Thermomineral water promotes axonal sprouting but does not reduce glial scar formation in a mouse model of spinal cord injury
Dubravka Aleksić, Milan Aksić, Nevena Divac, Vidosava Radonjić, Branislav Filipović, Igor Jakovčevski
1 Institute of Anatomy “Niko Miljanić”, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
2 Institute of Pharmacology, Clinical Pharmacology and Toxicology, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
3 Center for Molecular Neurobiology Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Thermomineral water promotes axonal sprouting but does not reduce glial scar formation in a mouse model of spinal cord injury
Dubravka Aleksić1, Milan Aksić1, Nevena Divac2, Vidosava Radonjić1, Branislav Filipović1, Igor Jakovčevski3
1 Institute of Anatomy “Niko Miljanić”, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
2 Institute of Pharmacology, Clinical Pharmacology and Toxicology, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
3 Center for Molecular Neurobiology Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Thermomineral water from the Atomic Spa Gornja Trepča has been used for a century in the treatment of neurologic disease. The thermomineral water contains microelements, including lithium and magnesium, which show neural regeneration-promoting effects after central nervous system injury. In this study, we investigated the effects of oral intake of thermomineral water from the Atomic Spa Gornja Trepča on nerve regeneration in a 3-month-old mouse model of spinal cord injury. The mice receiving oral intake of thermomineral water showed better locomotor recovery than those without administration of thermomineral water at 8 and 12 weeks after lower thoracic spinal cord compression. At 12 weeks after injury, sprouting of catecholaminergic axons was better in mice that drank thermomineral water than in those without administration of thermomineral water, but there was no difference in glial reaction to injury between mice with and without administration of thermomineral water. These fi ndings suggest that thermomineral water can promote the nerve regeneration but cannot reduce glial scar formation in a mouse model of spinal cord injury.
astrocyte scar; catecholaminergic innervations; lithium; magnesium; thermomineral water; locomotor recovery; microglia; neuroprotection; magnesium; spinal cord injury; nerve regeneration
Funding: Aleksić D, Aksić M and Divac N were supported by the Southeast Europe Cooperation, Hamburg, Germany.
Aleksić D, Aksić M, Divac N, Radonjić V, Filipović B, Jakovčevski I. Thermomineral water promotes axonal sprouting but does not reduce glial scar formation in a mouse model of spinal cord injury. Neural Regen Res. 2014;9(24):2174-2181.
Introduction
For a long time period, severe spinal cord injury with paralysis has been considered an irreversible condition, because neurons in the central nervous system (CNS), unlike peripheral nerves, do not regrow their once injured axons (Steuer and Guertin, 2009). Additionally, glial scar which forms after spinal cord injury by the reactive astrocytes to prevent further in fl ammation and neurodegeneration (Bush et al., 1999; Faulkner et al., 2004) is an active barrier which hinders axonal regeneration by containing numerous growth-inhibiting molecules (Silver and Miller, 2004). The range of available treatments for spinal cord injury is very limited, and the provision of medical care for individuals with injury burdens society with high expenses. Present research is focused on advanced interventions that provide hope for regeneration and functional restoration, as well as chronic treatments which could promote partial functional rehabilitation. Therefore, more research efforts focusing on combinatorial approach are needed to improve the clinical outcome after injury (Xu et al., 2011; Lee et al., 2012).
Because thermomineral water from the Atomic Spa Gornja Trepča has been used for a century in patients with autoimmune disorders such as multiple sclerosis and rheumatoid arthritis, as well as after acute CNS trauma, we hypothesized that it might have regeneration-promoting effects after spinal cord injury. The thermomineral water of the Atomic Spa is volcanic water, mixed with infiltration water on its way through the ground. The hot curative waters of Gornja Trepča had been known to local population since the ancient times, while it enjoyed the reputation of a monastery spa during the Middle Ages. These waters contain Mg and Ca as main cations, and hidrocarbonate and Cl as main anions. The water also contains microelements, including Li, Cs, Se, known for their neuroprotective properties (Bräuer and Savaskan, 2004; Su et al., 2007; Chiu and Chuang, 2011; Godoi et al., 2013). The temperature of the water is 27–31°C and pH is 7.4–7.5, so the water is considered to be neutral with alkaline tendency. It has low radioactivity with the emission of 29.6 Bq/L. The water is low mineralized because of the low concentration of anions like HCO3–(385 mg/mL) and cations like Mg2+(61 mg/mL), and is free from H2S andFe. The dominating microelement in the water is Li with the concentration of 0.0032 mg/mL. Recent studies have reported that Li is bene fi cial in animal models of brain injury (Yu et al., 2012), stroke (Chiu and Chuang, 2010, 2011), Alzheimer’s disease (Chuang, 2004), amyotrophic lateral sclerosis (Dill et al., 2008), spinal cord injury (Su et al., 2007), and that it stimulates neurogenesis both in vitro and in vivo (Hashimoto et al., 2003). Lithium also protects neurons from glutamate-induced excitotoxicity and so prevents apoptosis (Chalecka-Franaszek et al., 1999; Young, 2009). Additionally, magnesium, a dominant macroelement in the water, has already been used in clinical trials in patients with spinal cord injury as an adjuvant treatment (Kwon et al., 2010).
