乳腺癌脑转移47例三维适形放疗疗效及预后分析
侯 俊,冯林春,马 林,曲宝林,彭 亮,王运来,许卫东,权建华,张富利,王雅棣北京军区总医院 放疗科,北京 00700;解放军总医院 放疗科,北京 00853
乳腺癌脑转移47例三维适形放疗疗效及预后分析
侯 俊1,冯林春2,马 林2,曲宝林2,彭 亮2,王运来2,许卫东1,权建华1,张富利1,王雅棣11北京军区总医院 放疗科,北京 100700;2解放军总医院 放疗科,北京 100853
目的 评价三维适形放疗(three-dimensional conformal radiotherapy,3D-CRT)治疗乳腺癌脑转移临床疗效、不良反应以及预后。方法 回顾性分析北京军区总医院放疗科及解放军总医院放疗科1995年5月- 2010年10月行全脑放疗(whole brain radiotherapy,WBRT)的47例乳腺癌脑转移患者(PTV 40 ~ 50 Gy/20 ~ 25 F或PTV 40 Gy/20 F),脑转移灶(pGTV)同步加量至60 Gy/20 F(或后补量至60 Gy/30 F),每周放疗5次。部分颅内复发患者再行立体定向放疗10 ~ 24 Gy/1 ~ 3 F。放疗结束1 ~ 3个月后评价疗效、不良反应,观察远期不良反应。结果 47例均完成放疗计划,其中CR 5例(10.6%)、PR 26例(55.3%)、SD 13例(27.7%)、PD 3例(6.4%),总有效率(CR + PR)66.0%,临床获益率(CR + PR + SD)93.6%。1、2、3年放疗生存率分别为53.2%(25例)、25.5%(12例)、2.1%(1例),中位生存期13个月。急性不良反应主要为脑水肿、脱发、急性中耳炎、听力下降、皮肤反应、乏力、轻度骨髓抑制,2例(4.3%)出现Ⅲ~Ⅳ级神经系统晚期不良反应。单因素分析显示,放疗生存期与KPS相关。多因素分析显示,患者放疗生存期与KPS、临床分期、脑转移个数(<3或≥3)、颅内转移灶进展相关。结论 三维适形放疗治疗乳腺癌脑转移能提高局控率,延长生存期;全脑照射40 Gy/20 F安全有效,不良反应可耐受。一般情况越好、临床分期越早、脑转移瘤个数越少、颅内病变进展越晚,生存期越长。
乳腺肿瘤转移;全脑放疗;三维适形放疗;预后
近年来随着乳腺癌综合治疗水平的进步,乳腺癌患者的生存期进一步延长,同时也增加了乳腺癌脑转移的发病率。乳腺癌脑转移多为多发,治疗上以放疗治疗为主,其他包括手术、激素及脱水等。本文收集整理了北京军区总医院及解放军总医院1995年5月- 2010年10月47例乳腺癌脑转移患者三维适形放疗临床资料,现对其近期疗效、不良反应及预后因素进行回顾性分析。
资料和方法
