食品药品监督管理总局医疗器械技术评审中心,北京 100044
目的 探讨5-羟色胺(5-hydroxytryptamine,5-HT)在胆管上皮细胞(biliary epithelia cells,BECs)与门管区成纤维细胞(portal fibroblasts,PFs)之间自分泌/旁分泌效应中的意义,阐述两种细胞之间的相互作用。方法 体外细胞培养分为6组:1)BECs组单独培养;2)BECs + TGF-β1组,BECs单独培养,用2 ng/ml重组TGF-β1干预24 h后更换培养液;3)BECs + 5-HT组,BECs单独培养,以60 ng/ml的5-HT干预48 h后更换培养液;4)PFs组单独培养;5)PFs + 5-HT组,PFs单独培养,以60 ng/ml的5-HT干预48 h后更换培养液;6)BECs + PFs,共同培养。各组均在培养72 h后,以酶联免疫吸附分析法(ELISA)检测培养介质内5-HT、TGF-β1含量;实时荧光定量多聚酶链反应(QRT-PCR)检测BECs内色氨酸羟化酶(TPH1、TPH2)和5-HT受体1A、1B表达;以BrdU、α-SMA分别作为BECs增殖及PFs转化为肌纤维母细胞(myofibroblasts,MFs)的标志,免疫细胞化学检测。结果 单独培养的BECs表达5-HT合成限速酶TPH1、TPH2及5-HTR1A、5-HTR 1B,5-HT分泌较高而BECs增殖不明显;经TGF-β1处理或与PFs共培养后,TPH1、TPH2表达各减少80%和87%,5-HTR1A、5-HTR 1B表达分别减少75%和85%,BECs增殖明显。单独培养的PFs分泌TGF-β1,部分呈α-SMA阳性的MFs;经5-HT处理或与BECs共培养后,TGF-β1表达及MFs显著增加。结论 BECs来源的5-HT以及PFs来源的TGF-β1介导BECs与PFs之间的自分泌与旁分泌效应,维持BECs增殖和PFs向MFs的转化,在胆管病发病机制中可能具有重要意义。
5-羟色胺;胆管上皮细胞;门管成纤维细胞;自分泌;旁分泌
胆管病是以肝内胆管树慢性炎症及其导致的以胆汁淤积、胆管增生或丧失、胆管纤维化或胆管细胞的恶性转化为病理表现的疾病,临床治疗效果不佳,常因肝功能衰竭需要行肝移植术[1]。即便如此,肝移植术后的胆管纤维化、胆管狭窄以及原发病的复发依然是导致移植肝功能丧失和患者死亡的重要原因。胆管上皮细胞(biliary epithelia cells,BECs)是胆管病的首要靶点,在受到损伤时启动细胞的再生修复机制,发生细胞增殖。近年发现增殖的BECs能获得神经内分泌细胞表型,分泌单胺类神经递质5-羟色胺(5-hydroxytryptamine,5-HT)[2]。色氨酸先被色氨酸羟化酶(hydroxylase,TPH)催化生成5-羟色氨酸,再经5-羟色氨酸脱羧酶催化成5-HT,因此TPH是5-HT合成的限速酶,包括TPH1、TPH2亚型。某种细胞产生的细胞因子作用于该细胞本身称为自分泌,作用于邻近细胞则称为旁分泌。研究表明BECs来源的5-HT与BECs上的5-HT受体1A(5-HT receptor 1A,5-HTR1A)结合后,抑制BECs增殖,发挥自分泌作用;另一方面,研究发现5-HT也促进肝星状细胞(hepatic stellate,HSC)分化为肌纤维母细胞(myofibroblasts,MFs)并分泌转化生长因子(transforming growth factor,TGF-β1),发挥旁分泌作用,在肝纤维化病理过程中起重要作用[3-4]。