The aim of this study was to investigate the effects of the oral intake of the thermomineral water from the Atomic Spa Gornja Trepča on the injured spinal cord of the C57BL/6J wild-type mice. We studied how oral intake of this mineral water influences the outcome of spinal cord injury using motor behavior testing and morphometric approach.
Materials and Methods
Animals
Twenty-four female 3-month-old C57BL/6J mice, weighing 21.4 ± 1.2 g, were obtained from the breeding colony at the animal facility of the Institute for Biological Research “Siniša Stanković”, Belgrade. All experiments were conducted in accordance with the European Community laws on protection of experimental animals, and the procedures used were approved by the responsible committee of the Serbian Ministry of Agriculture. All animal treatments and data acquisition were performed by observers blinded to treatment groups.
Surgical procedures
All surgical procedures were performed with special attention to the sterile technique inside of the laminar flow cabinet. For surgery, the mice were anesthetized by intraperitoneal injections of ketamine (100 mg/kg body weight; Ketanest®, Parke-Davis/Pfizer, Karlsruhe, Germany) and xylazine (5 mg/kg body weight; Rompun®, Bayer, Leverkusen, Germany). Laminectomy was performed at the T7–9level, corresponding approximately to spinal cord segments T10–12, with mouse laminectomy forceps (Fine Science Tools, Heidelberg, Germany), dorsal and lateral vertebral laminae were carefully removed. A mouse spinal cord compression device was used to elicit compression injury at the level of spinal cord segments T10–11(Curtis et al., 1993). The device is composed of a pair of watchmaker forceps mounted in a metal block attached to a stereotaxic frame. Compression force (degree of closure of the forceps) and duration are controlled by an electromagnetic device. The spinal cord was maximally compressed (100%) according to the operational de fi nition of Curtis et al. (1993) for 1 second by a time-controlled current flow (12 V – maximum voltage) through a custom-made electromagnetic device. The skin was then surgically closed using 6-0 nylon stitches (Ethicon, Norderstedt, Germany). After surgery, mice were kept in a heated room (35°C) for several hours to prevent hypothermia and thereafter singly housed in a temperature-controlled (22°C) room with water and standard food provided ad libitum. During the postoperative time period, the bladders of the animals were manually voided twice daily. Immediately after surgery, the mice were randomly divided into two groups: an experimental group mice in which were given thermomineral water (Atomic Spa, Gornja Trepča, Serbia) and a control group mice in which were allowed to drink standard water ad libitum. The volume of water drank by each mouse was approximately 5 mL daily, in both experimental and control groups, therefore showing that mice had no preference for, or repulsion from thermomineral water. During the immediate post-operative phase and in the fi rst week after injury, four mice from each group died due to severity of injury and post-surgical complications, leaving eight mice per group for the analysis.
Analysis of motor function
The recovery of ground locomotion was evaluated using the Basso Mouse Scale (BMS) score (Basso et al., 2006), which is a modification of Basso, Beattie, Bresnahan (BBB) locomotor rating scale, originally developed for rats (Basso et al., 1995). This method includes evaluation of mice with spinal cord injury observed in the open fi eld for 4 minutes pre-operatively and at least weekly for 84 days post-operatively. The scale estimates movements of the ankle joint, ability of plantar stepping and walking coordination, by giving score values 0–9 (Basso et al., 2006). Maximal value of the score 9 is given for normal walking with frequent or consistent plantar stepping, normal trunk stability and tail always up, whereas a minimal score of 0 is given for the complete absence of ankle movement (Basso et al., 2006). This approach allows identifying all visually discernable attributes of locomotor recovery for both mildly and severely injured mice. Assessment of all parameters was performed before and at 3 days, and 1, 2, 4, 8 and 12 weeks after the injury. Values for the left and right extremities were averaged.
Tissue fi xation and sectioning
At 12 weeks after spinal cord injury, mice were anesthetized with 16% solution of sodium pentobarbital (Narcoren, Merial, Hallbergmoos, Germany, 5 µL/g body weight). The animals were transcardially perfused with fi xative consisting of 4% formaldehyde and 0.1% CaCl2in 0.1 mol/L cacodylate buffer, pH 7.3, for 15 minutes at room temperature. Following perfusion, the spinal cords were left in situ for 2 hours at room temperature, after which they were dissected out and post- fi xed overnight (18–22 hours) at 4°C in the same solution used for perfusion. Tissue was then immersed into 15% sucrose solution in 0.1 mol/L cacodylate buffer, pH 7.3, for 2 days at 4°C, embedded in Tissue Tek (Sakura Finetek, Zoeterwoude, NL, USA), and frozen by 2-minute immersion into 2-methyl-butane (isopentane) precooled to –80°C. Serial transverse or longitudinal sections were cut using a cryostat (Leica CM3050, Leica Instruments, Nußloch, Germany). Sections, 25-µm-thick, were collected on SuperFrost Plus glass slides (Roth, Karlsruhe, Germany). Sampling ofsections was always done in a standard sequence so that six sections 250 µm apart were present on each slide.
