Lamotrigine versus carbamazepine in treating newly diagnosed epilepsy:A meta-analysis
LIU Ya,PENG Ya-Wen,ZHOU Ke-Cheng,QU Cheng-Hao,HU WeiOutpatient Department,Xijing Hospital,Fourth Military Medical University of PLA,Xi'an 700,China;Student Brigade,Fourth Military Medical University of PLA,Xi'an 700,China;Research Laboratory of Chinese Medicine Compound,China Pharmaceutical University,Nanjing 98,China
·临床与转化医学·
Lamotrigine versus carbamazepine in treating newly diagnosed epilepsy:A meta-analysis
LIU Ya1,PENG Ya-Wen2,ZHOU Ke-Cheng3,QU Cheng-Hao2,HU Wei21Outpatient Department,Xijing Hospital,Fourth Military Medical University of PLA,Xi'an 710032,China;2Student Brigade,Fourth Military Medical University of PLA,Xi'an 710032,China;3Research Laboratory of Chinese Medicine Compound,China Pharmaceutical University,Nanjing 211198,China
First-line therapy for newly diagnosed epilepsy(e.g.carbamazepine)is generally considered effective.However,in a significant proportion of patients(especially in the elderly),usage may be limited by unwanted adverse events.To synthesize evidence regarding efficacy and tolerability of lamotrigine as first line,monotherapyorprophylacticantiepileptic.MEDLINE,
PsycINFO,Scopus,EMBASE,and the Cochrane Central Register of Controlled Trials(CENTRAL)were searched from inception to June 2014.Randomised controlled trials(RCTs)comparing lamotrigine with carbamazepine monotherapy for newly diagnosed epilepsy.Eligible studies were independently selected and methodological quality was independently evaluated by two reviewers.Effects were summarized using standardized hazard ratio(HR)or odds ratio(OR)with suitable effect models.Pre-specified sensitivity analyses were performed to explain heterogeneity.Nine studies involving 2793 participants met the inclusion criteria.The effects of lamotrigine compared with carbamazepine in patients with newly diagnosed seizures were investigated in all studies.We found that carbamazepine was inferior in comparison to lamotrigine when measuring the proportion of remaining seizure free in the elderly(hazard ratio(HR)1.71;95%confidence interval(CI)
1.27 to 2.29)but notthe children and the adult.There was strong evidence for the tolerability profile of lamotrigine compared with carbamazepine in the Retention rates(HR 1.67;95%CI 1.43 to 1.94).Moreover,lamotrigine lead to less adverse events.Lamotrigine and carbamazepine showed similar efficacy on newly diagnosed epilepsy but better efficacy in the elderly than carbamazepine.Furthermore,lamotrigine was better tolerated.
lamotrigine;carbamazepine;newly diagnosed epilepsy;meta-analysis
1 Introduction
Standard first-line therapy for newly diagnosed epi-lepsy,e.g.carbamazepine(CBZ),is generally considered effective.However,in a significant proportion of patients,especially in the elderly,usage may be limited by unwanted adverse events.
Lamotrigine(LTG)is a kind of benzene thiazine derivatives,the chemical properties differ from other new antiepileptic drugs.Experimental results suggested that LTG inhibits amyl four nitrogen and electrical stimulation of convulsion,shorten lesions,cortex and hippocampus excited after the discharge time,against part and generalized epilepsy.The drug effect was suggested to be mediated via multiple mechanisms:(1)inhibiting voltage dependent II type a sodium channel,stabilizing cell membrane to prevent abnormal discharge;(2)Stablizing presynaptic membrane,thus preventing evoked excitatory neurotransmitter release(especially glutamate);(3)inhibiting voltage dependent calcium channel.LTG has been used to control various types of epilepsy,including simple partial onset seizure,complex partial onset seizure,and secondary body stiffness seizure.However,whether it can be used to treat newly diagnosed epilepsy is not concluded.In some previous RCTs(randomized controlled trial),the efficacy of LTG in treating newly diagnosed epilepsy compared with CBZ was investigated,however,without consolidated conclusion.