1 资料 1995年5月- 2010年10月北京军区总医院及解放军总医院47例女性患者中,发生脑转移时年龄33 ~ 82岁,中位年龄48岁;采用AJCC分期标准行TNM分期;KPS评分20 ~ 70分,中位评分50;原发病均经病理组织学或细胞学检查确诊,脑转移均经增强MRI证实,未合并其他肿瘤病史;所有患者均完成三维适形放疗。患者一般情况详见表1。
2 放疗方法 热塑头模固定,激光灯定位,Philips Brilliance CT定位机下行CT扫描,处方剂量为PTV 40 ~ 50 Gy/20 ~ 25 F,2 Gy/F或PTV 40 Gy/20 F,2 Gy/F,pGTV 60 Gy/20 ~ 30 F,2 ~ 3 Gy/F,5 F/W。部分患者放疗后颅内复发再行转移灶立体定向放疗10 ~ 24 Gy/1 ~ 3 F。
3 观察指标 1)近期疗效:放疗结束后1 ~ 3个月复查颅脑MRI,并记录患者症状、体征及放疗不良反应,采取WHO疗效判定标准评价,疗效分为完全缓解(CR)、部分缓解(PR)、疾病稳定(SD)、疾病进展(PD)。2)不良反应:评价按照CTCAE 3.0不良反应评价标准,采用美国放疗肿瘤组织中枢神经系统不良反应评价标准评价急性和晚期不良反应。3)生存时间:包括发生远处转移时间、发生脑转移时间和放疗生存期。远处转移时间和脑转移时间的起始时间为患者乳腺癌确诊时,放疗生存期起始时间为患者乳腺癌放疗开始时,终点为患者死亡或末次随访时间。4)预后分析:ER/PR表达通过免疫组织化学法鉴定,ER/PR任何一项阳性即为阳性,HER-2测定采用免疫组化法或荧光原位杂交法鉴定,“+”即为阳性。根据美国放射治疗肿瘤协作组(radiation therapy oncology group,RTOG)提供的预后分级评分(graded prognostic assessment,GPA),乳腺癌脑转移根据年龄、KPS、ER/PR、HER-2评分,GPA分为0 ~ 1,1.5 ~ 2,2.5 ~3,3.5 ~ 4四级。评分标准见表2。
4 统计学方法 用SPSS17.0软件,Kaplan-Meier法计算生存率,Log-rank法检验并单因素分析,多因素采用COX回归模型,P<0.05为差异有统计学意义。
结 果
1 疗效 随访2 ~ 49个月,中位随访时间13个月,末次随访时间2013年3月,其中12例(25.5%)存活,17例(36.2%)死于颅内病变进展,18例(38.3%)死于肺、骨、肝、肾上腺等转移及全身多脏器衰竭。其中7例(14.9%)颅内病变进展后行立体定向放疗。治疗结束后1个月评价疗效,其中CR 5例(10.6%)、PR 26例(55.3%)、SD 13例(27.7%)、PD 3例(6.4%),总有效率(CR + PR)66.0%,临床获益率(CR + PR + SD)93.6%。1、2、3年放疗生存率分别为53.2%(25例)、25.5%(12例)、2.1%(1例),中位生存期13(1 ~33)个月。