门管区成纤维细胞(portal fibroblasts,PFs)是近年来引起关注的另一类肝内间质细胞,在邻近肝门区域分布更多,而此处是肝移植术后胆管狭窄的多发部位[5]。虽有较多研究证实PFs向MFs的大量转化并分泌TGF-β1是介导胆管纤维化的重要机制,但此过程中是否涉及5-HT介导的自分泌与旁分泌作用,目前未见报道。本研究通过建立原代大鼠BECs与PFs共培养体系,观察BECs增殖及PFs向MFs的转化,探讨5-HT和TGF-β1在两种细胞之间自分泌与旁分泌中的作用,为胆管病尤其胆管纤维化、肝移植术后缺血型胆病的发病机制提供细胞学依据。
材料和方法
1 主要试剂与仪器 大鼠5-HT ELISA试剂盒购于南京森贝佳生物科技有限公司(SBJ-R0128);TGF-β1ELISA试剂盒购于江苏普诺生生物科技有限公司(PK-EL-63506R);溴化去氧尿苷(5'bromo-2'-deoxyuridine BrdU)购于美国Sigma-Aldrich公司(B5002);5-HT(ab120528)、重组人TGF-β1(ab50036)、鼠抗α-SMA(ab28052)、HTR1A(ab79230)、HTR1B (ab13896)、TGF-β1(sc-52893)抗体购自美国abcam公司;兔抗TPH1抗体(SAB1105052)、TPH2抗体(SAB1105053)、CK-19(SAB4501670)、鼠抗Elastin (E4013)抗体购自美国Sigma-Aldrich公司;ChemMate TM Envision免疫组织化学试剂盒为丹麦Dako公司产品;ECFTM Western blot试剂盒式购于美国Amersham公司;Light Cycler定量PCR仪由美国Roche公司制造。
2 大鼠原代BECs和PFs分离与培养 肝内BECs分离与培养按文献方法:大鼠腹腔注射硫喷妥钠50 mg/kg麻醉,用不含Ca2+/Mg2+的Hanks液灌注肝,然后以含有0.04%胶原酶S-1和胰蛋白酶抑制剂的Ham's F-12液消化10 min[6]。用柔软毛刷轻轻刷除肝实质细胞,将残余组织转移至离心管中,用移液管适当吹打,之后将悬液过200目网筛,分离出白色的肝内胆管树置于15 ml离心管中,加入5 ml混合消化液(0.25%胰蛋白酶+ 0.1%Ⅳ型胶原酶),37℃振荡消化1 h后加入血清终止消化,待未完全消化的较大组织块沉降至管底后,吸取上层悬液转移至另一离心管中,1 000 r/min离心5 min,弃去上清液,无血清培养基重悬离心管底的沉淀,以50%与30% Percoll非连续密度梯度法离心(1 800 r/min 30 min),吸取中间层细胞,用含双抗的1×PBS(pH=7.4)清洗后,1 000 r/min离心5 min,弃去上清液,用完全的上皮培养基重悬细胞,转移至用胶原包被的T-25培养瓶中,置于37℃、5% CO2的培养箱中培养培养72 h。
3 门管成纤维细胞分离与培养 按文献方法:肝原位灌注、消化方法同原代BECs分离,其后取沉降至管底未完全消化的组织,用含双抗的1×PBS(pH=7.4)清洗后均匀贴在未包被的T-25培养瓶的培养面上,加入2 ml成纤维细胞培养基后倒置于37℃、5% CO2的培养箱中2 h,之后轻轻地翻转培养瓶,使培养基浸没贴于瓶中的组织块培养72 h,在2 d时换液一次[7]。待爬出的细胞数量较多时(铺瓶率30% ~ 40%),吹起组织块后将之吸弃,第2天将细胞重铺一次,细胞长满即可正常传代。细胞传代1次后,1×105/ml的PFs和BECs分别接种在Transwell插入式共培养系统的上层和下层。分别采用CK-19免疫细胞化学法鉴定BECs、Elastin免疫荧光染色法鉴定PFs[8]。