We thus performed the current meta-analysis of published and unpublished RCTs in which the efficacy and tolerability of CBZ and LTG,as monotherapy,were compared in patients with newly diagnosed epilepsy.Since the methodologies used and the patients recruited were similar in these studies,we compared these overall estimates between LTG and CBZ,allowing a broad estimate of their comparative efficacy and tolerability.Through comparison,the best comparative data available on these drugs to date can be provided(though in no way replacing RCTs comparing active treatments).A systematic approach and statistical synthesis of available data seems least“bad”at this time and a better approach than only reliance on the views of“opinion leaders”.
2 Methods
We performed the current meta-analysis based on the QUORUM guidelines[1](Quality of Reporting of Meta-analyses)and the recommendations of the Cochrane Collaboration[2].
2.1 Data sources and searches The electronic databases screened were MEDLINE(1990 to September 2014),PsycINFO(1990 to September 2014),Scopus (1988 to September 2014),EMBASE(1990 to September 2014),and The Cochrane Library(Issue 9 of 12,September 2014),including the Cochrane Central Register of Controlled Trials(CENTRAL),Cochrane Database of Systematic Reviews(CDSR),Database of Abstracts of Reviews of Effects(DARE),and Health Technology Assessments(HTA).Searches were limited to human and performed for all languages,year of publication and type of publication.The key words,epilepsy and LTG,were used in combination with RCT or controlled clinical trial or review.Limiting the number of reports with a filter did not seem reasonable considering the potential number of studies,thus we did not use a filter such as the highly sensitive search strategy.The procedure of study selection was demonstrated in Fig.1 and two of us manually and independently screened reference sections of relevant original articles,reviews,and meta-analyses,leading to the inclusion of a total of 9 RCTs in this analysis.
2.2 Analyzed studies Studies were included according to the following criteria:LTG were applied to treat the newly diagnosed epilepsy;Any RCT,controlled clinical trial,or open label trial(OLT)designed with a control group receiving first-line treatment drugs for epilepsy;and another group receiving LTG.
The literature search yielded 116 citations.Initially,40 publications met our inclusion criteria.The excluded 76 publications were not about the newly diagnosed epilepsy.On more detailed review,an additional 16 papers were excluded because the comparison between LTG and CBZ were not performed.Then,another 5 papers were excluded because the focus of these studies was not onefficacy or tolerability of both drugs.Ten more publications were further excluded because of repeated or deficiency data(the repeated and deficiency data was derived from the abstract published on Epilepsia).The remained 9 studies[3-11]met our selection criteria and were included in the meta-analysis and their characteristics were presented in table 1.In total,1744 patients were randomly assigned to receive any form of oral administration of LTG,1393 were assigned to CBZ groups.
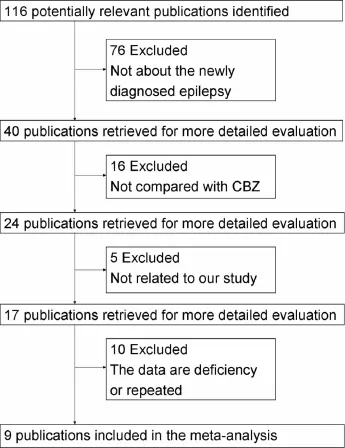
Fig.1 Study selection
2.3 Data Extraction Two reviewers independently screened the titles and abstracts of potentially eligible studies.The full text articles were examined independently by 2 of us to determine whether they met the inclusion criteria.Two of us independently extracted data (study characteristics and results)using data extraction forms,and then the collected data were entered into RevMan 5.0 using the double-entry system.Point estimates for selected variables were extracted and checked by other 2 reviewers.All discrepancies were recheckedand consensus was achieved by discussion with a third author involved.A record of reasons for excluding studies was kept.Cohen's kappa was applied for calculating inter-rater agreement.