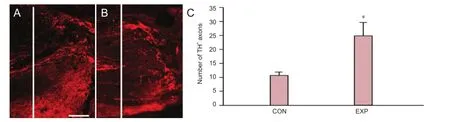
Figure 2 Sprouting of tyrosine hydroxylase (TH)-expressing axons caudal to the lesion site 12 weeks after injury.

Figure 3 NeuN+neurons rostral and caudal to the lesion site at 12 weeks after spinal cord injury.

Figure 1 Time course of functional recovery after compressive spinal cord injury in mice from the experimental (EXP) and control (CON) groups.
Immunohistochemistry
Procedures for immunohistochemistry have been described previously (Jakovcevski et al., 2007). Water-bath antigen de-masking was performed in 0.01 mol/L sodium citrate solution, pH 9.0, for 30 minutes at 80°C for all antigens. Non-specific binding was blocked using 5% normal goat serum dissolved in PBS, pH 7.3 and supplemented with 0.2% Triton X-100, 0.02% sodium azide for 1 hour at room temperature. Incubation with the primary antibody, diluted in PBS containing 0.5% lambda-carrageenan (Sigma/Aldrich) and 0.02% sodium azide, was carried out for 3 days at 4°C. The following primary antibodies were used: for detection of cathecholaminergic axons, rabbit anti-tyrosine hydroxylase (Chemicon, Temecula, CA, USA; 1:800), for detection of neurons, mouse monoclonal anti-neuron-speci fi c nuclear antigen (NeuN; Chemicon; 1:1,000), for detection of astrocytes and glial scar, mouse anti-glial fi brillary acidic protein (GFAP; Sigma-Aldrich, St Louis, MO, USA; 1:1,000), and rabbit anti-ionized calcium binding adaptor-1, for detection of microglia/macrophages (Iba-1; Sigma/Aldrich; 1:1,500). After washing in PBS (3 × 15 minutes at room temperature), goat anti-mouse or anti-rabbit Cy-3 conjugated secondary antibody (Dianova, Hamburg, Germany) diluted 1:200 in PBS-carrageenan solution was applied for 2 hours at room temperature. After a subsequent wash in PBS, cell nuclei were stained for 10 minutes at room temperature withbis-benzimide solution (Hoechst 33258 dye; Sigma/Aldrich; 5 µg/mL in PBS). Finally, the sections were washed again, mounted in anti-fading medium (Fluoromount G; Southern Biotechnology Associates, Biozol, Eching, Germany) and stored in the dark at 4°C.

Figure 4 Glial fi brillary acidic protein (GFAP) expression around the lesion site at 12 weeks after spinal cord injury.
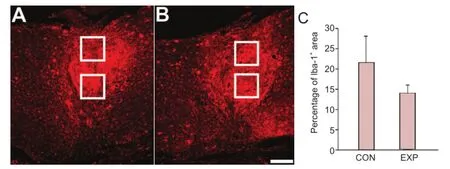
Figure 5 Iba-1+microglia within the lesion site at 12 weeks after spinal cord injury.
Quanti fi cations of axons at the lesion site
Parasagittal spinal cord sections stained for tyrosine hydroxylase (TH) were used to analyze the numbers of catecholaminergic axons in the thoracic spinal cord caudal to the lesion site at 12 weeks after spinal cord injury. All TH+axons crossing an arbitrarily selected border 250 µm caudally to the lesion site were counted in every 5thparasagittal serial section from the spinal cord of each mouse on an Axiophot microscope (Zeiss, Oberkochen, Germany) equipped with a motorized stage and Neurolucida software-controlled computer system (MicroBrightField, Magdeburg, Germany). This method for estimation of axonal sprouting beyond the lesion site was previously shown to correlate well with locomotion recovery score (Apostolova et al., 2006; Jakovcevski et al., 2007), and gives a reasonable estimate of overall axonal sprouting in the lumbar spinal cord (Pan et al., 2014).