4 实验分组 1)BECs组:BECs单独培养3 d;2)BECs + TGF-β1组:BECs单独培养,以2 ng/ml重组TGF-β1干预24 h;3)BECs + 5-HT组:BECs单独培养,以60 ng/ml的5-HT干预48 h;4)PFs组:PFs单独培养;5)PFs + 5-HT组:PFs单独培养,以60 ng/ml的5-HT干预48 h;6)BECs + PFs:BECs和PFs培养[9]。
5 ELISA法测定培养介质中5-HT和TGF-β1含量 细胞培养3d后,采用ELISA法测定各组培养介质内5-HT和TGF-β1含量,具体操作按试剂盒说明书要求。
6 实时荧光定量多聚酶链反应(QRT-PCR) 采用QRT-PCR法测定TPH1、TPH2、5-HTR1A、5-THR1B mRNA在BECs内的表达。RNA抽提及逆转录均按照试剂说明书操作,以实时荧光定量PCR法在Light Cycler仪上对目的基因进行扩增。对含有靶基因已知拷贝数的cDNA进行倍比稀释,构建标准曲线。样本拷贝数通过标准曲线来计算,采用与β-actin的相对比值作为靶基因的表达量。各靶基因的引物序列见表1。

表1 各基因引物扩增序列Tab. 1 Amplification sequences of various primers
7 免疫细胞化学检测 分别以BrdU、α-SMA作为BECs增殖及PFs转化为MFs的标志,BrdU在检测前4 h加入BECs培养介质。将鼠尾胶原包被的盖玻片放入6孔培养板内,待培养的细胞铺满玻片80%时取出,用4%多聚甲醛室温固定20 min,以0.1%的Triton-X穿透10 min,之后按照二步法免疫组化试剂盒说明书操作。增殖BEC细胞核BrdU染色阳性,MFs胞质α-SMA染色阳性,采用平均光密度作为阳性细胞表达程度的半定量工具。
8 统计学处理 所有数据应用SPSS10.0软件进行统计分析,计量资料以±s表示,多组均数间的比较采用单因素方差分析,P<0.05为差异有统计学意义。
结 果
1 大鼠原代BECs与PFs鉴定 锥虫蓝染色证实分离出的原代BECs和PFs活细胞率>97%,细胞纯度>98%。BECs呈CK-19免疫组化染色胞质表达阳性,PFs呈梭形,Elastin免疫荧光染色胞质表达阳性。传至第2代的细胞用于后继实验,细胞纯度100%,鉴定结果见图1。
2 RT-qPCR检测各组5-HT与TGF-β1含量5-HT在PFs单独培养介质中含量极微,在BECs单独培养介质中含量最高;BECs经TGF-β1处理或与PFs共培养后,培养介质内5-HT含量明显下降。BECs单独培养时分泌少量TGF-β1,在5-HT刺激下分泌略增加;TGF-β1在PFs单独培养介质中以一定水平存在,当受到5-HT刺激或与BECs共培养时TGF-β1分泌明显增加。见图2。
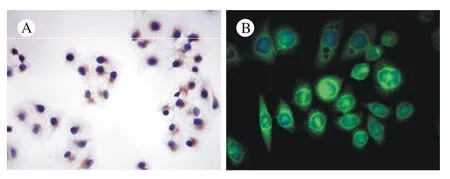
图 1 BECs与PFs鉴定(×400) A: BECs胞质棕染,为CK-19染色阳性; B: 绿色荧光显示PFs胞质elastin表达阳性Fig. 1 Identification of BECs (A) and PFs (B) A: BECs cytoplasm showing CK-19 positive staining; B: green fluorescence showing PFS cytoplasm as elastin positive staining
