Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses
Qi Yang, Min Luo, Peng Li, Hai Jin
1 China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Mechanics, School of Mechanical Science and Engineering, Jilin University, Changchun, Jilin Province, China
Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses
Qi Yang1, Min Luo1, Peng Li2, Hai Jin1
1 China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Mechanics, School of Mechanical Science and Engineering, Jilin University, Changchun, Jilin Province, China
A brachial plexus injury model was established in rabbits by stretching the C6nerve root. Immediately after the stretching, a suspension of human amniotic epithelial cells was injected into the injured brachial plexus. The results of tensile mechanical testing of the brachial plexus showed that the tensile elastic limit strain, elastic limit stress, maximum stress, and maximum strain of the injured brachial plexuses were signi fi cantly increased at 24 weeks after the injection. The treatment clearly improved the pathological morphology of the injured brachial plexus nerve, as seen by hematoxylin eosin staining, and the functions of the rabbit forepaw were restored. These data indicate that the injection of human amniotic epithelial cells contributed to the repair of brachial plexus injury, and that this technique may transform into current clinical treatment strategies.
nerve regeneration; peripheral nerve injury; brachial plexus injury; animal model; human amniotic epithelial cells; forepaw function; morphology; tensile mechanics; neural regeneration
Funding: This study was financially supported by a grant from the Science and Technology Development Project of Jilin Province of China, No. 20110492.
Yang Q, Luo M, Li P, Jin H. Transplantation of human amniotic epithelial cells repairs brachial plexus injury: pathological and biomechanical analyses. Neural Regen Res. 2014;9(24):2159-2163.
Introduction
Brachial plexus injury is a dif fi cult-to-treat peripheral nerve injury that often leads to severe dysfunction of the upper extremity, causing permanent disability in some patients (Terzis and Papakonstantinou, 2000). The quality of life achieved in patients with brachial plexus injury after conventional treatment is not satisfactory (Kitajima et al., 2006; Bailey et al., 2009; Bertelli and Ghizoni, 2010; Giuffre et al., 2010; Sibinski et al., 2010; Vekris et al., 2010; Bhandari and Deb, 2011; Flores, 2011; Goubier et al., 2011; Ngeow et al., 2011; Zhao et al., 2011; Zhou and Kong, 2011; Terzis and Barmpitsioti, 2012; Zhou et al., 2013). Therefore, the development of more effective treatment programs is urgently needed. Transplantation of stem cells, such as neural stem cells and embryonic stem cells, has been of wide interest for the treatment of central and peripheral nerve injuries, but the application of stem cells is limited by source scarcity, ethical concerns, potential immune rejection, and security (Yang et al., 2007; Fan et al., 2010; Liu et al., 2011b; Xu et al., 2011; Han, 2012). Recently, more attention has been focused on human amniotic cells (Miki et al., 2007; Hou et al., 2008) as a cell source for transplantation. Several previous studies have shown that amniotic epithelial cells can differentiate into mature nerve cells, secrete neurotransmitters such as dopamine, acetylcholine, and norepinephrine (Miki et al., 2007; Fu et al., 2010; Niknejad et al., 2010), and have similar biological properties as embryonic stem cells (Chen et al., 2011). Thus, we hypothesized that transplantation of human amniotic epithelial cells (hAECs) can restore the tensile mechanical properties of injured brachial plexus in an animal model of brachial plexus injury. The aim of the present study was to test this hypothesis.
Materials and Methods
Animals
Fifth-one healthy 5-month-old male Japanese white rabbits, weighing 2.5–2.8 kg, were provided by the Changchun Hightech Medical Experimental Animal Center (Changchun, Jilin Province, China; license No. SCXK (Ji) 2003-0004). The animals were housed in a temperature-controlled (24–25°C), air-circulated room with a relative humidity of 55–70% under natural lighting. The animals were allowed free access to water and food. All the protocols involving animals were approved by the Animal Ethics Committee of China-Japan Union Hospital of Jilin University (Changchun, Jilin Province, China). These rabbits were randomly and equally divided into control, brachial plexus injury model, and hAECs intervention groups, with 17 rabbits in each group.
