Characterization and subcellular localization of two 14-3-3 genes and their response to abiotic stress in wheat
Xiaodan Meng, Xin Chen, Yaying Wang, Ruixia Xiao, Hailun Liu, Xinguo Wang, Jiangping Ren, Yongchun Li, Hongbin Niu, Xiang Wang, and Jun Yin
National Engineering Research Centre for Wheat, Henan Agricultural University, Zhengzhou 450002, Henan, China
Introduction
The 14-3-3 proteins were originally isolated from bovine brain as a family of abundant acidic brain proteins in 1967[1]. The name is derived from its particular elution and migration pattern on two-dimensional DEAE-cellulose chromatography and its migration position in the subsequent starch-gel electrophoresis[2]. As members of the 14-3-3 protein family form a group of highly conserved, ubiquitously expressed proteins, which are found in all eukaryotic cells but not in bacteria[3-4]. These molecules form homo- or hetero-dimers to interact with a large amount of diverse target proteins by their specific phosphoserine/phosphothreonine-binding activity[5-6].The phosphorylation-dependent protein binding mode allows the proteins to play significant roles in many biological processes like signal transduction,cell cycle control, carbon/nitrogen metabolism and apoptosis[7]. Therefore, the 14-3-3 proteins have received increased attention in recent decades.
In the early work, 14-3-3 proteins were found to be activators of tyrosine and tryptophan hydroxylases-enzymes involved in the biosynthesis of neurotransmitters[8]. Later on, the proteins were described as inhibitors of protein kinase C (PKC)[9]and stimulators of calcium-dependent exocytosis[10].A notable milestone was the discovery in 1996 that 14-3-3 proteins bind to specific phosphorylated motifs of their protein targets[11], which fundamentally advanced the identification of partners of 14-3-3 proteins. Nowadays, 14-3-3 proteins are regarded as a main kind molecular chaperones, with over 300 proteins being targeted[12-13], and extensive proteomic analysis further revealed the signaling pathways and networks in which 14-3-3 proteins were involved,progressively unveiling their significant roles in cell signaling.
In plant, current research clearly suggests 14-3-3s play important regulating roles in key physiological processes, in particular, abiotic and biotic stress responses, metabolism, as well as various aspects of plant growth and development[14].For this reason, various 14-3-3 isoforms have been isolated from diverse species including 15 known protein isoforms in Arabidopsis[15], six in cotton[16],eight in rice[17], five in barley[18]and 17 potential isoforms in tobacco[19]. The research results show thatArabidopsis14-3-3 isoforms have a high degree of organization and cell specificity, and located in the cytoplasm and organelles such as the chloroplast,Golgi apparatus and nuclei. The expression specificity and intracellular compartmentalization may lead to its diverse interactions with their partners and the different functions of the cell activities[20]. However, our understanding of the significance for 14-3-3 proteins in the regulation of wheat development and environmental adaptation is still rudimentary, and limited research has been undertaken on the 14-3-3 proteins in wheat.
Wheat (Triticum aestivum) is a staple food crop that is grown widely around the world.However, in many wheat-producing areas of the world, abiotic stresses such as drought, cold, heat and salinity often limit wheat productivity[21].Consequently it is important to understand the mechanisms of molecular adaptation of organisms to abiotic stress. Current research indicate that the plant phosphoserine-binding 14-3-3 proteins have important functions in plant responses to abiotic stress[20,22]. Although the expression of 14-3-3 genes under abiotic stress has been studied in a few plant species[22], little is known about the 14-3-3 genes in wheat. In this study, two cDNAs(designated asTa14R1andTa14R2) encoding putative 14-3-3 proteins were isolated from wheat.The molecular characterization of the two 14-3-3 genes in wheat and their expression patterns in different tissues and under abscisic acid (ABA),drought, high and low temperature treatments were investigated to further examine the role of these 14-3-3 proteins in development and abiotic stress responses of wheat.
1 Materials and methods
1.1 Plant samples preparation
Wheat cultivar Zhoumai 18 was used for gene cloning and expression analysis. Zhoumai 18 seed was surface sterilized for 1 min with 70% (V/V)ethanol and rinsed three times with sterile, deionized water, treated for 5 min with 0.1% (W/V) HgCl2, and rinsed five times with sterile distilled water. They were then germinated and cultured with 1/2-strength Hoagland solution in Petri dishes placed in a temperaturegrowth controlled chamber at 25℃withand under a 12 h photoperiod. Seedlings were watered daily with appropriate volumes of Hoagland solution. At the two-leaf stage, abiotic stress treatments were applied by transferring seedlings to appropriate solutions for 0.5, 1, 2, 6, 12, or 24 h.Drought and exogenous hormones (ABA) treatments were applied by culturing transferring seedlings in a Hoagland solution containing either 20% (W/V)PEG6000 or 100 µmol/L ABA. Cold and heat treatments were applied by transferring seedlings to 4 ℃ and 42 ℃ environment respectively. Control seedlings were treated only with Hoagland solution,cultured at 25 ℃ and under a 12 h photoperiod over the same time span. Afterward, leaves and roots were harvested from both stressed and control plants.To investigated the tissue expression patterns of the wheat 14-3-3 genes, some untreated seedling were grown to pots in a naturally light glasshouse with normal irrigation and fertilization until mature.Samples of mature seed embryos, primary roots and shoots of one day after germination, roots and fully expanded leaves of 10 days after germination, leaves in tillering stage, stems in jointing stage, flag leaves,young panicles in the heading stage and developing seeds at 10 and 25 days after pollination were harvested, respectively. All the plant materials were frozen in liquid nitrogen immediately after harvesting and stored in -80 ℃ until RNA extraction. Three biologically independent replicates were assayed to ascertain reproducibility.