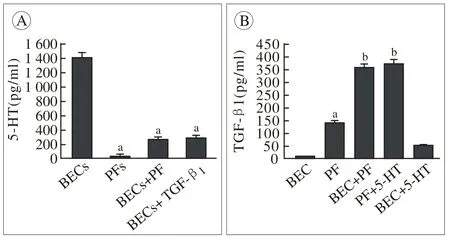
图 2 各组培养介质中5-HT及TGF-β1含量 (aP<0.05, vs BECs组,bP< 0.05, vs PFs组)Fig. 2 Production of 5-HT and TGF-β1in various culture medium (aP<0.05, vs BECs,bP< 0.05, vs PFs)
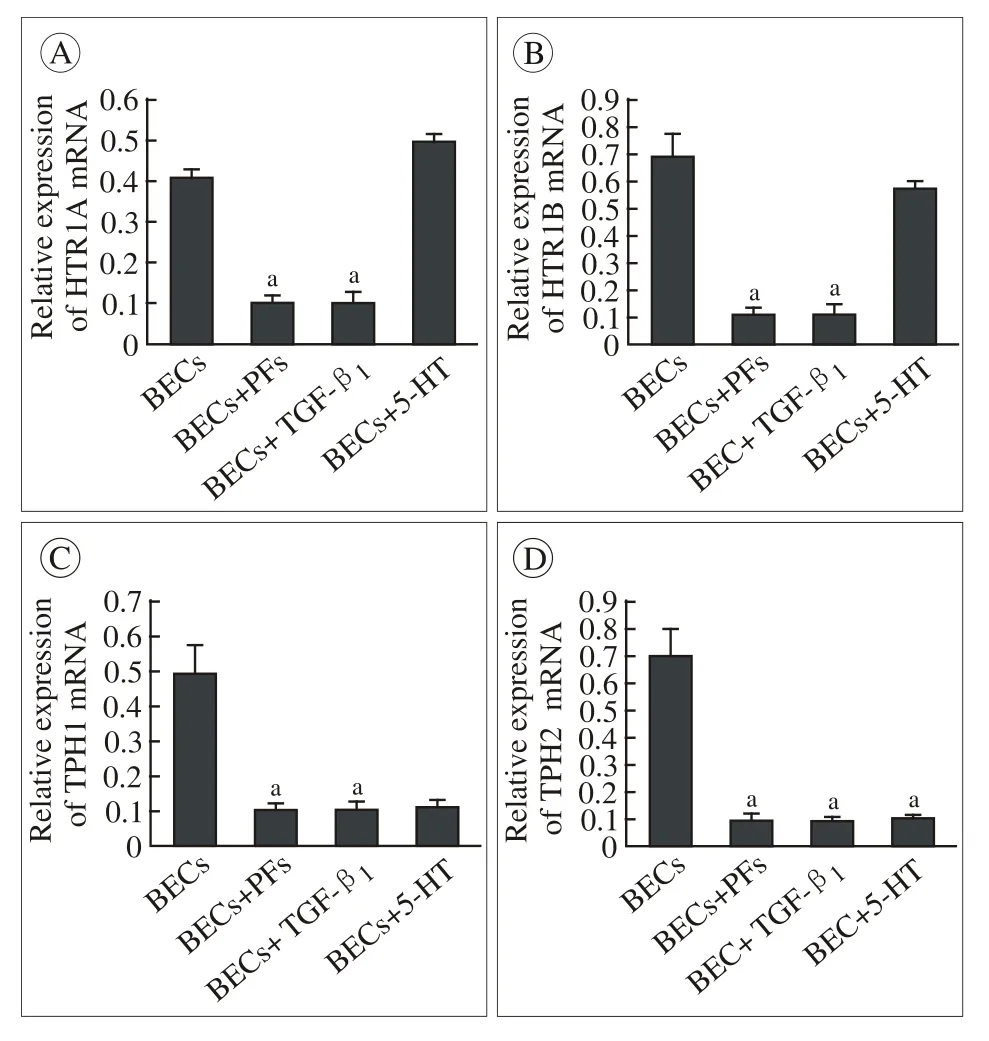
图 3 RT-qPCR检测各组BECs内5-HTR1A、 5-HTR1B、 TPH1、TPH2 mRNA表达(aP<0.05, vs BECs组)Fig. 3 Expression of 5-HTR1A, 5-HTR1B, TPH1, TPH2 mRNA in BECs by RT-qPCR (aP<0.05, vs BECs)
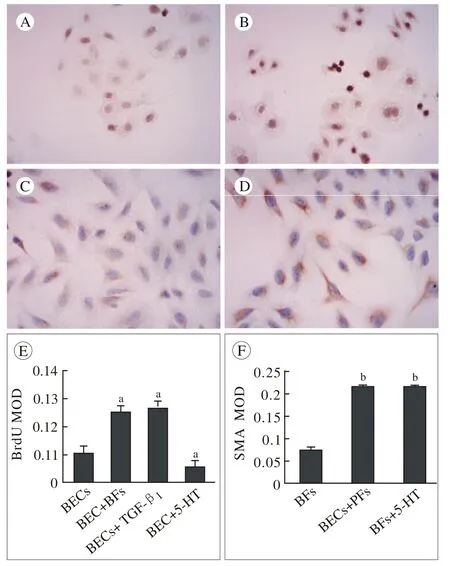
图 4 BECs增殖和PFs向MFs转化的免疫细胞化学检测(×400) A:单独培养的BECs仅有少量细胞增殖; B:与PFs共培养体系中,BECs增殖明显; C:PFs在单独培养3 d时部分细胞转化为α-SMA表达阳性的肌纤维母细胞(MFs); D:与BECs共培养时PFs向MFs转化显著增多; E ~ F:各组BECs增殖及PFs向MFs转化水平以BrdU和α-SMA表达的MOD值来表示(aP<0.05, vs BECs组,bP< 0.05, vs PFs组)Fig. 4 BECs proliferation and PF myofibroblastic transdifferentiation were evaluated by immunocytochemisry A: Monocultured BECs showing small amount of proliferation; B: BECs showing significant proliferation when co-cultured with PFs; C: Mono-cultured PFs showing some MFs with α-SMA positive staining; D: Abundant of MFs were showed when co-cultured with BECs; E-F: Presentation of BECs proliferation and MFs was quantified by the mean optical density (MOD) of expression of BrdU and α-SMA (aP<0.05, vs BECs,bP< 0.05, vs PFs)
3 各组BECs内5-HTR1A,5-HTR1B,TPH1,TPH2mRNA表达 单独培养的BECs较高水平地表达5-HT1A和5-HT1B受体,外源性5-H处理对二者的表达无显著影响;与PFs混合培养或给予TGF-β1处理后,BECs内5-HT1A mRNA表达分别约下调75%,5-HT1B mRNA表达约下调85%,差异有统计学意义。在单独培养时,BECs内5-HT合成限速酶TPH1、TPH2 mRNA表达较高,经5-HT处理后TPH1表达约减少80%,TPH2约减少87%,差异有统计学意义。BECs + PFs组与BECs + TGF-β1组之间的TPH及5-HTR表达差异无统计学意义。见图3。