2.4 Outcome measures Based on the outcome measurements reported in the RCTs,we stratified main outcomes including withdrawal rate,retention rate,proportion of remaining seizure free,the time to first attack,the time to withdrawal and adverse effects.
2.4.1 Efficacy:efficacy was evaluated by the primary outcome withdrawal rate and retention rate.Furthermore,proportion of remaining seizure free,the time to first attack and the time to withdrawal were recorded.
2.4.2 Tolerability:tolerability was measured with the withdrawal rate,retention rate and the time to withdrawal.
2.4.3 Adverse effects(AEs):rash,dizziness,headache,somnolence,fatigue,nausea and vomiting,diarrhea and poor coordination were included.Furthermore,the number of patients experiencing any AEs was recorded.
The Jadad test(5 items)[12]was applied to assess methodological quality as high(score 5),moderate (score 4),or low(scores 1-3).
2.5 Statistical analysis Because most outcomes were presented as incontinuous data,we used either the hazard ratios(HRs)or odds ratios(ORs)with 95%confidence intervals(CI)as effect measures.HRs were used to evaluate the time to first attack and the time to withdrawal.ORs as well as their 95%CIs were calculated.Data were analyzed using Review Manager Analyses software(RevMan 5.0.25)according to Cochrane Handbook for Systematic Reviews of Interventions.
A sensitivity analysis was conducted to determine whether the meta conclusion were influenced by including low Jadad score papers.
Publication bias may lead to asymmetrical funnel plots[13].Visual assessment of the funnel plot calculated by RevMan Analyses software was used to investigate the potential publication bias(i.e.,the association of publication probability with the statistical significance of study results).
3 Results
3.1 Baseline characteristics The baseline demographic characteristics,dosage of LTG and CBZ,exclusion criteria,and Jadad score for every study were demonstrated in Table 1.
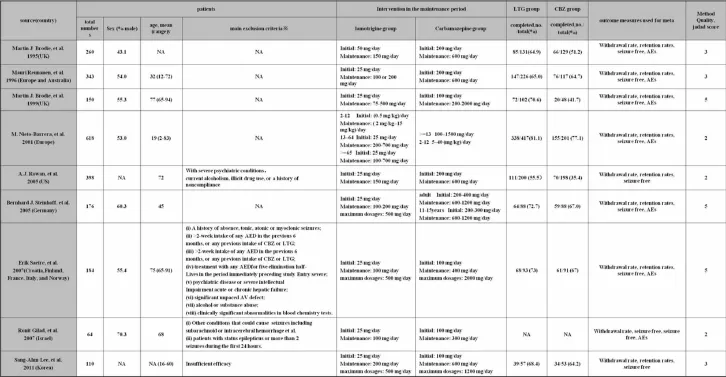
Table 1 Main study characteristics
3.2 The time to first attack,withdrawal or retention The time to first attack or withdrawal were presented in Fig.3.Due to the lack of data,we could not build the survival curve;we just listed the currently a-vailable data from individual studies.Although no confirmative conclusion can be reached,LTG treatment led to longer time to withdrawal or the first attack,suggesting that the efficacy of LTG might be better than that of CBZ.
The higher retention rate of LTG also showed its advantages over CBZ(OR,1.67(95%CI,1.43 to 1.94;P<0.000 01)).Furthermore,as can be seen from Fig.3,LTG had much more efficacy and tolerability in the elderly[OR,2.21(95%CI,1.64 to 2.98;P<0.000 01)]and adult[OR,1.53(95%CI,1.28 to 1.84;P<0.000 01)]than the children[OR,1.12 (95%CI,0.51 to 2.46;P=0.78)].

Fig.2a Risk of bias graphFig.2 b Risk of bias summary
3.3 Seizure free rate A moderate influence of LTG on the seizure free rate was revealed in the adult(Fig.4).Overall,LTG significantly increased the seizure free rate in the elderly[OR,1.71(95% CI,1.28 to 2.30;P=0.000 3)]but not the adult[OR,1.04 (95%CI,0.75 to 1.46;P=0.80].However,LTG treatment insignificantly decrease the seizure free rate in children[OR,0.73(95%CI,0.41 to 1.28;P=0.27)].