1.2 RNA extraction and the first-strand cDNA synthesis
Total RNA extraction from embryos and seeds were carried out using a hot-phenol method[23]and from leaves and roots using Trizol Reagent(Invitrogen, Shanghai, China) according to the manufacturer’s instructions. The quality and concentration of total RNA was measured by spectrophotometer (NanoDrop ND-1000,Wilmington, USA) and agarose gel electrophoresis.
Equal amounts (2 µg) of total RNA were transcribed into cDNA in a 20 μL reaction system containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L MgCl2, 10 mmol/L DTT, 50 mmol/L dNTPs, 200 U MMV reverse transcriptase (Promega, Madison,America), and 50 pmol Oligo-dT(15) anchor primer.Reverse transcription was performed for 60 min at 42 ℃ with a final denaturation step at 95 ℃ for 5 min.
1.3 Rapid amplification of cDNA ends (RACE)
The mRNA was purified through oligotex chromatography (Clontech, Beijing, China) from total RNA and 5'-RACE was performed using the SMART-RACE cDNA amplification kit (Clontech,Beijing, China) according to the manufacturer's protocol. Gene specific primers were designed according to expressed sequence tags (EST)obtained by cDNA chip. Primer used for the first and second round PCR were 5'-GSP1, 5'-GSP3 and 5'-GSP2, 5'-GSP4, respectively (Table 1). PCR reaction system was performed according to the manufacturer's protocol (Clontech). PCR condition used for the first round amplification was as follows:94 ℃ for 4 min, followed by 6 cycles: 94 ℃ for 30 s, 60 ℃/58 ℃ 30 s, 72 ℃ 3 min, next followed by 20 cycles: 94 ℃ for 30 s, 56 ℃ 30 s,72 ℃ 3 min, and fi nally 72 ℃ for 10 min. The second round PCR condition was 94 ℃ 4 min,followed by 36 cycles: 94 ℃ for 30 s, 54 ℃ 30 s,72 ℃ 3 min, and then 72 ℃ for 10 min. The PCR products were separated by 1% agarose gel electrophoresis, and recovered with DNA Recovery Kit (Sangon, Shanghai, China). Then, the recovered fragments were subcloned into the pMD18-T vector(TaKaRa, Dalian, China) and sequenced.
1.4 Cloning of full-length cDNA encoding Ta14R1 and Ta14R2
According to the sequence information obtained by 5'-RACE, two gene full-length cDNA sequence were spliced using the software DNAStar(DNAstar Inc. Madison WI USA). To verify the correctness of the inferred fragments, two pairs of specific primers (Table 1) for the amplification of open reading frame (ORF) were designed based on known the cDNA sequence of 5' and 3'-UTRs. PCR was performed using 2 μL of the cDNA as templates and 125 pmol of each primer in a 20 μL reaction system containing 0.2 mmol/L of each dNTPs,1.5 mmol/L MgCl2, and 1UTaqpolymerase. The PCR program was as follows: 95 ℃ for 4 min,followed by 35 cycles of 95 ℃ for 30 s, 55 ℃ 30 s,72 ℃ 90 s, and then 72 ℃ for 10 min. Methods of the PCR products’s separation, recovery and sequencing were similar as described above (Section 1.3).

Table 1 Primer sequences in this study
1.5 Real-time quantitative RT-PCR
Aliquots of 1 μg of total RNA were used for first-strand cDNA synthesis using Primecript Reagent Kit with gDNA Eraser (Perfect Real Time) according to the manufacturer’s instructions (TaKaRa, Dalian,China). Real-time quantitative RT-PCR was performed using an Eppendorf Realplex4 Mastercycler Epgradient S machine (Eppendorf, Hamburg, Germany). The forward and reverse primers (Table 1) for real-time PCR were designed according to theTa14sgene’s 3'-UTR using the software Pimer Premier 5.0. For normalization, wheatβ-actingene (GenBank Accession No. AB181991) was amplified as an endogenous control (Table 1).
PCR was performed with the SYBR Green quantitative RT-PCR kit (TaKaRa, Dalian, China),following the manufacturer’s instructions. PCR cycling conditions comprised an initial cycle at 95 ℃ for 30 s,followed by 40 cycles at 95 ℃ for 15 s and at 60 ℃for 35 s and a final extension of 7 min at 72 ℃.No-template negative controls (H2O control) were set up using gene-specific primer pairs, and the no-RT-PCR controls were set up in duplicate using primers foractingene. For each sample, reactions were set up in triplicate to ensure the reproducibility of the results.
The cycle threshold (Ct), defined as the PCR cycle at which a statistically significant increase of reporter fluorescence is first detected, was used as a measure for the starting copy number of the target gene.Relative quantity of the targetTa14sexpression levels were determined using the comparativeCt method.The relative quantification for each target gene were calculated by the 2-ΔΔCtmethod, usingβ-actinas an internal reference gene for comparing data from different PCR runs or cDNA samples[24].
1.6 Transient expression of GFP fusion constructs in Arabidopsis protoplasts
The green fl uorescent protein (GFP) expression vector pJIT163-hGFP is a recombinant derivative of pJIT163/hGFP. The coding sequences ofTa14R1andTa14R2were amplified from a cDNA clone and then subcloned into pJIT163-hGFP vector to generate aTa14R1-GFPand aTa14R2-GFPfusion proteins.The constructed expression vectors and pJIT163-hGFP (as a control) were then introduced into the protoplast ofArabidopsis. Protoplast preparation followed the method of Sheen[25]. For the transformation, 100 μL of protoplast suspension was carefully mixed with 10 µg of column-purified plasmid DNA and 110 μL of polyethylene glycol(PEG)/Ca2+solution [4 g of PEG 4000, 3 mL of H2O,2.5 mL of 0.8 mol/L mannitol and 1 mL of 1 mol/L CaCl2]and incubated for 15–20 min at 23 ℃. Then the mixture was washed with 0.44 mL of W5(154 mmol/L NaCl, 125 mmol/L CaCl2, 5 mmol/L KCl and 2 mmol/L MES ⁄KOH at pH 5.7) solution and spun at 100 g for 1 min to remove PEG. The protoplasts were resuspended in 100 μL of W5 solution and added to 1 mL of W5. The protoplasts were then incubated for 12–16 h in the dark at 23 ℃and fl uorescence images were captured with confocal laser scanning microscopy[26].