4 免疫细胞化学检测各组BECs增殖及PFs向MFs的转化 BECs在单独培养时仅有少量细胞增殖,给予5-HT处理后细胞增殖进一步减少;与此相反,在和PFs共同培养或受TGF-β1刺激时增殖明显。PFs单独培养3 d时有部分细胞转化为α-SMA表达阳性的肌纤维母细胞,在与BECs共培养或5-HT刺激时MFs数量明显增多(图3A ~图3D)。各组BEC增殖以及PFs向MFs的转化以BrdU和α-SMA表达的平均光密度来量化(图3E ~图3F)。
讨 论
胆管上皮细胞与肝内间质细胞之间的交互作用与胆管病密切相关,而器官纤维化的本质是实质细胞再生以及间质细胞纤维化之间失衡[10]。在胆管病早期,BECs的增殖可来自多种胆管刺激,与此相关的显著而持久的间质细胞活化是胆管纤维化进展最重要的原因[11]。BECs与肝星状细胞(hepatic stellate,HSC)之间的自分泌与旁分泌作用曾被认为是介导胆源性肝纤维化的机制[12]。然而随着PFs分离技术的成熟,越来越多的研究证实在胆源性纤维化或胆管纤维化中,PFs向肌纤维母细胞的持续转化(活化)才是关键因素[13]。因此廓清BECs与PFs之间的交互作用,对探讨胆管病的发病机制是至关重要的。文献报道5-HT抑制BECs增殖,本研究也发现正常情况下BECs表达5-HT合成与利用的成分TPH1、TPH2以及5-HT1A、5-HT1B受体亚型,分泌较多5-HT,但仅有少量细胞增殖[2]。尽管抑制BECs增殖,本研究却显示5-HT有促进PFs向MFs转化的作用:不仅在给予5-HT刺激时有较多的PFs分化成MFs,在PFs和BECs共同培养体系中MFs也明显增多。作为促纤维化的关键因子,TGF-β1在多个组织中均被证实是成纤维细胞向肌纤维母细胞转化的关键因子,并且是MFs分泌的主要细胞因子之一[14]。我们发现在给予5-HT处理或与BECs共同培养时,PFs分泌TGF-β1较单独培养时显著增加,在共培养体系中BECs来源的5-HT作用于PFs,上调TGF-β1的表达并促进PFs向MFs转化,呈现旁分泌效应。
本研究还观察到TGF-β1有利于BECs增殖:在给予TGF-β1干预或与PFs共同培养时,培养介质内5-HT浓度较之前显著降低,TPH1和TPH2的表达减少80%以上,BrdU阳性的BECs显著增加。因此,PFs来源的TGF-β1通过抑制5-HT合成限速酶TPH1和TPH2的表达来减少BECs分泌5-HT,伴随着5-HT1受体表达的减少,从而消除5-HT抑制BECs增殖的自分泌效应,BECs得以增殖从而成为“活化的胆管细胞”,在胆管病理中可能具有重要意义:作为对胆道损伤的代偿性反应,BECs增殖和小胆管的增生一方面有利于胆汁引流从而减轻胆汁淤积,另一方面也通过5-HT促进管周MFs的活化与聚集;而MFs也可通过TGF-β1消除5-HT的自分泌效应,维持BECs的增殖状态[3,15]。