Stereological analysis of neuronal density
Cell counts were performed on an Axioscope microscope (Carl Zeiss Meditec, Inc., Dublin, CA, USA) equipped with a motorized stage and Neurolucida software-controlled computer system (MicroBrightField) using the optical disector method as described (Jakovcevski et al., 2009; Mehanna et al., 2010; Wu et al., 2012). We counted NeuN-immunoreactive cells 1.5 mm rostral and 1.5 mm caudal to the edges of the lesion site, in the spinal cord segment comprising a rectangle of 0.5 mm in length. Therefore, we did not measure neuronal death in immediate vicinity to lesion site caused by injury, but rather the amount of remote neurodegeneration at 3 months after injury. Longitudinal spinal cord sections from the 0.5-mmlong segment starting at the beginning of the lumbar enlargement, as well as the segment of the same length of the thoracic spinal cord were used for counting. The sections were observed under low-power magnification (10× objective) with a 365/420 nm excitation/emission filter set (blue fluorescence). The nuclear staining was used to delineate spinal cord area. The numerical density of NeuN-immunoreactive neurons was estimated by counting nuclei of immunolabeled cells within systematically randomly spaced optical disectors. The parameters for this analysis were guard space depth 2 µm, base and height of the dissector 900 µm2and 10 µm, respectively, distance between the optical dissectors 30 µm, using the objective Plan-Neo fl uar × 40/0.75. Left and right spinal cord areas were evaluated in six sections 250 μm apart each. All results shown are averaged bilateral values. The counts wereperformed by one observer in a blinded fashion.
Estimation of the glial scar volume and the expression of GFAP and Iba-1 in the glial scar
Spaced serial 25-µm-thick longitudinal spinal cord sections 250 µm apart were immunostained for GFAP and used for estimations of the scar volume using the Cavalieri’s principle. This method for estimation of the volume of irregularly shaped objects as a sum of regularly sampled areas multiplied by the distance between them was adopted by Howard and Reed (1998) and has been previously used to assess brain and spinal cord volumes (e.g., Apostolova et al., 2006; Djogo et al., 2013). Areas of the scar required for volume estimation were measured directly under the microscope using the Neurolucida software (MicroBrightField). To investigate expression of GFAP and Iba-1 rostral and caudal to the lesion site, we estimated the area of the immunopositive structures normalized to the total image area using ImageJ software. This estimation was performed on images obtained on an LSM 510 confocal microscope (Carl Zeiss) using a 40× oil-immersion objective and a digital resolution of 1,024 × 1,024 pixels. The confocal images were converted to grey scale to consistently adjust the threshold based on the histogram shape of the optimal color intensity. Images of the GFAP or Iba-1 immunostained area rostral and caudal to the lesion site taken from each of six longitudinal sections were analyzed per animal, and mean values from individual animals were used to calculate group mean values.
Photographic documentation
Photographic documentation was made on an Axiophot 2 microscope equipped with a digital camera AxioCam HRC and Axio Vision Software (Zeiss) at the resolution of 1,024 × 1,024 pixel (RGB) and with a Leica confocal laser scanning microscope (Leica). The images were processed using Adobe Photoshop CS5.1 software (Adobe System Inc., San Jose, CA, USA) and manipulations were limited to crop and brightness/contrast functions.
Statistical analysis
Statistical analysis was performed using Sigma Plot 12.5 software (SPSS, IBM, Armonk, NY, USA). Data are shown as the mean ± SEM, and were tested by the two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test or twotailed t-test, as appropriate. For all comparisons, the degree of freedom was determined by the number of animals. The accepted level of signi fi cance was 5%.
Results
Mice that drank thermomineral water show improved locomotor recovery after spinal cord injury
Spinal cord compression caused severe disabilities in both groups of mice as assessed by BMS scores which were close to 0 at 3 days after injury (Figure 1). Between 1 and 12 weeks, the walking abilities improved in both groups, but the improvement was significantly better in the group of mice that drank thermomineral water than in control mice, as revealed by analyses of BMS scores at 4, 8 and 12 weeks after injury (P < 0.05;Figure 1). This represents the difference between the slight ankle movements seen in the majority of mice in the control group, while in the experimental group, the majority of animals displayed extensive ankle movement. Recovery of locomotor function was analyzed by two-way analysis of variance with treatment and time as factors. Data show that the main effect of the treatment was statistically signi fi cant (P = 0.002), i.e., mice that drank thermomineral water recovered significantly better when compared with control animals. Time after injury had also signi fi cant in fl uence on recovery (analysis of variance; P = 0.047), indicating that in both groups, locomotor function improved over time, whereas analysis showed no signi fi cant interaction effect between the treatment and time factors (P = 0.30). Subsequent Tukey’s post-hoc tests showed statistical signi fi cance for differences between treatments at 4 weeks (P = 0.036), 8 weeks (P = 0.018) and 12 weeks (P = 0.011). Overall, our results indicate a better locomotor recovery after spinal cord injury in mice that drank thermomineral water compared with controls.