Establishing the brachial plexus injury model
The brachial plexus injury model was produced in the rabbits following the methods described by Zhang et al. (2013). All rabbits were anesthetized with 6% chloral hydrate via an intraperitoneal injection (6 mL/kg). The rabbits in the model and hAECs intervention groups were fi xed in a pronatedposition on the operating table, and the skin at the surgical area was disinfected prior to the surgery. First, a left side laminectomy was performed at the C5–8level, exposing the dural sac. The brachial plexus was exposed to the nerve root along the humeral callus. The C6nerve root on the left side was stretched with a force of 0.9 N for 1 minute using a KL-0.25-type spring dynamometer (Beijing Tianchuang Shangbang Instrument and Equipment Co., Ltd., Beijing, China). The nerve and incision were then sutured closed. The success of the brachial plexus injury model was determined using the grooming test evaluation criteria (Zheng and Xiao, 2011). For the control group, the animals were fed normally and received no surgical model or drug intervention.

Table 1 Effect of human amniotic epithelial cells (hAECs) transplantation on the rabbit forepaw motor functions after brachial plexus injury

Table 2 Brachial plexus tensile testing results at 24 weeks after human amniotic epithelial cell transplantation
Transplantation of hAECs
Following the methods described by Zhang et al. (2013), a suspension of hAECs (Shanghai Bioleaf Biotech Co., Ltd., Shanghai, China) was slowly injected into the rabbits using a micro-syringe immediately after creating the brachial plexus injury. The two injection sites were within 4.0 mm of the cephal side and caudal side of the C6brachial plexus to a depth of 1.25 mm. Each injection included 3 µL (75,000 cells) of suspension, and the needle was retained for 5 minutes to prevent cell re fl ux.
Motor function assessment
After creating the brachial plexus injury model, the animals were recovered from the anesthesia. At that time (2 hours after surgery) and at 2, 6, 12, 18, and 24 weeks after the surgery, their motor functions were evaluated and graded according to the grooming test evaluation criteria (Bertelli and Mira, 1993; Zheng and Xiao, 2011). Brie fl y, the scoring system was: forepaws paralyzed (score 0); forepaws could touch the mouth (score 1); forepaws could touch the space between the mouth and the eyes (score 2); forepaws could touch the eyes (score 3); forepaws could touch the preauricular tissue (score 4); and forepaws could touch the postauricular tissue (score 5).
Tissue specimens

Figure 2 Effect of human amniotic epithelial cells transplantation on the pathological morphology of the injured brachial plexus in rabbits (hematoxylin-eosin staining, × 400).
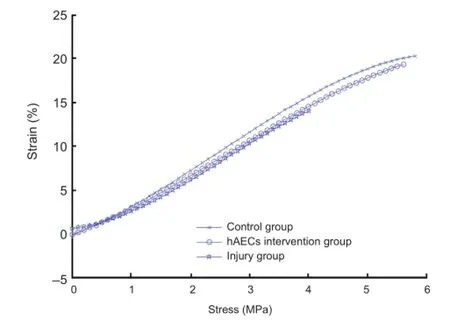
Figure 1 The stress-strain curves of the rabbit brachial plexus specimens in each group.
At 24 weeks after brachial plexus injury, the rabbits in each group were anesthetized with 6% chloral hydrate via an intraperitoneal injection (6 mL/kg) and exsanguinated from the abdominal aorta. A 30-mm specimen was harvested from the injured brachial plexus (C6) in each rabbit (n = 17 per group), which were fixed in saline. Among the 17 specimens in each group, 15 were randomly chosen for mechanical tensile testing, and the remaining two were used to observe the tissue morphology. The specimens were cut using an S-5 sterile plastic-handled scalpel (HuaiAn Uniecom Medical Supplies Co., Ltd., Xuyi County, Jiangsu Province, China). The specimen length and diameter were 25 mm and 0.98–1.06 mm using a CGH-3-type reading microscope (Changchun Third Optical Instruments Co., Ltd., Changchun, Jilin Province, China).
Brachial plexus tensile testing
Brachial plexus tensile testing was performed using a MODEL55100-type automatic electronic universal testing machine (Changchun Research Institute for Mechanical Sciences Co., Ltd., Changchun, Jilin Province, China). Each specimen was preconditioned as previously described (Liu et al., 2011a, 2012; Ding et al., 2012; Peng et al., 2012; Li et al., 2013a, b; Xu et al., 2013). The testing was conducted at 36.5 ± 1°C. The specimens were secured into the clamping chuck of the testing machine and were loaded under tension at 5 mm/min. During testing, the specimens were soaked with saline to maintain their humidity. After the experiment was fi nished, the maximum load, maximum stress, maximum strain, elastic limit strain, elastic limit load, elastic limit stress, and the stress-strain curve were automatically saved to a computer.