Fig.3 Summary the efficacy

Fig.4 Seizure free during different time
3.4 The withdrawal Figure 5 demonstrated the strong evidence for the safety of oral LTG in treating the newly diagnosed epilepsy.LTG significantly reduced the withdrawal for any cause,all the events and rash[OR,0.54(95%CI,0.46 to 0.63;P<0.000 01);OR,0.41(95%CI,0.32 to 0.52;P<0.000 01);and OR,0.36(95%CI,0.24 to 0.55;P<0.000 01)],respectively.Concerning the withdrawal for all causes and AEs,LTG was considered as no significant advantage over CBZ in the children[OR,0.89(95%CI,0.41 to 1.96;P=0.78);OR,0.41(95%CI,0.32 to 0.52;P=0.62)],but LTG was better in the adult [OR,0.57(95%CI,0.47 to 0.68;P<0.000 01);OR,0.43(95%CI,0.33 to 0.57;P<0.000 01)]and the elderly[OR,0.45(95%CI,0.34 to 0.61;P<0.000 01);OR,0.32(95%CI,0.20 to 0.51;P<0.000 01)].
In addition,the withdraw for rash was also significantly decreased in LTG than CBZ in the adult and elderly[OR,0.43(95% CI,0.33 to 1.57;P<0.000 01);OR,0.32(95%CI,0.20 to 0.51;P<0.000 01)]respectively.

Fig.5 The withdrawal
3.5 The adverse events The AEs of LTG and CBZ were summarized in Fig 6.Seven studies[3-5,7-8,10-11]reported adverse events data including the number of patients experiencing any AE,rash,dizziness,headache,somnolence,fatigue,nausea or vomiting,diarrhea,poor coordination compared to CBZ in a total of 2359 patients.Strong evidence was obtained that the LTG didn't increase the incidence of any AE[OR,0.62;(95%CI,0.44 to 0.87,P=0.006)],rash[OR,0.45;(95%CI,0.26 to 0.80,P<0.000 1)],somnolence[OR,0.33;(95%CI,0.20 to 0.54,P=0.87)],fatigue[OR,0.58;(95%CI,0.44 to 0.76,P<0.000 1)],poor co-ordination[OR,0.25;(95% CI,0.13 to 0.47,P<0.000 1)].However,there is high heterogeneity detected between studies in rash(I2 =72%,P=0.006)and somnolence(I2=60%,P<0.000 1).
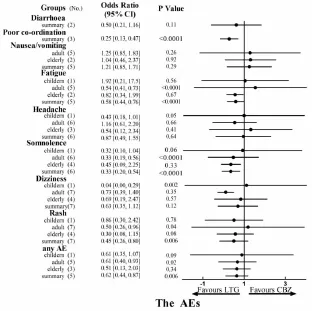
Fig.6 The AEs
The outcome of the number of adult experiencing any AE showed LTG was safer than CBZ[OR,0.61;(95%CI,0.40 to 0.93,P=0.02)].
Moreover,LTG significantly decreased rate of rash [OR,0.50;(95%CI,0.26 to 0.96,P=0.04)],somnolence[OR,0.33;(95%CI,0.20 to 0.54,P<0.000 1)]or fatigue[OR,0.54;(95%CI,0.41 to 0.73,P<0.000 1)]in the adult.In the children,the dizziness(OR,0.04;95%CI,0.00 to 0.29,P=0.002)and headache(OR,0.43;95%CI,0.18 to 1.01,P=0.05)were significantly decreased by LTG treatment.
3.6 Bias assessment Summary assessment of the risk of bias tables is presented in Fig.2a,Fig.2b and Fig.3.High risk of bias might be derived from the study of Selim [14]because incomplete outcome data were given due to the lack of control group.Funnel plots for subgroup analysis assessing AEs revealed a symmetric distribution suggesting no publication bias(Fig.4).However,other funnel plots could not be provided because the data from included studies in each comparison group were inadequate.