1.7 Sequence and data analysis
Blast search was completed in NCBI(http://www.ncbi.nlm.nih.org). The deduced amino acid sequence and their structure prediction were performed at ExPaSy (http://www.expasy.org). ClustalW(http://www.ebi.ac.uk/clustalw/) was used for alignment analysis. MEGA (ver.4.0) was used for phylogenetic analysis. The data statistical analysis was carried out by means of SPSS10.0 software.
2 Results
2.1 Cloning of Ta14-3-3s cDNA
In our preliminary work, two drought-related ESTs were obtained by cDNA chip analysis, which had high similarity to Hv14-3-3 (GenBank Accession No. X93170 and X62388) in barley. In order to isolate the full-length 14-3-3 genes of wheat,the gene specific primers were designed based on the EST sequences, and the cDNA from roots were used as the template. RACE was performed to isolated the 5' end fragments. Agarose gel analysis of amplification of 5'-RACE end with specific primers(Table 1) gave bands at 540 bp and 480 bp.According to the overlap between the 5'-fragments and EST sequences, two full-length sequences were spliced using the software DNAStar. A 999 bp and an 897 bp full-length 14-3-3 cDNA were inferred,respectively. As anticipated, a 999 bp and an 897 bp of specif i c DNA bands were amplified by PCR using full length primers Ta14-F1 and Ta14-R1, Ta14-F2 and Ta14-R2, respectively. Sequences of these amplicons coincided completely with the deduced full-length cDNA sequence.
2.2 Characteristics of wheat 14-3-3s
The analysis of cloned genes at NCBI(http://www.ncbi.nlm.nih.org) and ExPaSy(http://www.expasy.org) indicated that the two cDNA clones encode putative 14-3-3 proteins. Both the cDNAs,designated asTa14R1andTa14R2(GenBank Accession No. KC121319 and KC121320), contain a coding region,a 5'-UTR and a 3'-UTR.Ta14R1cDNA contains an open reading frame (ORF) of 789 bp encoding an acidic protein of 262 amino acids (29.747 kDa, pI 4.62).Ta14R2cDNA contains an ORF of 786 bp encoding an acidic protein of 261 amino acids (pI 4.75). The calculated molecular weight of this protein is 29.263 kDa.
2.3 Multi-alignment comparison of 14-3-3 proteins
Four wheat 14-3-3 proteins were obtained from GenBank database (GenBank Accession No.AY386126, AB042193, AB042194, JN650603, Table 2). The sequences are all full-length judging from ORF analysis (http://www.ncbiikol.nlm.nih.gov/gorf/gorf.html). Ta14R1 and Ta14R2 proteins share high homology with these wheat 14-3-3 proteins and the identities of Ta14R1 and Ta14R2 ranged from 73%–91% and 75%–97%, respectively.
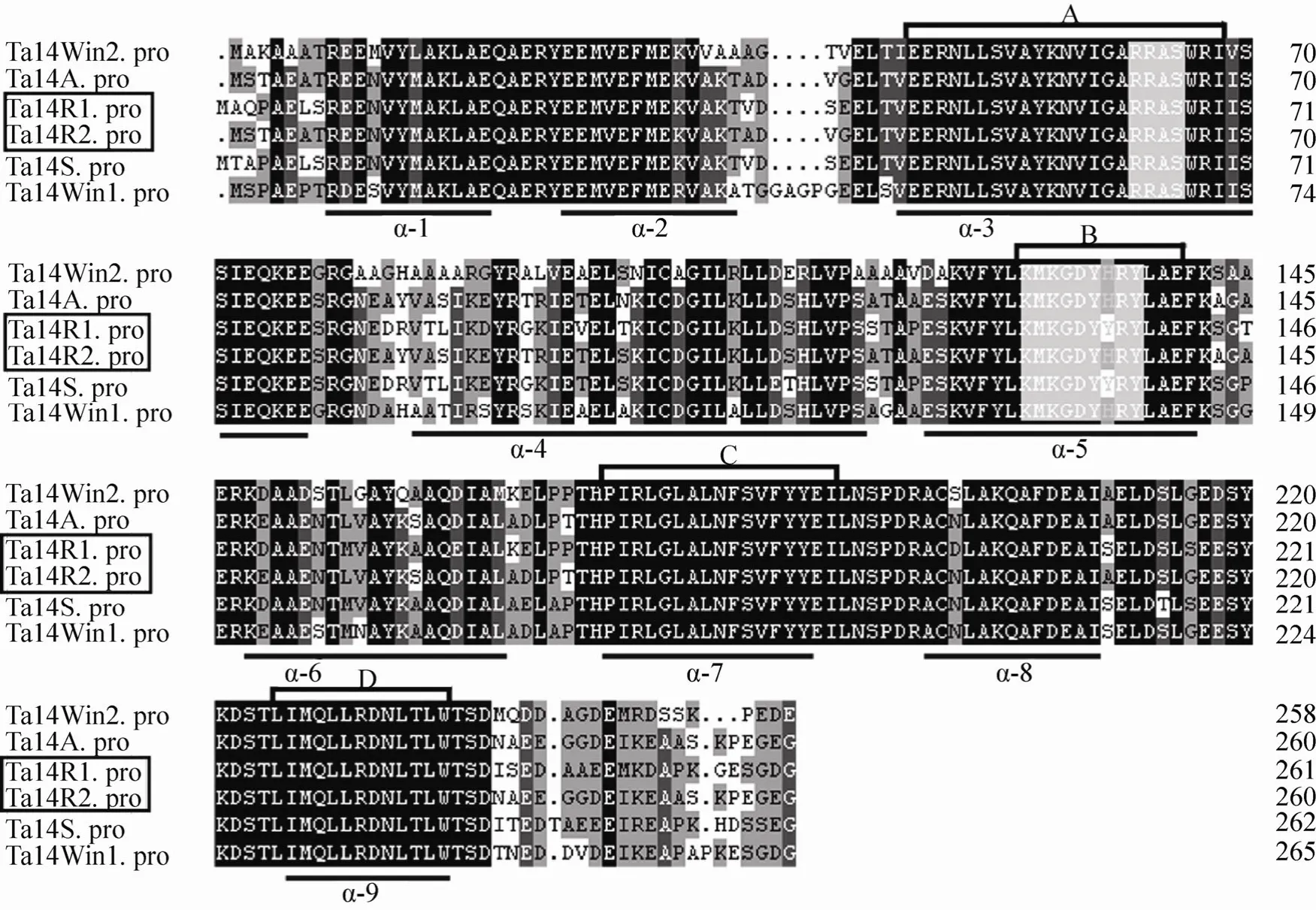
Fig. 1 Sequence alignment among the six 14-3-3 proteins from wheat. Multiple sequence alignment was performed with the DNAMAN program. The amino acid residues identical among the sequences are indicated in black, while similar residues are shown in grey. Small black spots indicate gaps in the sequences to allow for maximal alignment, and the regions of the conversed antiparallel α-helices (α-1-9) are underlined. Two white regions represent two phosphorylation motifs conserved among the sequences. Four highly conserved domains (A, B, C and D) represent potential pseudosubstrate for protein kinase C, annexin-like domain, domain to bind transcription factor and nuclear export signal, respectively.