2 不良反应 急性不良反应主要有脑水肿、脱发、急性中耳炎、听力下降、急性皮肤反应、乏力、轻度骨髓抑制,大部分可恢复正常。远期反应主要为神经系统不良反应,放疗结束3 ~ 6个月2例(4.3%)出现Ⅲ~Ⅳ级神经系统不良反应,其中1例58岁,PTV 50 Gy/25 F,放疗前有脑梗死病史,放疗后血压、血脂未控,出现进行性记忆力、认知力下降,现出现中度痴呆,大小便失禁,复查颅脑CT提示脑萎缩、脑白质呈低密度改变,放疗后22个月仍存活;1例75岁,PTV 40 Gy/20 F,pGTV 60 Gy/20 F,放疗前有双手不自主抖动等早期帕金森症状,放疗结束后6个月出现进行性运动性失语、情感障碍、自闭症、痴呆,放疗后存活48个月,死于颅内转移。
3 生存分析 单因素分析显示放疗生存期与KPS(P<0.00)相关(表1)。多因素COX分析显示放疗生存期与KPS、临床分期、脑转移个数及颅内转移灶进展相关(表3)。生存曲线见图1。
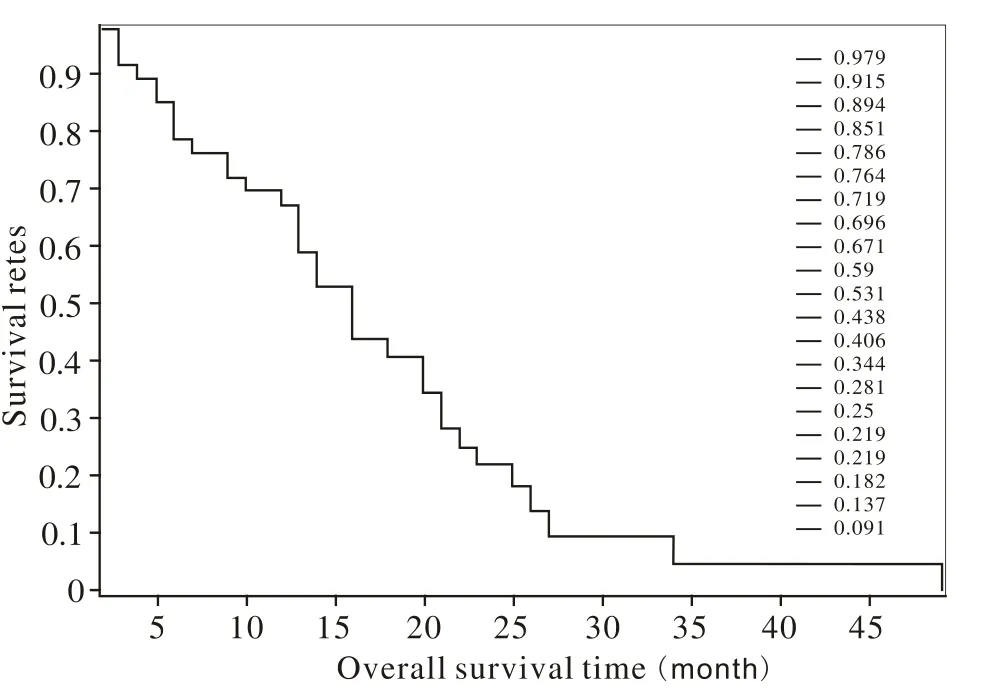
图 1 乳腺癌脑转移患者的放疗生存曲线Fig. 1 Overall survival curves of breast cancer patients with brain metastases
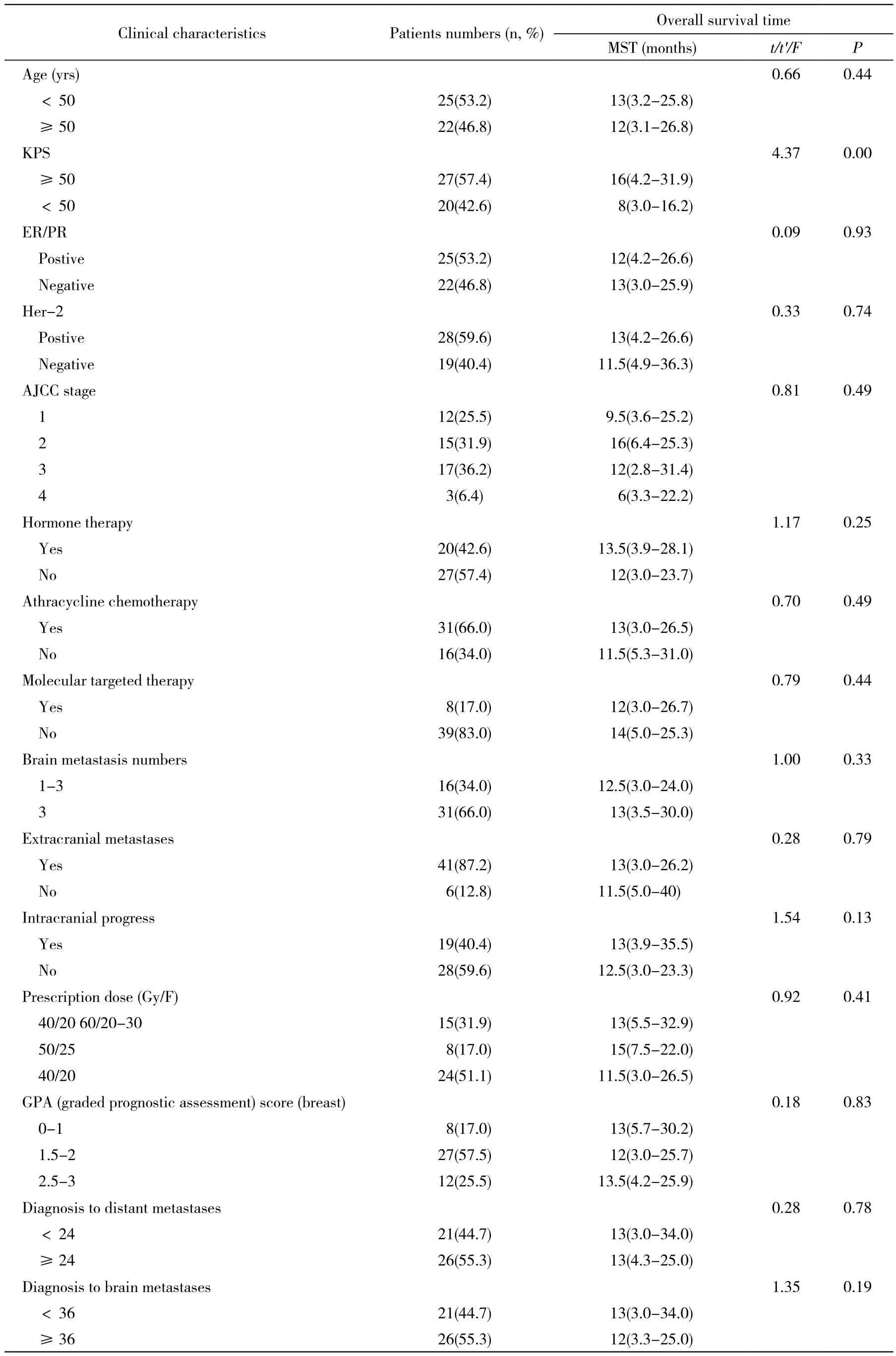
表1 47例乳腺癌脑转移患者临床基线特征Tab. 1 Baseline characteristics of 47 breast cancer patients with brain metastases
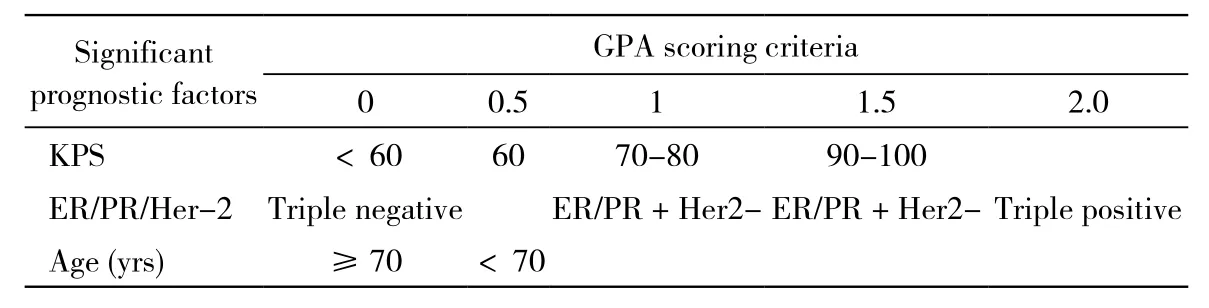
表2 乳腺癌预后分级评分标准Tab 2 Diagnosis-specific GPA of breast cancer
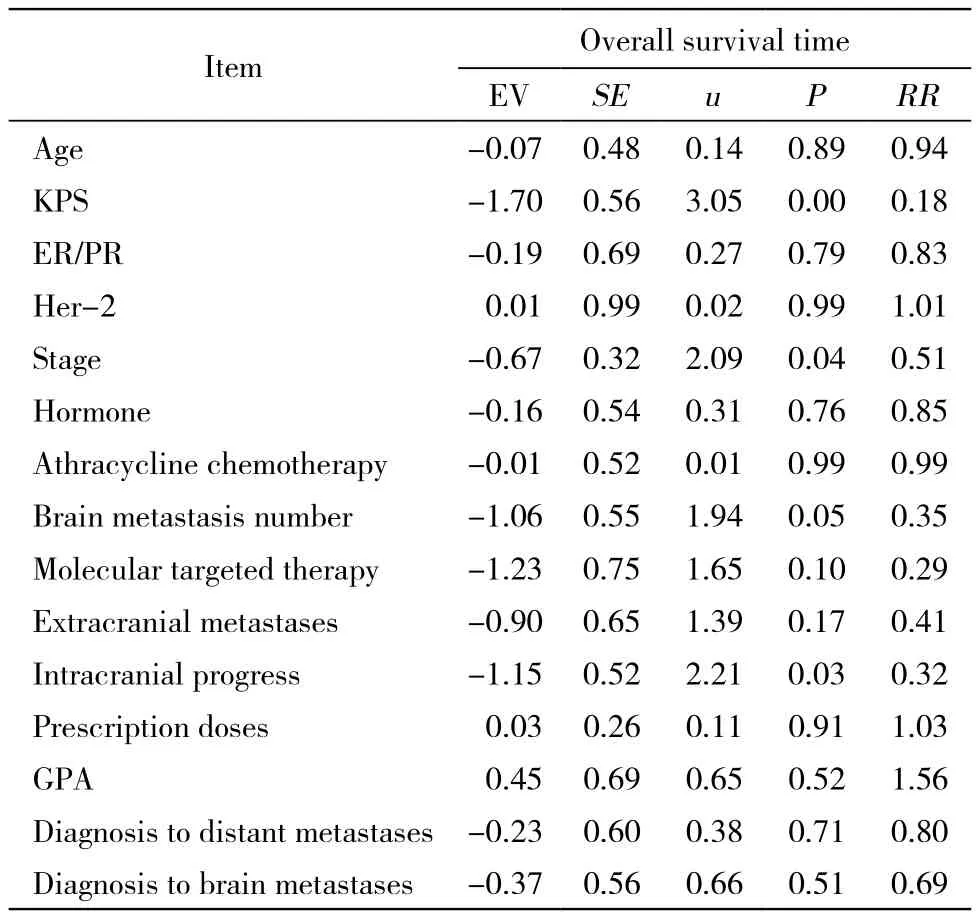
表3 47例乳腺癌脑转移患者预后Cox 模型Tab. 3 Prognosis parameters of 47 breast cancer patients with brain metastasis analyzed by Cox multivariate model
讨 论
乳腺癌脑转移发病率为13% ~ 20%,单发占14%,多发占78%,脑膜转移为8%,平均生存期2 ~ 16个月,1年生存率为32.5%[1-4]。由于乳腺癌脑转移已属疾病晚期阶段,一般情况差,多有肺、骨、肝等转移,可能对内分泌、化疗及分子靶向等药物耐药,因此对颅内转移灶治疗以姑息性治疗为主,力求减轻患者临床症状,减少治疗并发症,提高生存质量,进而有望提高生存期。