4 Discussion
LTG has wide therapeutic spectra,fewer sideeffects,and lesser drug-to-drug interactions compared with the older typical antiepileptic drugs,thus has been used to treat some kinds of epilepsy,but seldom used in treating the newly diagnosed epilepsy.We performed the current meta-analysis to address whether LTG is superior to CBZ in treating newly diagnosed epilepsy.Through the analysis of the nine articles,we can come to the preliminary conclusion that LTG at least have the same curative effect with CBZ and a better tolerability than CBZ.However,the high heterogeneity of the current RCTs can not be ignored.We think,more studies should be done in the Europe and in every country independently,not multicenter.And this indicates LTG doesn't have the same efficacy and tolerability on patients from different countries in the Europe.It will be a significant measure to clear up this matter.Similarly more studies need to be done both in Asia and in America.We cannot reach reliable conclusion just by the limited studies.
Old age is the most common time to develop the seizure disorder.In elderly patients,there is a particu-lar need to avoid central nervous system adverse events,which have been linked to increased risk of falls[15].Furthermore,the management of epilepsy in old patients is complicated by interactions between many well-established anti-epileptic drugs and treatments used for concomitant conditions.Unlike other antiepileptic treatments,LTG does not inhibit or induce hepatic enzymes,so reducing the potential for drug interactions[16].LTG shows more remarkable advantage over CBZ in the elderly and children than the adult.Its advantage has been convinced in the elderly,however,it need more studies to be supported.
During this study,we also found some other conclusions about the newly diagnosed epilepsy treated by LTG.Bonnett L et al found that the sex was a significant factor that influences the time to 12 months of remission [17].Saetre E et al studied the quality of life in the elderly treated with antiepileptic drugs.The finding showed neither LTG nor CBZ seemed likely to cause significant changes in health-related quality of life measures after 40 weeks at therapeutic doses[11,18].However RUTH GILLHAM et al concluded that LTG offers the newly diagnosed patient with partial and/or generalized tonicclonic seizures significant benefits of greater tolerability and better quality of life,compared with CBZ[19].We think there are more trails needed conducting to study this question.In an international randomized doubleblind 40-week trial,Saetre E et al demonstrated clinically significant ECG changes were not common during treatment with CBZ or LTG in elderly patients with no preexisting significant AV conduction defects[20].Hussein Z and Posner J compared the oral clearance between Asians and Caucasian;it was lower in Asians than Caucasian[21].We can infer that the efficacy and tolerability among patients also differ in different districts.This also reminds us more studies are needed in different districts separately.
There are also some other studies comparing LTG with other antiepileptic.There were no significant differences with regard to efficacy and tolerability of LEV(levetiracetam)and LTG in newly diagnosed focal and generalised epilepsy in Rosenow F et al's study[22].LTG had similar tolerability and seemed to have better efficacy to pregabalin for the treatment of newly diagnosed partial seizures in adults in Kwan P et al's study[23].Ethosuximide and valproic acid were more effective than LTG in the treatment of childhood absence epilepsy and ethosuximide was associated with fewer adverse attentional effects in Glauser TA et al's study[24].No difference in efficacy was found between sodium valproate and LTG in the patients with newly diagnosed epilepsy,however,LTG appeared to be better tolerated in Stephen LJ et al's study[25].Brodie MJ et al reached the conclusion that gabapentin and LTG monotherapy were similarly effective and well tolerated in patients with newly diagnosed epilepsy[26].LTG and PHT(phenytoin)monotherapy were similarly effective against these seizure types in patients with newly diagnosed epilepsy and LTG was better tolerated,more frequently causing rash,but with a lower incidence of central nervous system side effects in Steiner TJ's study[27].Coppola G et al also compared valproic acid with LTG,they also found VPA and LTG can be efficacious against absence seizures[28].Among so many antiepileptics,LTG doesn't have the best efficacy and tolerability among all the studies with newly diagnosed epilepsy.However,it is effective and well tolerated compared with placebo[29].And among all the first-line therapy for newly diagnosed epilepsy,especially CBZ,LTG is more effective and tolerated.The reason we chose CBZ as compare was its wide acceptance and there was relatively enough trails for us to take this meta-analysis.All other studies compared LTG with other antiepileptic need more evidences.