By comparing the amino acid sequences (Fig. 1)of Ta14R1 and Ta14R2 with 14-3-3 proteins above,we found that the amino acid sequences of these proteins are highly conserved except for the C-terminal and N-terminal regions. All the proteins contain nine antiparallel alpha-helices and two highly conserved phosphorylation-dependent binding motifs, which were found to lie within helices 3 and 5. The amino acids in the helices 3, 7 and 9 are highly conserved, and in helices 2, 4, 6 and 8 are less conserved. In addition, as found in known plant 14-3-3 proteins[27-28], the dimeric structure of 14-3-3 also contain many potential protein-binding domains, such as protein kinase C binding,annexin-like, transcription factor binding and nuclear export signal domains (Fig. 1). These functional domains are located within the helices 3,5, 7 and 9, respectively. These conserved structures may be related to the regulation pathway of 14-3-3 proteins.
2.4 Phylogenetic relationship of 14-3-3 proteins
To investigate the divergence of wheat 14-3-3 proteins with other known 14-3-3 proteins from monocotyledon andArabidopsisduring evolution,an unrooted phylogenetic relationship of 33 14-3-3 proteins (Table 2) were generated by Mega 4.0 program by the neighbor-joining method. As shown in Fig. 2, the tree was clearly divided into two distinct branches (epsilon and non-epsilon). The non-epsilon group contained most of the proteins,while the epsilon group only had four proteins(GF14epsilon,Iota,muandOmicron). The non-epsilon group can be further divided into two subgroups. Among them, it can be observed that each subgroup contained both monocotyledons and dicotyledons. It appears that Ta14R1 and Ta14R2 belong to non-epsilon group and closely resemble Hv14B and Hv14C, respectively.
In addition, other three 14-3-3 proteins of barley (Hv14A, Hv14D and Hv14E) form the same small branch together with three reported 14-3-3 proteins of wheat (Ta14S, Ta14Win2 and Ta14Win1),respectively, indicating that wheat and barley may have a close phylogenetic relationship.

Table 2 List of 14-3-3 sequence used in the present work

Fig. 2 A phylogenetic analysis of 14-3-3 proteins from monocot plants and Arabidopsis thaliana. The tree was constructed with the Mega 4.0 software by using the neighbor-joining method and branch lengths are shown in each branch. The sequences used in this analysis were shown in Table 2.
2.5 Subcellular localization of Ta14R1/R2
The putative subcellular localization of the two 14-3-3 proteins in wheat were predicted online with software TargertP (http://www.cbs.dtu.dk/services/TargetP/) and PredSL (http://hannibal.biol.uoa.gr/PredSL/). Prediction results showed that two 14-3-3 proteins were less likely localized in the chloroplasts,mitochondria and other organelles involved in secretory pathway such as endoplasmic reticulum (Table 3). To ensure the subcellular localization ofTa14R1andTa14R2,Ta14R1-GFPandTa14R2-GFPconstructs with the GFP fused at the C terminal of the two genes were used for transient expression inArabidopsisprotoplast.As evident in Fig. 3, the GFP signals elicited by the protoplasts of the two genes were all evidently distributed in the cytoplasm and membranes. Also, we did not observe any overlapping of between the green GFP fl uorescence from theTa14s-GFPand the red chlorophyll signal, which confirms the two genes are not expressed in the chloroplast.