WBRT中位生存期为4 ~ 6个月,立体定向放疗联合WBRT中位生存期为12.4个月[5-6]。本组资料处方剂量全脑40 Gy/20 F,转移灶60 Gy/20 ~ 30 F组、全脑50 Gy/ 25 F组和全脑40 Gy/20 F组3组中位生存期分别为13个月、15个月和11.5个月,差异无统计学意义,不同处方剂量生存期无差异,与Gaspar等[7]荟萃分析一致。本资料放疗中位生存期高于上述文献报道,可能与近年来肿瘤综合治疗进步有关[8]。近年来乳腺癌患者生存期的提高,主要得益于包括手术、放疗、化疗、内分泌、分子靶向、止痛及积极支持等综合治疗的提高。Her-2阳性是乳腺癌发生脑转移的独立危险因素[9]。Her-2阳性患者应用赫赛汀治疗可以提高中位生存期[10],Sperduto等[11]2012年总结了乳腺癌脑转移预后因素,肿瘤表型ER/ PR(-)和HER-2(-)三阴预后最差,其次是ER/PR(+)和HER-2(-),ER/PR(-)和HER-2(+),最好的是ER/PR(+)和HER-2(+)。本组资料HER-2阳性患者占59.6%,高于文献报道的20% ~ 30%[12]。新一代分子靶向药物拉帕替尼通过血脑屏障能力比赫赛汀强,联合卡培他滨能提高HER-2阳性乳腺癌脑转移患者生存时间,推荐作为HER-2阳性乳腺癌脑转移患者一线治疗方案[13]。本资料单因素及多因素分析显示,脑转移放疗生存时间与ER/PR阳性与否无关,可能因为发生脑转移时多数患者对内分泌治疗药物已经耐药,使用内分泌药物对颅内转移灶作用有限。放疗生存期与Her-2阳性与否无关,可能因为本组资料Her-2阳性率偏高及使用靶向药物人数少(8/47)有关。本资料单因素显示患者一般状况越好,预后越好;多因素显示,一般状况越好、临床分期越早、脑转移个数越少、颅内进展时间越晚,预后越好。早发现、临床分期早,早期治疗预后好。患者一般情况好、对手术、放化疗、内分泌及靶向治疗的耐受性好、患者免疫力强,预后好。脑转移个数越少、可以行手术、立体定向放疗等治疗肿瘤的局控率高,预后好。放疗后颅内病灶进展晚、肿瘤表型好、对放疗敏感,预后好。
对WBRT的神经系统晚期毒性一直存在争议。回顾性研究认为WBRT会降低神经认知功能[14]。前瞻对照实验表明WBRT组认知功能较对照组无下降[15]。颅内转移病灶进展引起神经认知功能障碍远较全脑放疗引起并发症明显[16]。Tallet等[17]系统分析了截至2011年9月的前瞻性研究文献,发现神经认知功能下降大多出现在放疗后4个月内,取决于颅内转移灶的控制,多为1度神经认知功能下降,2度及以上损伤只有8%。有研究报告全脑照射可以引起近记忆力下降,但不会引起认知功能明显下降,对于存在高危因素患者(如高龄、伴发脑血管及退行性疾病、有多次行头部放疗史)神经认知功能下降明显[18]。本组资料全脑照射40 ~ 50 Gy/20 ~ 25 F,转移灶加量至60 Gy/20 ~30 F未出现明显认知功能下降,未明显影响患者的生存质量,2例(4.5%)出现Ⅲ~Ⅳ级神经系统不良反应,与上述报道一致。