Enhanced axonal regrowth/sprouting of catecholaminergic axons at the site of injury in the spinal cord of mice treated with thermomineral water
To assess monoaminergic reinnervation of the spinal cord caudal to the injury site, previously shown to correlate well with motor recovery (Jakovcevski et al., 2007, 2013; Devanathan et al., 2010), we counted numbers of catecholaminergic (dopaminergic and noradrenergic, TH+) axons crossing an arbitrarily selected border 250 µm caudal to the lesion site in spaced serial parasagittal sections at 12 weeks after the injury (Figure 2A, B). Numbers of TH+fi bers at 12 weeks were more than double in mice that drank thermomineral water compared with control mice (P < 0.05;Figure 2C).
Number of NeuN+neurons in the spinal cord, rostral and caudal to the site of injury
To investigate whether the intake of thermomineral water could protect neurons from dying after injury, we performed stereological counts of neurons in the thoracic and lumbar spinal cord at 12 weeks after injury. Neurons were counted in both the ventral and dorsal horn of the spinal cord gray matter (Figure3A, B). There was no significant difference in the density of neurons (number of cells per volume) between the two treatment groups, either rostral or caudal to the injury site (Figure3C). Thus, thermomineral water intake did not in fl uence the number of surviving neurons after spinal cord injury.
Glial scar volume and GFAP expression at the site of injury
To investigate whether thermomineral water intake leads to alterations in the formation of glial scar, we estimated GFAP+astrocyte scar volume at 12 weeks after injury. This is deemed important, because functional recovery after spinal cord injury strongly depends on the lesion severity, usually assessed by size of the astroglial scar (Jakovcevski et al., 2007;Wu et al., 2012). The volume of the GFAP+astroglial scar was similar between the experimental (0.92 ± 0.17 mm3) and control mice (1.1 ± 0.23 mm3). We also sampled areas rostral and caudal to the lesion center and measured the size of the area immunostained for GFAP (Figure 4A, B). Congruent with measurements of the scar volume, there was no signi fi cant difference between both groups (Figure 4C). Thus, GFAP+astroglial scar was not in fl uenced by the thermomineral water intake.
Iba-1+microglia/macrophages at the site of injury
It is well recognized that immune response after injury infl uences the regenerative response of the CNS (Ankeny and Popovich, 2009; Wu et al., 2012). Microglia/macrophages were immunofluorescently labeled with Iba-1 antibody, which labels both activated and quiescent cells (Streit et al., 1999), and the size of immunostained area was measured at the lesion site (Figure 5A, B). There was a tendency towards lower Iba-1 expression in animals that drank thermomineral water, but it did not reach statistical signi fi cance (Figure 5C).
Discussion
Injury to the spinal cord causes massive tissue destruction and total neuronal loss at the site of injury, coupled with progressive neurodegeneration of the structures caudal to the site of injury (Rossignol et al., 2007; Blesch and Tuszynski, 2009; Filli and Schwab, 2012). Because no therapies are available to cure spinal cord injury, any approach that would bene fi t locomotor function and improve rehabilitation after injury presents a considerable advance. The objective of this study was to examine if the balance of minerals present in the Atomic Spa water improves locomotor recovery in an experimental animal model, thus eliminating possible placebo effects that could be present in patients. Additionally, we aimed to show that not only single elements in high therapeutic doses, but also mixtures of such elements in much lower concentrations are capable of producing beneficial effects, avoiding possible adverse side effects.
The improvement of locomotor recovery after injury was modest in mice of both experimental and control groups, indicating high severity of injury. However, a large difference in BMS scores between the mice that drank thermomineral water and control mice at 2 and 3 months after injury indicates that the effects of thermomineral water can improve locomotion even upon severe spinal cord injury. The results of histological analysis of the injured spinal cords at 3 months upon injury indicate that thermomineral water improved axonal sprouting, but did not alter neuronal survival or glial response to injury. Notably, monoaminergic axons were sprouting markedly more in mice treated with thermomineral water than in controls. These axons, originating in brainstem nuclei, were previously shown to be important for recovery of motor function after spinal cord injury (Jakovcevski et al., 2007, 2013; Lieberoth et al., 2009; Chen et al., 2010; Devanathan et al., 2010; Pan et al., 2014). This increase in fi ber numbers could be due to regrowth of axons across the lesion scar or sprouting of spared axons caudal to the lesion site, indicating a more vigorous regenerative response in the injured spinal cord of mice treated with thermomineral water. The number of NeuN+neurons, however, was not affected by the thermomineral water intake, either rostral or caudal from the site of injury. The loss of neurons, and in particular motoneurons, due to deafferentiation is well documented in several animal models of spinal cord injury (Young, 1966; Eidelberg et al., 1989; Dong Teng et al., 1999), but it is less clear whether the protection from neuronal loss would impact motor recovery (Dong Teng et al., 1999; Mehanna et al., 2010). Previous studies that have reported neuroprotective effects always coupled increase in neuronal numbers with increased axonal sprouting, with the latter being more likely involved in improved restoration of motor function (Cui et al., 2011; Xu et al., 2011).