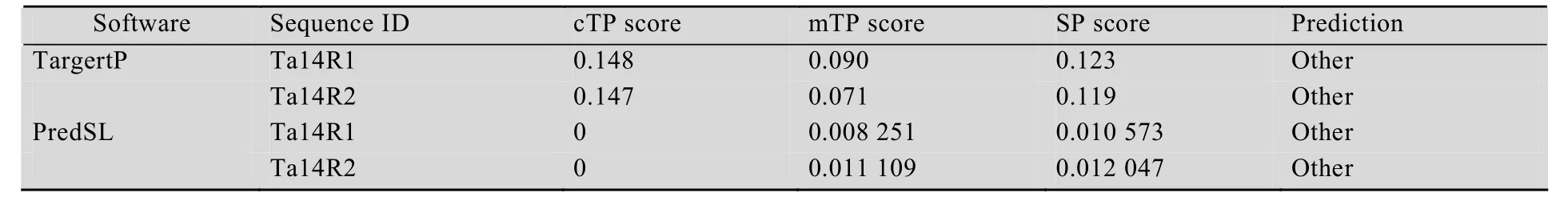
Table 3 Predictions for subcellular localization of Ta14R1/Ta14R2 generated by PredSL software(http://hannibal.biol.uoa.gr/PredSL/) and TargeTP (http://www.cbs.dtu.dk/services/TargetP/)
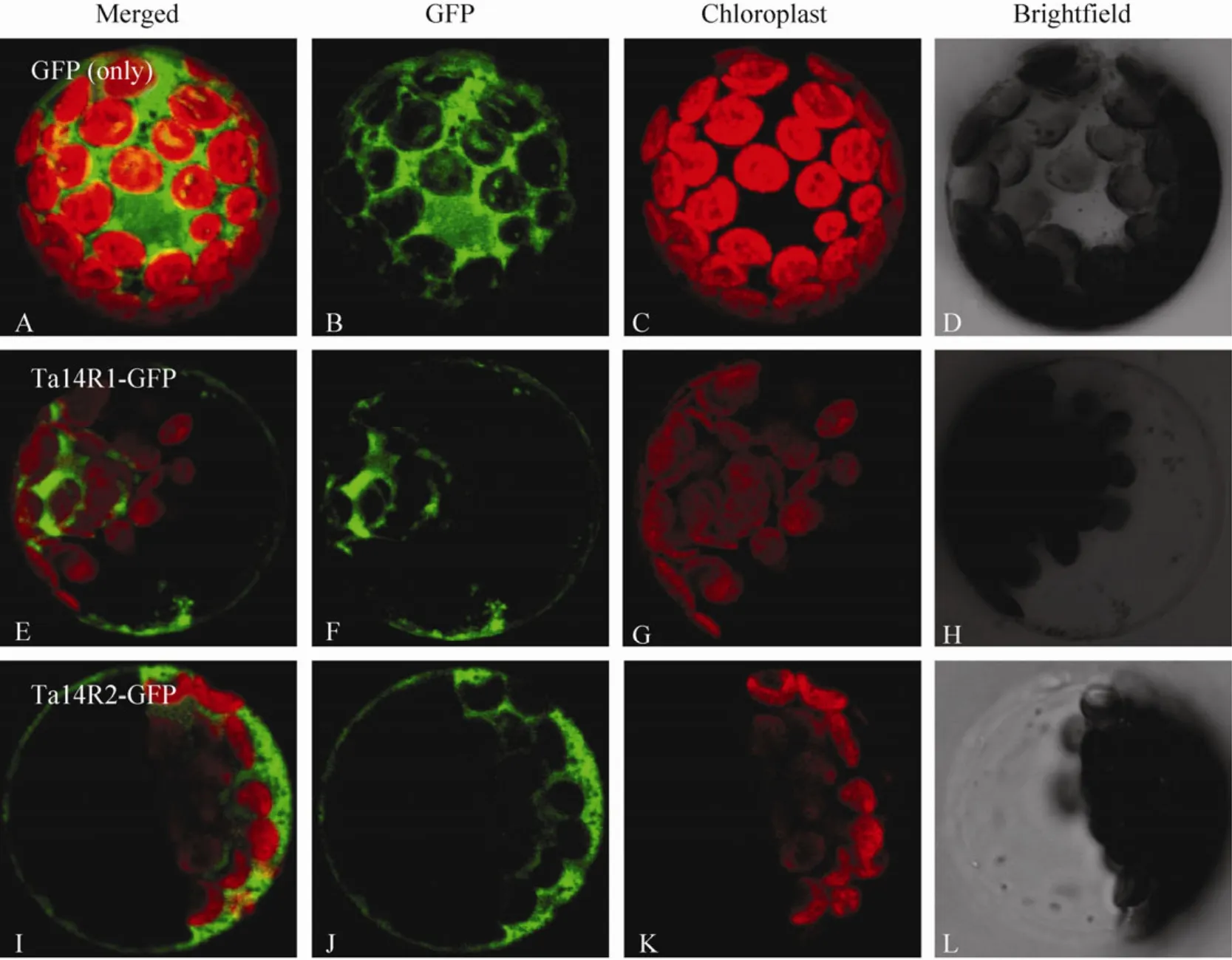
Fig. 3 Subcellular localization of Ta14R1 and Ta14R2. The fusion construct of Ta14R1 and Ta14R2 with pJIT163-hGFP and pJIT163-hGFP alone were introduced into Arabidopsis protoplasts. (A–D) GFP alone. (E–L) Transient expression of GFP fused to the Ta14R1 and R2 in Arabidopsis protoplast. (B, F and J) GFP fluorescence. (C, G and K) Chlorophyll fluorescence. (D, H and L)Bright field images of protoplasts. (A, E and I) Merged images of B and C, F and G, as well as (J) and (K).
2.6 Spatial expression patterns of Ta14R1/2 genes in different tissues
To determine the temporal and spatial expression patterns of the isolatedTa14R1/Ta14R2genes, total RNA were extracted from mature seed embryos,primary roots and shoots of one day after germination,roots and fully expanded leaves of 10 days after germination, leaves in tillering stage, stems in jointing stage, flag leaves, young panicle spikes in the heading stage and developing seeds at 10 and 25 days after pollination. The expression patterns of twoTa14-3-3cDNA clones were analyzed by real-time quantitative RT-PCR using gene-specific primers as described in Table 1. The relative transcript levels ofTa14R1andTa14R2genes are shown in Fig. 4 using the wheat actin gene as a standard control to normalize differences in RNA template concentrations. The results indicated that bothTa14R1andTa14R2were expressed in all the tested tissues.Ta14R1andTa14R2were predominantly expressed in primary shoots of one day after germination while relatively lower expressed in mature embryos and seeds in filling stage. TheTa14R1mRNA was detected mainly in primary shoots of one day after germination, but also expressed at relatively high level in roots of one day after germination. The expression levels ofTa14R2was relatively higher than that ofTa14R1in primary shoots of one day after germination, fully expanded leaves of 10 days after germination, tillering-stage leaves, jointing-stage stems and flag leaves, while relatively lower thanTa14R1in roots of one day after germination. These tissue-wide expression patterns of the two genes are probably associated with their different functions.