Morphology of brachial plexus
At 24 weeks after creating the brachial plexus injury, tissue specimens were harvested from the injured brachial plexus of all animals, fi xed with 4% paraformaldehyde for 5 minutes, and prepared as frozen sections. The sections were stained with hematoxylin for 2–5 minutes, separated with hydrochloric acid and ethanol, developed in bluing reagent, and stained with eosin for 20 seconds to 3 minutes. Then, they were dehydrated through a gradient of ethanol, cleared in xylene, and mounted. The sections were rinsed with tap water between every two steps. Finally, the specimens were observed with a BX51 optical microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± SD and were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Differences between the two groups were compared with one-way analysis of variance and Scheffe’s tests. P-values less than 0.05 level were considered to indicate signi fi cant differences. The stress-strain function expression was determined using linear regression.
Results
hAECs transplantation improved rabbit forepaw motor function after brachial plexus injury
The grooming test scores showed that the rabbits lost forepaw motor functions after brachial plexus injury. Compared with the injury group, the motor functions were slightly recovered at 2 weeks in the hAECs intervention group and were clearly improved at 24 weeks (Table 1).
hAECs transplantation improved the tensile mechanical properties of injured brachial plexus in rabbits
The tensile mechanical test results showed that, compared with the control group, the maximum load, maximum stress, maximum strain, elastic limit load, elastic limit strain, and elastic limit stress of the rabbit brachial plexus were signi ficantly decreased in the injury group at 24 weeks, comparedwith the uninjured controls (P < 0.05). Intervention with hAECs signi fi cantly increased the maximum load, maximum stress, maximum strain, elastic limit load, elastic limit strain, and elastic limit stress of the rabbit brachial plexus specimens (P < 0.05;Table 2).
The stress-strain curves of the rabbit brachial plexus specimens from each group were plotted after the tensile testing data were fi t to a general equation (Figure 1). In the control group, the stress-strain curves of the brachial plexus specimens were exponential from 0 to 7.62% strain, approximately linear from 7.63% to 15.80% strain, and experienced plastic deformation from 15.81% to 22.69% strain, losing load-bearing capacity and indicating tissue destruction. In the injury group, the stress-strain curves of the brachial plexus specimens were exponential from 0 to 4.21% strain, approximately linear from 4.22% to 10.13% strain, and experienced plastic deformation from 10.14% to 14.64% strain, losing load-bearing capacity and indicating tissue destruction. In the hAECs intervention group, the stress-strain curves were exponential from 0 to 7.53% strain, approximately linear from 7.54% to 14.54% strain, and experienced plastic deformation from 14.55% to 21.34% strain, losing load-bearing capacity and indicating tissue destruction.
The stress-strain function expressions of the brachial plexus specimens in each group were established using unary linear regression.
Control group: σ(ε) = 0.0988e5+ 0.7307e4+ 2.6155e3–0.1431e2
Injury group: σ(ε) = 0.1739e5+ 1,344e4+ 0.7573e3+ 0.6122e2
hAECs intervention group: σ(ε) = 0.0745e5+ 0.5970e4+ 2.4617e3– 0.0907e2
hAECs transplantation improved the pathological morphology of injured brachial plexus in rabbits
Hematoxylin-eosin staining showed that the rabbit brachial plexus nerve fi bers in the control group were neatly arranged, the myelin sheath was covered with the endoneurium formed by connective tissue at the surface of the nerve fibers, the axons were wrapped by a myelin sheath, and the axons and other components were clearly visible. At 24 weeks after injury, the rabbit brachial plexus endoneurium, myelin sheath, axons, and other components in the injury group were altered, and the connective tissue, axons, and myelin sheath at the surface of the nerve fi bers had begun to fracture, leading to obstruction of the basement membrane cavity. At 24 weeks after transplantation of the cells in the hAECs intervention group, the majority of the connective tissue formed an endoneurium at the surface of the brachial plexus nerve, the endoneurium encased the myelin sheath, most of the axons were distributed around the myelin sheath, and the axons and other components were clearly visible (Figure 2).