Reactive astrocytes are prominent feature of the cellular response to spinal cord injury (Faulkner et al., 2004). Scar tissue formed in part by reactive astrocytes has long been implicated a major impediment to axon regeneration (Ramon y Cajal, 1928; Clemente and Windle, 1954; Reier et al., 1983; Liuzzi and Lasek, 1987; Rudge and Silver, 1990). Numerous studies report that inhibition of astrocyte reaction to injury has beneficial effect on recovery (Jakovcevski et al., 2007; Hellal et al., 2011; Cregg et al., 2014). Our current study, however, did not show an effect of thermomineral water on astrocyte scaring. Another component of glial response to spinal cord injury is microglia/macrophages proliferation/invasion of injured tissue (Streit and Kreutzberg, 1988; Streit et al., 1999). Previous studies on the infl uence of microglia/macrophages on recovery after injury have been inconclusive, revealing both pro-inflammatory and neuroprotective roles of microglia. While some view microglia/macrophages as integral components of the regenerative response, others emphasize their contribution to delayed neuronal apoptosis and demyelination in secondary response during subchronic phase after injury (Ankeny and Popovich, 2010; David and Kroner, 2011; Streit, 2002; Wu et al., 2012). Activated microglia/macrophages secrete extracellular matrix components, such as keratin, dermatan and chondroitin sulfate proteoglycans which have an overall negative impact on axonal regeneration (Grimpe and Silver, 2002; Jones and Tuszynski, 2002; Akyüz et al., 2013). Our finding of non-significantly lower microglia/macrophage in fi ltration of injury site in mice treated with thermomineral water could be due to lower rate of elimination of damaged fi bers compared with controls, thus not being a direct effect of thermomineral water intake on microglia/macrophages.
Lithium has been used for more than 60 years to treat bipolar disorders due to its complex activity in modulation of the neurotransmitter systems at multiple levels of signaling in the brain (Manji and Lenox, 1998; Jope, 1999; Geddes and Miklowitz, 2013). Neuroprotective effects of lithium have been reported in the 1990s (Jope, 1999), but only recently the possible mechanisms of these effects have been described (Chuang, 2004; Chiu and Chuang, 2010). Chronic treatment with lithium reduces microglia and macrophage activation (Su et al., 2007), and also increases neurogenesis, both incultured brain neurons and in vivo, and prevents inhibition of proliferation induced by glutamate or glucocorticoids (Hashimoto et al., 2003; Chiu and Chuang, 2010). Longterm treatment with lithium effectively protects primary cultures of neurons from glutamate-induced toxicity mediated by N-methyl-D-aspartate (NMDA) receptors. Lithium prevents the activation of Cdk5 which regulates signaling mediated by NMDA receptors and whose sustained activity is believed to be involved in the pathogenesis of many neurodegenerative diseases (Cruz and Tsai, 2004; Chiu and Chuang, 2010). Additionally, lithium induces the expression of the brain-derived neurotrophic factor and its receptor and stimulates neurogenesis in primary cultures of neurons (Hashimoto et al., 2002).
Many other ions which are present in the thermomineral water from the Atomic spa have neuroprotective/neurotrophic properties. The neuroprotective effects of magnesium in fetal CNS development have been well established and the use of magnesium sulfate in the prevention of brain injury in preterm infants is supported by relevant, evidence-based clinical guidelines (Salmeen et al., 2014). However, despite plenty of evidence for neuroprotective bene fi ts of magnesium in animal models of spinal cord injury, its clinical use in human medicine has been restricted by the fact that doses needed for neuroprotection (300–600 mg/kg) far exceed human tolerability for magnesium (Kwon et al., 2009), thus a necessity for developing special formulations, such as combination with polyethylene glycol. This formulation allows much lower doses of magnesium to be clinically effective (Kwon et al., 2009). However, the concentration of magnesium in the Atomic spa water is relatively low (61 mg/mL), so the neuroprotective properties of this water observed in patients cannot be attributed solely to its magnesium content.
Another important consideration regarding the thermomineral water is that in patients with spinal cord injury, it has been extensively used during the chronic phase, i.e., during rehabilitation after injury. Our results in a rodent model demonstrate that, taking individual health status of patients into consideration, it could also contribute to the restoration of innervation in the acute and sub-chronic phases after injury.
Finally, we would like to emphasize that none of the macro- or microelements contained within the thermomineral water tested here has previously been reported to be therapeutically effective in such low doses. Thus, it is our hypothesis that the optimal ratio between the ions contained within this natural spring water, rather than single ions, has benefi cial effect. Although further studies are needed to con fi rm this hypothesis, our study provides evidence that the thermomineral water from the Atomic Spa Gornja Trepča promotes recovery after spinal cord injury in a mouse model.