2.7 Expression patterns of Ta14R1/2 under abiotic stress and exogenous ABA

Fig. 4 Expression analysis by real time RT-PCR of the Ta14R1 and Ta14R2 at different developmental stages. 1: mature seed embryos; 2: roots of one day after germination; 3: shoots of one day after germination; 4: roots of 10 days after germination; 5:fully expanded leaves of 10 days after germination; 6: leaves in tillering stage; 7: stems in jointing stage; 8: flag leaves in heading stage; 9: immature ears in heading stage; 10: seeds at 10 DAP; 11: seeds at 25 DAP.

Fig. 5 Expression patterns of Ta14R1 and Ta14R2 in leaves and roots monitored by real time RT-PCR under ABA, drought, heat and cold stress. The relative transcript levels were calculated by the formula R=2-ΔΔCt (ΔΔCt=(CtTa14 treatment–Ct Actin)–(Ct Ta14 CK–Ct Actin)). *P<0.05, **P<0.01.
Responses of plant to abiotic stress are composed of multiple signaling pathways[29], and various signaling pathways have cross-talks[30-32]. In order to further determine whether theTa14R1andTa14R2are responsive to abiotic stress, the expression of these 14-3-3 genes in wheat in roots and leaves under abiotic stress, including ABA,drought, heat and cold treatments were analyzed by real-time quantitative PCR. The two 14-3-3 genes in wheat were found to be expressed differently under ABA and abiotic stress treatments, as well as between roots and leaves (Fig. 5). In roots,Ta14R1was strongly responsive to the various abiotic stresses. It was significantly up-regulated in general and its expression level was increased by up to 11, 6,3.5 and 2.2-fold at the 0.5 h intervals after ABA,drought, heat and cold stress treatments were applied compared to that of control (Fig. 5A, C, E and G).TheTa14R2showed a similar expression profile in all the stress treatments except heat. Under heat-stress,Ta14R2did not respond significantly(Fig. 5E), and the expression level was effectively unchanged across the assay intervals. In leaves,Ta14R1andTa14R2exhibited similar expression patterns in response to all stress treatments. The expressions of bothTa14R1andTa14R2were steadily up-regulated until to the highest level at 6 h and 24 h time points after ABA and cold treatments were applied compared to the control (Fig. 5B and H). TheTa14R1andTa14R2were responded rapidly responsive to application of drought stress and their expression levels increased significantly at 0.5 h and 1 h after application of the treatment, then decreased gradually. Under heat treatment,Ta14R1andTa14R2responded strongly with the transcript levels were higher at all assay times compared to the control(Fig. 5F). These results indicated thatTa14R1andTa14R2might play an important role in response to ABA, as well as heat, cold and drought stress in wheat.
3 Discussion
Wheat is an important food crop in the world as well as in China, where it accounts for 20%–27%food crop and 21% of total food production.However, drought and high temperature frequently occurs in wheat in planting areas, especially in North China, and drought has become a main factor limiting the yield and quality of wheat.
Previous studies have shown that the 14-3-3 proteins may play important roles in plant response to environmental stress[3,7]. Therefore, isolation and functional analysis of 14-3-3 genes are critical steps toward understanding their regulating mechanism.The 14-3-3 is a family of highly homologous proteins encoded by separate genes and many higher plants have at least one 14-3-3 isoforms. Currently, genome sequencing and annotation indicated that 15 and 8 14-3-3 genes exist inArabidopsisand rice,respectively[8,10]. In barley, five 14-3-3 genes have been identified and their spatial expression patterns have been characterized[11]. In this study, two wheat 14-3-3 genesTa14R1andTa14R2were isolated and sequenced. The analysis of the cloned 14-3-3 genes from wheat showed that theTa14R1andTa14R2genes have a full-length of 999 and 897 bp with a complete ORF of 789 and 786 bp and encodes 262 and 261 amino acids, respectively, and the deduced amino acid sequences ofTa14R1andTa14R2contain typical 14-3-3 protein structural features such as nine alfa-spiral, two highly conserved phosphorylationdependent binding motifs and four highly conserved function domains. These results illustrated thatTa14R1andTa14R2are members of the family of 14-3-3 genes in wheat. The phylogenetic analysis of 14-3-3 proteins revealed that Ta14R1 and Ta14R2 clustered together to 14-3-3 proteins from other cereals and the majority of 14-3-3 proteins fromArabidopsis, which belong to the non-epsilon group.These results are in agreement with the previous research[5,15]. As the non-epsilon isoform both exist in monocotyledonous and eudicotyledon species, the gene divergence of epsilon and non-epsilon isoforms may had occurred before the dicopatric speciation of monocotyledon and eudicotyledon plants.
14-3-3 protein is an important regulator of plant growth and development. In a given physiological environment, 14-3-3 protein is involved in the cell regulation through specific spatial and temporal expression[5]. An early research had showed that at least 12 out of the 15 14-3-3 genes inArabidopsiswere expressed and exhibited high tissue and strict developmental specificity[7,15]. Unlike 14-3-3 isoforms inArabidopsis, in this study, we found thatTa14R1andTa14R2display minimal tissue specificity and homogenously expressed in mature seed embryos,primary roots and shoots of one day after germination,roots and fully expanded leaves of 10 days after germination, leaves in tillering stage, stems in jointing stage, flag leaves, young panicles in the heading stage and seeds at 10 and 25 days after pollination of wheat, and with stronger expression in shoots in germination period. A similar result has been observed by other authors, who reported that the other two 14-3-3 isoforms from wheat (TaWin1andTaWin2) were ubiquitously expressed in different tissues and cell types[33]. However, the tissue specific expression of some 14-3-3 isoforms has been observed. Wang et al. (2008) reported that another wheat 14-3-3 geneTa14Awas expressed mostly in stem and leaf tissues without signal detected in root tissues. This indicates that structure divergence and spatial expression of 14-3-3 could be the important factors to determine its functional specificity[34].