Sperduto等[19]荟萃分析显示,影响乳腺癌脑转移患者放疗生存期的预后因素有年龄、KPS、ER/PR/HER-2;GPA四级评分中,中位生存期分别为3.4个月、7.7个月、15.1个月、25.3个月,平均中位生存期为13.8个月,本组资料GPA 0 ~1、1.5 ~ 2、2.5 ~ 3三组放疗后中位生存期分别为13个月、12个月、13.5个月,高于上述文献报道,与近年来乳腺癌综合治疗的进步有关。患者一般情况越好,免疫力越强,对全身综合治疗耐受性越好。乳腺癌患者发生脑转移时,一般情况差,免疫力低下,肿瘤对内分泌药物、化疗药物及分子靶向药物均可能耐药,颅外往往肿瘤未控,发生肺、骨、肝等多处转移,加上长期的放化疗损伤,此时对颅内转移灶根治性放疗或手术,对进一步提高生存期作用有限。
三维适形放疗治疗乳腺癌脑转移患者能提高局控率,延长生存期,全脑照射40 Gy/20 F安全有效,不良反应可耐受。存在高危因素(高龄、既往有脑血管及退行性疾病)患者可能较易出现晚期严重神经系统不良反应,更多资料还需临床进一步积累。
1 Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer[J]. J Clin Oncol, 2004, 22(17):3608-3617.
2 Evans AJ, James JJ, Cornford EJ, et al. Brain metastases from breast cancer: identification of a high-risk group[J]. Clin Oncol (R Coll Radiol), 2004, 16(5):345-349.
3 Shaffrey ME, Mut M, Asher AL, et al. Brain metastases[J]. Curr Probl Surg, 2004, 41(8):665-741.
4 Altundag K, Bondy ML, Mirza NQ, et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis[J]. Cancer, 2007,110(12):2640-2647.
5 Wadasadawala T, Gupta S, Bagul V, et al. Brain metastases from breast cancer: management approach[J]. J Cancer Res Ther,2007, 3(3):157-165.
6 Frazier JL, Batra S, Kapor S, et al. Stereotactic radiosurgery in the management of brain metastases: an institutional retrospective analysis of survival[J]. Int J Radiat Oncol Biol Phys, 2010, 76(5):1486-1492.
7 Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline[J]. J Neurooncol, 2010, 96(1):17-32.
8 Kim HJ, Im SA, Keam B, et al. Clinical outcome of central nervous system metastases from breast cancer: differences in survival depending on systemic treatment[J]. J Neurooncol, 2012, 106(2):303-313.
9 Stemmler HJ, Kahlert S, Siekiera W, et al. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer[J]. Breast, 2006, 15(2):219-225.
10 Gupta GP, McLane A, Stewart L, et al. Stereotactic Radiosurgery for Breast Cancer Brain Metastases: Improved Outcomes in Her2 Amplified Patients[J]. Int J Radiat Oncol Biol Phys, 2010, 78(S3):S5.
11 Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosisspecific tool to estimate survival for patients with brain metastases[J]. J Clin Oncol, 2012, 30(4):419-425.
12 Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG)[J]. Ann Oncol, 2006, 17(6):935-944.
13 Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study[J]. Lancet Oncol, 2013, 14(1):64-71.