综上所述,本研究显示:增殖的BEC通过分泌5-HT而促进PFs向MFs的转化而发挥旁分泌效应,同时BEC来源的5-HT抑制自身增殖而发挥自分泌效应;PFs来源的TGF-β1一方面继续促进细胞向MFs的转化,发挥自分泌效应,另一方面TGF-β1也抑制BECs分泌5-HT,从而消除5-HT对BECs的自分泌效应,此时TGF-β1又发挥了旁分泌效应。增殖的BECs继续通过5-HT与PFs发生交互作用,以自分泌与旁分泌的方式和PFs之间构成了一个闭合的环路,维持BECs的增殖和PFs向MFs的转化。由于TGF-β1信号通路的广泛存在,以TGF-β1为治疗靶点的抗器官纤维化常常效果不佳[16]。本研究提示5-HT合成酶或5-HT受体可能作为潜在的干预靶点以延缓胆管纤维化的病理进展,尚待今后的深入研究。
1 Park SM. The crucial role of cholangiocytes in cholangiopathies[J]. Gut Liver, 2012, 6(3): 295-304.
2 Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin[J]. Gastroenterology, 2005, 128(1): 121-137.
3 Jensen K, Marzioni M, Munshi K, et al. Autocrine regulation of biliary pathology by activated cholangiocytes[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(5): G473-G483.
4 Kim DC, Jun DW, Kwon YI, et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis[J]. Liver Int, 2013, 33(4): 535-543.
5 Demetris AJ, Fontes P, Lunz JG, et al. Wound healing in the biliary tree of liver allografts[J]. Cell Transplant, 2006, 15 Suppl 1:S57-S65.
6 Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis[J]. Hepatology, 2010, 51(4):1438-1444.
7 Bosselut N, Housset C, Marcelo P, et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts[J]. Proteomics, 2010, 10(5): 1017-1028.
8 Omenetti A, Yang L, Gainetdinov RR, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults[J]. Am J Physiol Gastrointest Liver Physiol, 2011, 300(2):G303-G315.
9 Wen JW, Olsen AL, Perepelyuk M, et al. Isolation of rat portal fibroblasts by in situ liver perfusion[J]. J Vis Exp, 2012, (64):pii: 3669.
10 Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence[J]. Wound Repair Regen, 2004, 12(2): 134-147.