Abiotic stress such as drought, high and low temperature are the major environmental conditions that adversely affect plant growth and crop yield.Various genes are involved in abiotic-stress response and their differential expression is suggested to contribute to the abiotic stress[35-36]. Currently,14-3-3 proteins are thought to be involved in a large range of abiotic stress signaling processes and to interact with many target proteins, mainly including plasma membrane H+-ATPase, ascorbate peroxidase(APX3), ion channels and abscisic acid (ABA).These target proteins are very important for plant adapted to adversity-stress[37-39]. Under osmotic stress, an increase in the enzyme activity of the plasma membrane H+-ATPase was accompanied by accumulation of 14-3-3 proteins in plasma membranes in maize roots[40]. When sugar beet cells were exposed to cold condition, an increase in the content of ATPase/14-3-3 complexes and H+-ATPase activity were also observed[41]. Thus, 14-3-3 proteins may also be involved in the regulation of the response of higher plants to abiotic stresses. In this study, we performed a real-time RT-PCR to investigate the expression pattern of the isolated wheat 14-3-3 genes in response to the above three stress conditions. Our results indicated that the two 14-3-3 genes in wheat were differentially expressed under drought and cold stress and the expression levels of the two 14-3-3 genes in wheat were significantly enhanced compared to the controls,indicating that the wheat 14-3-3 isoforms may be involved in the response to drought, as well as low temperature stress and playing similar roles to the known 14-3-3 proteins in other plants. Meanwhile,we also found that the two 14-3-3 genes in wheat were differentially expressed after high-temperature stress in wheat roots, while onlyTa14R1was responsive to heat stress. In addition, their expression profiles in roots were quite different from that in leaves. This indicated that different regulation pathways might exist in these two tissues, and the more sophisticated expression patterns in leaves than that in roots indicate that 14-3-3 genes in wheat might take part in more than one regulatory pathway in abiotic-stress response in leaf tissues. It is well known that many responses to biotic and abiotic stresses in plants are mediated by plant hormones.For example, ABA is an essential mediator for drought, cold and heat shock[38]. Our results showed that two wheat 14-3-3 genes were found to be up regulated by ABA treatment, which is consistent with a previous study uncovering the functional role of 14-3-3 proteins in drought tolerance ofVicia[42].We also found that the two 14-3-3 genes in wheat were differentially expressed after ABA treatment in roots and leaves, indicating these wheat 14-3-3 genes are regulated by ABA signal and might participate in different signaling pathways in the two tissues. Above data should prove useful in further study of the role of 14-3-3 genes in plant signal regulation.
In conclusion, two 14-3-3 genes were identified in wheat, and their expression patterns revealed in response to ABA, drought, heat, and cold stress. It will be a challenge to understand the actual roles and the intricate net of the interactions of 14-3-3 proteins in wheat and other plants. Therefore, further validating potential clients and identifying functions of the target proteins would provide clues and information for various 14-3-3-mediated life activities. Perhaps future studies should focus more on the role of 14-3-3 proteins phosphorylation and the environmental stimuli that impact in these processes.
[1]Moore BW, Perez VJ. Specific Acidic Proteins of the Nervous System. Physiological and Biochemical Aspects of Nervous Integratin. Englewood Cliffs,New Jersey: Prentice-Hall, 1967: 343–359.
[2]Ichimura T, Isobe T, Okuyama T, et al. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci USA, 1988, 85(19): 7084–7088.
[3]Chaudhri M, Scarabel M, Aitken A. Mammalian and yeast 14-3-3 isoforms form distinct patterns of dimersin vivo. Biochem Biophys Res Commun,2003, 300(3): 679–685.
[4]Jones DH, Ley S, Aitken A. Isoforms of 14-3-3 protein can form homo- and heterodimersin vivoandin vitro: implications for function as adapter proteins. FEBS Lett, 1995, 368(1): 55–58.
[5]Testerink C, van der Meulen RM, Oppedijk BJ, et al. Differences in spatial expression between 14-3-3 isoforms in germinating barley embryos. Plant Physiol, 1999, 121(1): 81–87.
[6]Oecking C, Jaspert N. Plant 14-3-3 proteins catch up with their mammalian orthologs. Curr Opin Plant Biol, 2009, 12(6): 760–765.
[7]Fulgosi H, Soll J, de Faria Maraschin S, et al.14-3-3 proteins and plant development. Plant Mol Biol, 2002, 50(6): 1019–1029.
[8]Ichimura T, Isobe T, Okuyama T, et al. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+,calmodulin-dependent protein kinase II. FEBS Lett,1987, 219(1): 79–82.
[9]Toker A, Ellis CA, Sellers LA, et al. Protein kinase C inhibitor proteins. Purification from sheep brain and sequence similarity to lipocortins and 14-3-3 protein. Eur J Biochem, 1990, 191(2): 421–429.
[10]Morgan A, Burgoyne RD. Exo1and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature,1992, 355(6363): 833–836.
[11]Muslin AJ, Tanner JW, Allen PM, et al. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell, 1996, 84(6):889–897.
[12]Chang IF, Curran A, Woolsey R, et al. Proteomic profiling of tandem affinity purified 14-3-3 protein complexes inArabidopsis thaliana. Proteomics,2009, 9(11): 2967–2985.