Author contributions:Aleksić D, Aksić M and Jakovčevski I designed the study, performed the experiments, analyzed the data and drafted the manuscript. Divac N, Radonjić V and Filipović B helped with the data analysis and manuscript writing. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Akyüz N, Rost S, Mehanna A, Bian S, Loers G, Oezen I, Mishra B, Hoffmann K, Guseva D, Laczynska E, Irintchev A, Jakovcevski I, Schachner M (2013) Dermatan 4-O-sulfotransferase1 ablation accelerates peripheral nerve regeneration. Exp Neurol 247:517-530.
Ankeny DP, Popovich PG (2009) Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 158:1112-1121.
Ankeny DP, Popovich PG (2010) B cells and autoantibodies: complex roles in CNS injury. Trends Immunol 31:332-338.
Apostolova I, Irintchev A, Schachner M (2006) Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci 26:7849-7859.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open fi eld testing in rats. J Neurotrauma 12:1-21.
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in fi ve common mouse strains. J Neurotrauma 23:635-659.
Blesch A, Tuszynski MH (2009) Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci 32:41-47.
Bräuer AU, Savaskan NE (2004) Molecular actions of selenium in the brain: neuroprotective mechanisms of an essential trace element. Rev Neurosci 15:19-32.
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration and neurite outgrowth after ablation of scarforming, reactive astrocytes in adult transgenic mice. Neuron 23:297-308.
Chalecka-Franaszek E, Chuang DM (1999) Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A 96:8745-8750.
Chen J, Joon Lee H, Jakovcevski I, Shah R, Bhagat N, Loers G, Liu HY, Meiners S, Taschenberger G, Kügler S, Irintchev A, Schachner M (2010) The extracellular matrix glycoprotein tenascin-C is bene fi cial for spinal cord regeneration. Mol Ther 18:1769-1777.
Chiu CT, Chuang DM (2010) Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther 128:281-304.
Chiu CT, Chuang DM (2011) Neuroprotective action of lithium in disorders of the central nervous system. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36:461-476.
Chuang DM (2004) Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol 16:83-90.
Clemente CD, WindleWF (1954) Regeneration of severed nerve fi bers in spinal cord of the adult cat. J Comp Neurol 101:691-731.
Cregg JM, DePaul MA, Filous AR, Lang BT, TranA, Silver J (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197-207.
Cruz JC, Tsai LH (2004) A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol 14:390-394.
Cui YF, Xu JC, Hargus G, Jakovcevski I, Schachner M, Bernreuther C (2011) Embryonic stem cell-derived L1 overexpressing neural aggregates enhance recovery after spinal cord injury in mice. PLoS One 6:e17126.
Curtis R, Green D, Lindsay RM, Wilkin GP (1993) Up-regulation of GAP-43 and growth of axons in rat spinal cord after compression injury. J Neurocytol 22:39-50.
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388-399.
Devanathan V, Jakovcevski I, Santuccione A, Li S, Lee HJ, Peles E, Leshchyns’ka I, Sytnyk V, Schachner M (2010) Cellular form of prion protein inhibits reelin-mediated shedding of Caspr from the neuronal cell surface to potentiate Caspr-mediated inhibition of neurite outgrowth. J Neurosci 30:9292-9305.
Dill J, Wang H, Zhou F, Li S (2008) Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci 28:8914-8928.
Djogo N, Jakovcevski I, Müller C, Lee HJ, Xu JC, Jakovcevski M, Kügler S, Loers G, Schachner M (2013) Adhesion molecule L1 binds to amyloid beta and reduces Alzheimer’s disease pathology in mice. Neurobiol Dis 56:104-115.
Dong Teng Y, Mocchetti I, Taveira-DaSilva A, Gillis R, Wrathall J (1999) Basic fi broblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after spinal cord injury. J Neurosci 19:7037-7047.
Eidelberg E, Nguyen LH, Polich R, Walden JG (1989) Transsynaptic degeneration of motoneurones caudal to spinal cord lesions. Brain Res Bull 22:39-45.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143-2155.
Filli L, Schwab ME (2012) The rocky road to translation in spinal cord repair. Ann Neurol 72:491-501.
Geddes JR, Miklowitz DJ (2013) Treatment of bipolar disorder. Lancet 381:1672-1682.
Godoi GL, de Oliveira Porciúncula L, Schulz JF, Kaufmann FN, da Rocha JB, de Souza DO, Ghisleni G, de Almeida HL Jr (2013) Selenium compounds prevent amyloid β-peptide neurotoxicity in rat primary hippocampal neurons. Neurochem Res 38:2359-2363.
Grimpe B, Silver J (2002) The extracellular matrix in axon regeneration. Prog Brain Res 137:333-349.
Hashimoto R, Takaei N,Shimazu K, Christ L, Lu B, Chuang DM (2002) Lithium induced brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: An essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology 43:1173-1179.
Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM (2003) Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience 117:55-61.
Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F (2011) Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331:928-931.
Howard CV, Reed MG (2005) Unbiased Stereology (second edition). Garland Science/BIOS Scienti fi c Publishers.