Discussion
The constitutive mechanical properties of peripheral nerves are defined by the specific nerve morphology, including a tortuous distribution of nerve stems at the tissue bed, tortuous pathway that the nerve bundles traverse at the outer membrane, and a tortuous arrangement of nerve fibers within the bundles (Li et al., 1991). This morphology makes the actual length of the nerve stem and nerve fi bers between any two fi xed points of the limbs longer than the length of a straight line between those two points. When a nerve is gradually stretched, the nerve entanglements and bundles disappear. However, nerve fibers themselves also relax, absorbing and neutralizing the injurious forces and protecting the nerve fi bers. When the nerves and bundles are stretched after being fully straightened, the tensile forces cause nerve fiber extension and even fracture. In addition, perineurial endothelium avulsion injury is an indicator of the loss of anti-tension strength and elasticity (Shen et al., 2010).
Okawa et al. (2001) reported that hAECs express nestin, a marker of neural stem cells, and amniotic epithelial cells can differentiate into neural stem cells in vitro (Guo and Xu, 2013). In addition, amniotic epithelial cells can also differentiate into other neural cells (Chen and Liu, 2011; Chen et al., 2012). Both amniotic epithelial cells and amniotic mesenchymal cells have previously been used as seed cells for cell transplantation and tissue engineering reconstruction of the cornea, lung, liver, cardiac muscle, nerve, and skin, as well as in applications in Parkinson’s disease, in animal experiments (Liu et al., 2001; Sankar and Muthusamy, 2003; Fliniaux et al., 2004; Sakuragawa et al., 2004; Zhao et al., 2005; Choong et al., 2007; Tamagawa et al., 2007; Marcus et al., 2008; Miki et al., 2009; Yang, 2009; Wan et al., 2011; Xia et al., 2011). These studies provide evidence for the use of amniotic epithelial cell transplantation to treat nerve injury. Zhang et al. (2013) reported that the transplantation of human amniotic epithelial cells clearly improved the rabbit forepaw functions after brachial plexus injury. The results of the present study demonstrate that, in animals with brachial plexus injuries, hAECs intervention recovered the maximum stress, maximum strain, elastic limit stress, and elastic limit strain of the injured brachial plexus. The cell transplantation also improved the pathological morphology of the brachial plexus endoneurium, myelin sheath, axons, and other neural tissue components, as well as reducing the fracture of the myelin sheath. This evidence indicates that hAECs transplantation is an effective means to repair the brachial plexus after injury.
Author contributions:Yang Q and Jin H were responsible for the study concept and design. Yang Q and Li P integrated and analyzed data. Jin H reviewed the study. Li P performed statistical analysis. Luo M was responsible for the funds. Luo M and Li P provided technical or information support. Jin H instructed the study. Yang Q drafted and was responsible for the article. All authors implemented the study and approved the final version of the manuscript.
Con fl icts of interest:None declared.
Bailey R, Kaskutas V, Fox I, Baum CM, Mackinnon SE (2009) Effect of upper extremity nerve damage on activity participation, pain, depression, and quality of life. J Hand Surg Am 34:1682-1688.
Bertelli JA, Mira JC (1993) Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. J Neurosci Methods 46:203-208.
Bertelli JA, Ghizoni MF (2010) Nerve root grafting and distal nerve transfers for C5-C6brachial plexus injuries. J Hand Surg Am 35:769-775.
Bhandari PS, Deb P (2011) Dorsal approach in transfer of the distal spinal accessory nerve into the suprascapular nerve: histomorphometric analysis and clinical results in 14 cases of upper brachial plexus injuries. J Hand Surg Am 36:1182-1190.
Chen XD, Liu HM (2011) In vitro differentiation of human amniotic epithelial cell into neuron-like cells. Shiyong Yixue Zazhi 27:1336-1338.
Chen XD, Hua XY, Wang XL (2012) The effects of astragalus on the differentiation and survival of human amniotic epithelial cells to neurocytes. Shenjing Jiepou Xue Zazhi 28:368-374.
Chen YY, Lu Y, Wang K, Wang Y, Wu DY, Liu B, Yang Y, Lv SH (2011) Optimization of in vitro culture conditions for human amniotic epithelial cells and expression of stem cell markers. Zhongguo Shiyan Xueye Xue Zazhi 19:464-468.
Choong PF, Mok PL, Cheong SK, Leong CF, Then KY (2007) Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro. Cytotherapy 9:170-183.
Ding HY, Wu Y, Li XY, Xu DH (2012) The study on the creep of fetus umbilical cord vein properties. Shengwu Yixue Gongcheng Yanjiu 31:103-106.