11 Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets[J]. Dig Liver Dis, 2004, 36(4): 231-242.
12 Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases[J]. Semin Liver Dis, 2011, 31(1):11-32.
13 Beaussier M, Wendum D, Schiffer E, et al. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries[J]. Lab Invest, 2007, 87(3): 292-303.
14 Li Z, Dranoff JA, Chan EP, et al. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture[J]. Hepatology, 2007, 46(4): 1246-1256.
15 Priester S, Wise C, Glaser SS. Involvement of cholangiocyte proliferation in biliary fibrosis[J]. World J Gastrointest Pathophysiol,2010, 1(2): 30-37.
16 Yabanoglu S, Akkiki M, Seguelas MH, et al. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors[J]. J Mol Cell Cardiol, 2009, 46(4): 518-525.
Role of serotonin in the autocrine / paracrine between cholangiocytes and portal fibroblasts
CHEN Li-ping1, WANG Ya-wen2, FAN Wen-mei1, XIAO Li1, SHI Bing-yi11Institute of Organ Transplantation, the 309th Hospital of Chinese PLA, Beijing 100091, China;2Center for Medical Device Evaluation, Beijing 100044, China
SHI Bing-yi. Email: shibingyi@medmail.com.cn
Objective To evaluate the role of serotonin (5-HT) in the crosstalk between cholangiocytes (biliary epithelia cells, BECs) and portal fibroblasts (PFs). Methods Cells were cultured and divided into 6 groups. 1) BECs were cultured alone; 2) BECs + TGF-β1: BECs were cultured alone and then were treated with 2 ng/ml of recombinant transforming growth factor β1(TGF-β1) for 24 h; 3) BECs + 5-HT: BECs were cultured alone and then were treated with 60 ng/ml of 5-HT for 48 h; 4) PFs were cultured alone; 5) PFs + 5-HT: PFs were cultured alone and then were treated with 60 ng/ml of 5-HT for 48 h; 6) BECs + PFs: BECs and PFs were co-cultured. After culturing for 72 h, the concentration of 5-HT or TGF-β1in culture medium was detected by ELISA. Expressions of TPH1, TPH2, 5-HTR1A, 5-HTR1B mRNA in BECs were detected by real-time QRT-PCR. BrdU and α-SMA as the marker of BECs proliferation and myofibroblastic transdifferentiation of PFs were evaluated by immunocytochemistry. Results Mono-cultured BECs expressed TPH1, TPH2, 5-HTR1A, 5-HTR1B mRNA with hypersecretion of 5-HT and slight proliferation of BECs. After being treated by TGF-β1or co-cultured with PFs, BECs reduced the expression of TPH1 by 80% and TPH2 by 87%. Moreover, 5-HTR1A and HTR1B mRNA expression in BECs also dropped by 75% and 85% respectively, companied by the increasment of BECs proliferation. Mono-cultured PFs produced TGF-β1with some MFs showing positive staining. However, after being treated by 5-HT or co-cultured with BECs, TGF-β1secretion and α-SMA-positive cells were significantly increased. Conclusion 5-HT derived from BECs and TGF-β1produced by PFs can mediate the autocrine and paracrine effects between BECs and PFs, and maintain the proliferation of BECs and the conversion of PFs to MFs, which may be meaningful to the pathogenesis of cholangiopathies.
5-hydroxytryptamine; cholangiocyte; portal fibroblast; autocrine; paracrine
R 575.7
A
2095-5227(2014)10-1044-05
10.3969/j.issn.2095-5227.2014.10.020
时间:2014-06-05 09:53
http://www.cnki.net/kcms/detail/11.3275.R.20140605.0953.001.html
2014-04-18
国家自然科学基金项目(81100322;81270509;81370572)
Supported by the National Natural Science Foundation of China(81100322; 81270509; 81370572)
陈莉萍,女,博士,副主任医师。研究方向:器官移植及外科重症监护的基础与临床。Email: paradiselily@163.com
石炳毅,男,主任医师。Email: shibingyi@medmail.com.cn