[13]Schoonheim PJ, Veiga H, Pereira DdaC, et al. A comprehensive analysis of the 14-3-3 interactome in barley leaves using a complementary proteomics and two-hybrid approach. Plant Physiol, 2007,143(2): 670–683.
[14]Denison FC, Paul AL, Zupanska AK, et al. 14-3-3 proteins in plant physiology. Semin Cell Dev Biol,2011, 22(7): 720–727.
[15]Sehnke PC, Rosenquist M, Alsterfjord M, et al.Evolution and isoform specificity of plant 14-3-3 proteins. Plant Mol Biol, 2002, 50(6): 1011–1018.
[16]Zhang ZT, Zhou Y, Li Y, et al. Interactome analysis of the six cotton 14-3-3s that are preferentially expressed in fibres and involved in cell elongation. J Exp Bot, 2010, 61(12):3331–3344.
[17]Yao Y, Du Y, Jiang L, et al. Molecular analysis and expression patterns of the 14-3-3 gene family fromOryza sativa. J Biochem Mol Biol, 2007, 40(3):349–357.
[18]Schoonheim PJ, Sinnige MP, Casaretto JA, et al.14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination.Plant J, 2007, 49(2): 289–301.
[19]Konagaya K, Matsushita Y, Kasahara M, et al.Members of 14-3-3 protein isoforms interacting with the resistance gene product N and the elicitor ofTobacco mosaic virus. J Gen Plant Pathol, 2004,70(4): 221–231.
[20]Ferl RJ, Manak MS, Reyes MF. The 14-3-3s.Genome Biol, 2002, 3(7): reviews 3010–reviews 3010.7.
[21]Tester M, Bacic A. Abiotic stress tolerance in grasses. From model plants to crop plants. Plant Physiol, 2005, 137(3): 791–793.
[22]Roberts MR, Salinas J, Collinge DB. 14-3-3 proteins and the response to abiotic and biotic stress. Plant Mol Biol, 2002, 50(6): 1031–1039.
[23]Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res, 1989, 17(6): 2362.
[24]Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCtmethod. Methods, 2001, 25(4):402–408.
[25]Sheen J. Methods for mesophyll and bundle sheath cell separation. Methods Cell Biol, 1995, 49:305–314.
[26]Levitan A, Trebitsh T, Kiss V, et al. Dual targeting of the protein disulfide isomerase RB60 to the chloroplast and the endoplasmic reticulum.Proc Natl Acad Sci USA, 2005, 102(17):6225–6230.
[27]Pan S, Sehnke PC, Ferl RJ, et al. Specific interactions with TBP and TFIIBin vitrosuggest that 14-3-3 proteins may participate in the regulation of transcription when part of a DNA binding complex. Plant Cell, 1999, 11(8):1591–1602.
[28]Rosenquist M, Sehnke P, Ferl RJ, et al. Evolution of the 14-3-3 protein family: does the large number of isoforms in multicellular organisms ref l ect functional specificity? J Mol Evol, 2000, 51(5):446–458.
[29]Singh KB, Foley RC, Luis OS. Transcription factors in plant defense and stress responses. Curr Opin Plant Biol, 2002, 5(5): 430–436.
[30]Shao HB, Chu LY, Shao MA. Calcium as a versatile plant signal transducer under soil water stress. BioEssays, 2008, 30(7): 634–641.
[31]Shao HB, Chu LY, Shao MA, et al. Advances in functional regulation mechanisms of plant aquaporins: their diversity, gene expression,localization, structure and roles in plant soil-water relations. Mol Membr Biol, 2008, 25(3): 179–191.
[32]Shao HB, Chu LY, Jaleel CA, et al. Water-deficit stress-induced anatomical changes in higher plants.C R Biol, 2008, 331(3): 215–225.
[33]Ikeda Y, Koizume N, Kusano T, et al. Specific binding of a 14-3-3 protein to autophosphorylated WPK4, an SNF1-related wheat protein kinase, and to WPK4-phosphorylated nitrate reductase. J Biol Chem, 2000, 275(41): 31695–31700.
[34]Wang C, Ma QH, Lin ZB, et al. Cloning and characterization of a cDNA encoding 14-3-3 protein with leaf and stem-specific expression from wheat. DNA Seq, 2008, 19(2): 130–136.
[35]Seki M, Kamei A, Yamaguchi-Shinozaki K, et al.Molecular response to drought, salinity and frost:common and different paths for plant protection.Curr Opin Biotech, 2003, 14(2): 194–199.
[36]Shinozaki K, Yamaguchi-Shinozaki K, Seki M.Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol, 2003, 6(5): 410–417.
[37]Yan J, He C, Wang J, et al. Overexpression of theArabidopsis14-3-3 protein GF14 lambda in cotton leads to a “stay-green” phenotype and improves stress tolerance under moderate drought conditions.Plant Cell Physiol, 2004, 45(8): 1007–1014.
[38]Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol,1994, 45: 113–141.
[39]van den Wijngaard PWJ, Sinnige MP, Roobeek I, et al. Abscisic acid and 14-3-3 proteins control K+channel activity in barley embryonic root. Plant J,2005, 41(1): 43–55.
[40]Shanko AV, Mesenko MM, Klychnikov OI, et al.Proton pumping in growing part of maize root: its correlation with 14-3-3 protein content and changes in response to osmotic stress. Biochemistry (Mosc),2003, 68(12): l320–1326.
[41]Chelysheva VV, Smolenskaya IN, Trofimova MC,et al. Role of the 14-3-3 proteins in the regulation of H+-ATPase activity in the plasma membrane of suspension-cultured sugar beet cells under cold stress. FEBS Lett, 1999, 456(1): 22–26.
[42]Takahashi Y, Kinoshita T, Shimazaki KI. Protein phosphorylation and binding of a 14-3-3 protein inViciaguard cells in response to ABA. Plant Cell Physiol, 2007, 48(8): 1182–1191.