Jakovcevski I, Wu J, Karl N, Leshchyns’ka I, Sytnyk V, Chen J, Irintchev A, Schachner M (2007) Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J Neurosci 27:7222-7233.
Jakovcevski I, Siering J, Hargus G, Karl N, Hoelters L, Djogo N, Yin S, Zecevic N, Schachner M, Irintchev A (2009) Close homologue of adhesion molecule L1 promotes survival of Purkinje and granule cells and granule cell migration during murine cerebellar development. J Comp Neurol 513:496-510.
Jones LL, Tuszynski MH (2002) Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci 22:4611-4624.
Jope RS (1999) Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry 4:117-128.
Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, Kindy MS (2009) Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: preclinical re fi nement and optimization. J Neurotrauma 26:1379-1393.
Kwon BK, Sekhon LH, Fehlings MG (2010) Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine (Phila Pa 1976) 35:S263-270.
Lee JH, Roy J, Sohn HM, Cheong M, Liu J, Stammers AT, Tetzlaff W, Kwon BK (2010) Magnesium in a polyethylene glycol formulation provides neuroprotection after unilateral cervical spinal cord injury. Spine (Phila Pa 1976) 35:2041-2048.
Lieberoth A, Splittstoesser F, Katagihallimath N, Jakovcevski I, Loers G, Ranscht B, Karagogeos D, Schachner M, Kleene R (2009) Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J Neurosci 29:6677-6690.
Liuzzi FJ, Lasek RJ (1987) Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 237:642-645.
Manji HK, Lenox RK (1998) Lithium: a molecular transducer of mood-stabilization in the treatment of bipolar disorder. Neuropsychopharmacology 19:161-166.
Mehanna A, Jakovcevski I, Acar A, Xiao M, Loers G, Rougon G, Irintchev A, Schachner M (2010) Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol Ther 18:34-43.
Pan HC, Shen YQ, Loers G, Jakovcevski I, Schachner M (2014) Tegaserod, a small compound mimetic of polysialic acid promotes functional recovery after spinal cord injury in mice. Neuroscience 277C:356-366.
Ramon y Cajal S (1928) Degeneration and regeneration of the nervous system. Oxford University Press: London, New York.
Reier PJ, Perlow MJ, Guth L (1983) Development of embryonic spinal cord transplants in the rat. Brain Res 312:201-219.
Rossignol S, Barrière G, Frigon A, Barthélemy D, Bouyer L, Provencher J, Leblond H, Bernard G (2007) Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Rev 57:228-240.
Rudge JS, Silver J (1990) Inhibition of neurite growth on astroglial scars in vitro. J Neurosci 10:3594-3603.
Salmeen KE, Jelin AC, Thiet MP (2014) Perinatal neuroprotection. F1000Prime Rep 6:6.
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146-156.
Steuer I, Guertin PA (2009) Spinal cord injury research in mice: 2008 review. Scienti fi cWorldJournal 9:490-498.
Streit WJ (2002) Microglia and the response to brain injury. Ernst Schering Res Found Workshop 39:11-24.
Streit WJ, Kreutzberg GW (1988) The response of endogenous glial cells to motorneuron degeneration induced by toxin ricin. J Comp Neurol 268:248-263.
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57:563-581.
Su H, Chu TH, Wu W (2007) Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp Neurol 206:296-307.
Wu B, Matic D, Djogo N, Szpotowicz E, Schachner M, Jakovcevski I (2012) Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp Neurol 237:274-285.
Xu JC, Bernreuther C, Cui YF, Jakovcevski I, Hargus G, Xiao MF, Schachner M (2011) Transplanted L1 expressing radial glia and astrocytes enhance recovery after spinal cord injury. J Neurotrauma 28:1921-1937.
Young IJ (1966) Morphological and histochemical studies of partially and totally deafferented spinal cord segments. Exp Neurol 14:238-248.
Young W (2009) Review of lithium effects on brain and blood. Cell Transplant 18:951-975.
Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM (2012) Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma 29:362-374.
Copyedited by Gransee H, Lewandowski G, Li CH, Song LP, Zhao M
10.4103/1673-5374.147950
Igor Jakovčevski, Center for Molecular Neurobiology Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, Hamburg D-20246, Germany, Igor.Jakovcevski@dzne.de.
http://www.nrronline.org/
Accepted: 2014-10-10
- 中国神经再生研究(英文版)的其它文章
- Hydrogen sul fi de controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases
- Activities of nicotinic acetylcholine receptors modulate neurotransmission and synaptic architecture
- A novel arti fi cial nerve graft for repairing longdistance sciatic nerve defects: a self-assembling peptide nano fi ber scaffold-containing poly(lactic-co-glycolic acid) conduit
- The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury
- Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses
- Long-term treatment with PP2 after spinal cord injury resulted in functional locomotor recovery and increased spared tissue