Our results cannot be extrapolated to patients with some concrete epilepsy and not newly diagnosed epilepsy,even some kinds of newly diagnosed epilepsy.There is clearly a need for studies in such patients.Then,we don't find a way to eliminate the high heterogeneity.Thirdly,we failed to contact some authors and didn't get the data.
5 Conclusions
LTG has the same efficacy and better tolerability compared with CBP.This is more obvious in the elderly patients.And LTG can be considered as the first-line therapy for the newly diagnosed epilepsy,especially for the elderly.
【Reference】
[1]Moher D,Cook DJ,Eastwood S,et al.Improving the quality of reports of meta-analyses of randomised controlled trials:the QUOROM statement.Quality of Reporting of Meta-analyses[J].Lancet,1999,354(9193):1896-1900.
[2]Bero L,Rennie D.The Cochrane Collaboration.Preparing,maintaining,and disseminating systematic reviews of the effects of health care [J].JAMA,1995,274(24):1935-1938.
[3]Brodie MJ,Overstall PW,Giorgi L.Multicentre,double-blind,randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy.The UK Lamotrigine Elderly Study Group[J].Epilepsy Res,1999,37(1):81-87.
[4]Brodie MJ,Richens A,Yuen AW.Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy.UK Lamotrigine/Carbamazepine Monotherapy Trial Group[J].Lancet,1995,345(8948):476-479.
[5]Gilad R,Sadeh M,Rapoport A,et al.Monotherapy of lamotrigine versus carbamazepine in patients with poststroke seizure[J].Clin Neuropharmacol,2007,30(4):189-195.
[6]Lee SA,Lee HW,Heo K,et al.Cognitive and behavioral effects of lamotrigine and carbamazepine monotherapy in patients with newly diagnosed or untreated partial epilepsy[J].Seizure,2011,20(1):49 -54.
[7]Nieto-Barrera M,Brozmanova M,Capovilla G,et al.A comparison of monotherapy with lamotrigine or carbamazepine in patients with newly diagnosed partial epilepsy[J].Epilepsy Res,2001,46(2):145-155.
这天晚上,阿东一直坐在床边,看着阿里睡着。他心里很难过,不知道怎样才能安慰阿里,也不知道怎样才能帮到阿里。
[8]Reunanen M,Dam M,Yuen AW.A randomised open multicentre comparative trial of lamotrigine and carbamazepine as monotherapy in patients with newly diagnosed or recurrent epilepsy[J].Epilepsy Res,1996,23(2):149-155.
[9]Rowan AJ,Ramsay RE,Collins JF,et al.New onset geriatric epilepsy:a randomized study of gabapentin,lamotrigine,and carbamazepine[J].Neurology,2005,64(11):1868-1873.
[10]Saetre E,Perucca E,Isojarvi J,et al.An international multicenter randomized double-blind controlled trial of lamotrigine and sustainedrelease carbamazepine in the treatment of newly diagnosed epilepsy in the elderly[J].Epilepsia,2007,48(7):1292-1302.
[11]Steinhoff BJ,Ueberall MA,Siemes H,et al.The LAM-SAFE Study:lamotrigine versus carbamazepine or valproic acid in newly diagnosed focal and generalised epilepsies in adolescents and adults[J].Seizure,2005,14(8):597-605.
[12]Jadad AR,Moore RA,Carroll D,et al.Assessing the quality of reports of randomized clinical trials:is blinding necessary?[J].Control Clin Trials,1996,17(1):1-12.
[13]Egger M,Davey Smith G,Schneider M,et al.Bias in meta-analysis detected by a simple,graphical test[J].BMJ,1997,315(7109):629-634.