14 DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases[J]. Neurology, 1989, 39(6):789-796.
15 Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR)and the European Organization for Research and Treatment of Cancer(EORTC)[J]. Eur J Cancer, 1997, 33(11):1752-1758.
16 Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function[J]. J Clin Oncol, 2007, 25(10):1260-1266.
17 Tallet AV, Azria D, Barlesi F, et al. Neurocognitive function impairment after whole brain radiotherapy for brain metastases:actual assessment[J]. Radiat Oncol, 2012, 7:77.
18 侯俊, 冯林春, 路娜, 等. 三维适形放疗非小细胞肺癌脑转移的疗效及预后分析[J]. 军医进修学院学报,2012, 33(10):1033-1036.
19 Sperduto PW, Xu Z, Sneed P, et al. The graded prognostic assessment for women with brain metastases from breast cancer(GPA-Breast): a diagnosis-specific prognostic index[J]. Int J Radiat Oncol Biol Phys, 2010, 78(S3): S6-S7.
Efficacy and prognosis of three-dimensional conformal radiotherapy in treatment of 47 breast cancer patients with brain metastases
HOU Jun1, FENG Lin-chun2, MA Lin2, QU Bao-lin2, PENG Liang2, WANG Yun-lai2, XU Wei-dong1, QUAN Jian-hua1, ZHANG Fu-li1, WANG Ya-di11Department of Radiation Oncology, The Military General Hospital of Beijing PLA, Beijing 100700, China;2Department of Radiation Oncology, Chinese PLA General Hospital, Beijing 100853, China
WANG Ya-di. Email: wangyadi@hotmail.com
Objective To evaluate the curative effect and adverse effects of three-dimensional conformal radiotherapy (3D-CRT) for breast cancer patients with brain metastases. Methods Clinical data about 47 breast cancer patients with brain metastases admitted to The Military General Hospital of Beijing PLA and Chinese PLA General Hospital from May 1995 to October 2010 who underwent the whole brain radio therapy (WBRT) were retrospectively analyzed. The doses of WBRT was 50 Gy in 2 Gy fractions, or 40 Gy in 2 Gy fractions with simultaneous integrated boost to multiple brain metastases (DT 60 Gy in 3 Gy fractions). Part of the patients underwent stereotactic radiotherapy with 10-24 Gy/1-3 F. The curative effect and adverse reactions were evaluated for one to three months after radiotherapy. Results All patients were followed up for 2-49 months. CR was observed in 5 cases (10.6%), PR in 26 cases (55.3%), SD in 13 cases (27.7%), PD in 3 cases (6.4%), and the clinical response rate was 66.0%. 1, 2 and 3 years survival rates of brain metastases to death were 53.2%, 25.5% and 2.1% respectively, and the median survival time was 13 months. The main adverse reactions were radiation-induced cerebral edema, alopecia, tympanitis, hearing loss, dermoreaction, acratia, marrow suppression, impaired memory and Ⅲ-Ⅳ nervous system side effects. The univariate analysis showed that KPS (<50 and ≥60) (t=4.37, P=0.00) was a significant prognostic factor of overall survival time. The mutivariate analysis showed that KPS (u=3.05, P=0.00), AJCC stage (u=2.09, P=0.04), brain metastasis number (<3 and ≥3) (u=1.94, P=0.05) and intracranial progress (u=2.21, P=0.03) were significant prognostic factors of overall survival time. Conclusion 3D-CRT can improve the local control rate of breast cancer patients with brain metastases, and prolong the survival time with few adverse effects. It is safe for patients treating with whole brain radiotherapy (DT 40 Gy, 2 Gy/fraction). Patients with good KPS, early stage, few brain metastasis number and occuring intracranial progress later may have longer overall survival time.
breast neoplasms metastasis; whole brain radiotherapy; three-dimensional conformal radiotherapy; prognosis
R 737.9
A
2095-5227(2014)10-1034-05
10.3969/j.issn.2095-5227.2014.10.017
时间:2014-06-17 14:55
http://www.cnki.net/kcms/detail/11.3275.R.20140617.1455.004.html
2014-04-18
侯俊,男,硕士,主治医师。专业方向:头颈部肿
瘤精细放疗。Email: howejun@163.com
王雅棣,女,博士生导师,教授,主任医师,主任。
Email: wangyadi@hotmail.com