[14]Selim MF,Elnabtity AM,Hasan AM.Comparative evaluation of epidural bupivacaine-dexmedetomidine and bupivacaine-fentanyl on Doppler velocimetry of uterine and umbilical arteries during labor[J].J Prenat Med,2012,6(3):47-54.
[15]Brodie MJ,Overstall PW,Giorgi L,et al.Multicenter double-blind randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy[J].Epilepsy Res,1999,37:81-87.
[16]Posner J,Holdich T,Crome P.Comparison of lamotrigine pharmacokinetics in young and elderly healthy volunteers[J].J Pharm Med,1991,1:121-128.
[17]Bonnett L,Smith CT,Smith D,et al.Prognostic factors for time to treatment failure and time to 12 months of remission for patients with focal epilepsy:post-hoc,subgroup analyses of data from the SANAD trial[J].Lancet Neurol,2012,11(4):331-340.
[18]Saetre E,Abdelnoor M,Perucca E,et al.Antiepileptic drugs and quality of life in the elderly:results from a randomized double-blind trial of carbamazepine and lamotrigine in patients with onset of epilepsy in old age[J].Epilepsy Behav,2010,17(3):395-401.
[19]Gillham R,Kane K,Bryant-Comstock L,et al.A double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy with health-related quality of life as an outcome measure[J].Seizure,2000,9(6):375-379.
[20]Saetre E,Abdelnoor M,Amlie JP,et al.Cardiac function and antiepileptic drug treatment in the elderly:a comparison between lamotrigine and sustained-release carbamazepine[J].Epilepsia,2009,50(8):1841-1849.
[21]Hussein Z,Posner J.Population pharmacokinetics of lamotrigine monotherapy in patients with epilepsy:retrospective analysis of routine monitoring data[J].Br J Clin Pharmacol,1997,43(5):457-465.
[22]Rosenow F,Schade-Brittinger C,Burchardi N,et al.The LaLiMo Trial:lamotrigine compared with levetiracetam in the initial 26 weeks of monotherapy for focal and generalised epil epsy an open-label,prospective,randomised controlled multicenter study[J].J Neurol Neurosurg Psychiatry,2012,83(11):1093-1098.
[23]Kwan P,Brodie MJ,Kalviainen R,et al.Efficacy and safety of pregabalin versus lamotrigine in patients with newly diagnosed partial seizures:a phase 3,double-blind,randomised,parallel-group trial[J].Lancet Neurol,2011,10(10):881-890.
[24]Glauser TA,Cnaan A,Shinnar S,et al.Ethosuximide,valproic acid,and lamotrigine in childhood absence epilepsy[J].N Engl J Med,2010,362(9):790-799.
[25]Stephen LJ,Sills GJ,Leach JP,et al.Sodium valproate versus lamotrigine:a randomised comparison of efficacy,tolerability and effects on circulating androgenic hormones in newly diagnosed epilepsy[J].Epilepsy Res,2007,75(2-3):122-129.
[26]Brodie MJ,Chadwick DW,Anhut H,et al.Gabapentin versus lamotrigine monotherapy:a double-blind comparison in newly diagnosed epilepsy[J].Epilepsia,2002,43(9):993-1000.
[27]Steiner TJ,Dellaportas CI,Findley LJ,et al.Lamotrigine monotherapy in newly diagnosed untreated epilepsy:a double-blind comparison with phenytoin[J].Epilepsia,1999,40(5):601-607.
[28]Coppola G,Auricchio G,Federico R,et al.Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures:an open-label,randomized,parallel-group study[J].Epilepsia,2004,45(9):1049-1053.
[29]Frank LM,Enlow T,Holmes GL,et al.Lamictal(lamotrigine)monotherapy for typical absence seizures in children[J].Epilepsia,1999,40(7):973-979.
2095-6894(2014)06-029-07
2014-04-23;接受时间:2014-05-19
Hu Wei,Student Brigade,Fourth Military Medical University of PLA,Xi'an,China.Tel:029-84774504 E-mail:xun1ta@sina.com