Fan GM, Zhang WB, Zhang S, Wang JJ (2010) Nerve growth factor combined with neural stem cell transplantation for treating spinal cord injury in rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 14:2572-2578.
Fliniaux I, Viallet JP, Dhouailly D, Jahoda CA (2004) Transformation of amnion epithelium into skin and hair follicles. Differentiation 72:558-565.
Flores LP (2011) Triceps brachii reinnervation in primary reconstruction of the adult brachial plexus: experience in 25 cases. Acta Neurochir (Wien) 153:1999-2007.
Fu G, Gu LQ, Qin BG, Li P, Xiang JP, Qi J, Zhu QT, Li ZY, Lao ZG, Liu XL, Zhu JK (2010) Investigation and analysis of the quality of life on brachial plexus injury patients. Zhonghua Xianwei Waike Zazhi 33:125-128.
Giuffre JL, Kakar S, Bishop AT, Spinner RJ, Shin AY (2010) Current concepts of the treatment of adult brachial plexus injuries. J Hand Surg Am 35:678-688.
Goubier JN, Teboul F, Khalifa H (2011) Reanimation of elbow extension with intercostal nerves transfers in total brachial plexus palsies. Microsurgery 31:7-11.
Guo B, Xu JJ (2013) Rat amniotic epithelial cells are induced to differentiate into neural-like stem cells in vitro. Zhongguo Zuzhi Gongcheng Yanjiu 17:3503-3507.
Han FB (2012) The applications of the induced pluripotent stem cells in studying the neurodegenerative diseases. Zhongguo Xibao Shengwu Xue Xuebao 34:403-414.
Hou Y, Huang Q, Liu T, Guo L (2008) Human amnion epithelial cells can be induced to differentiate into functional insulin-producing cells. Acta Biochim Biophys Sin (Shanghai) 40:830-839.
Kitajima I, Doi K, Hattori Y, Takka S, Estrella E (2006) Evaluation of quality of life in brachial plexus injury patients after reconstructive surgery. Hand Surg 11:103-107.
Li H, Shi JM, Li GL (1991) Tensile strength determination of human peripheral nerve. Jiepou Xue Zazhi 14:187-190.
Li S, Li P, Yuan Y (2013a) Comparison of mechanics characteristics on normal stress relaxation and pathological corneas. Shengwu Yixue Gongcheng Yanjiu 32:174-179.
Li YJ, Wang YF, Luo M (2013b) Study on creep test of tracheal cartilage. Shengwu Yixue Gongcheng Yanjiu 32:91-93.
Liu B, Tian XL, Wang XW, Yang F (2001) Regeneration of dopamine neuron by intrastriatal implantation of rat amnion cells. Zhongguo Laonian Xue Zazhi 21:358-360.
Liu GY, Zhang Q, Jin Y, Gao ZL (2011a) Autologous nerve anastomosis versus human amniotic membrane anastomosis A rheological comparison following simulated sciatic nerve injury. Neural Regen Res 6:2424-2429.
Liu GY, Zhang Q, Jin Y, Gao ZL (2012) Stress and strain analysis on the anastomosis site sutured with either epineurial or perineurial sutures after simulation of sciatic nerve injury. Neural Regen Res 7:2299-2304.
Liu N, Hu DL, Xu T, Yu SH, Sheng WB (2011b) Neural stem cells transplantation for the treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 15:1809-1813.
Marcus AJ, Coyne TM, Black IB, Woodbury D (2008) Fate of amnion-derived stem cells transplanted to the fetal rat brain: migration, survival and differentiation. J Cell Mol Med 12:1256-1264.
Miki T, Mitamura K, Ross MA, Stolz DB, Strom SC (2007) Identi fi cation of stem cell marker-positive cells by immunofluorescence in term human amnion. J Reprod Immunol 75:91-96.
Miki T, Marongiu F, Ellis ECS, Dorko K, Mitamura K, Ranade A, Gramignoli R, Davila J, Strom SC (2009) Production of hepatocyte-like cells from human amnion. Methods Mol Biol 481:155-168.
Ngeow WC, Atkins S, Morgan CR, Metcalfe AD, Boissonade FM, Loescher AR, Robinson PP (2011) The effect of Mannose-6-Phosphate on recovery after sciatic nerve repair. Brain Res 1394:40-48.
Niknejad H, Peirovi H, Ahmadiani A, Ghanavi J, Jorjani M (2010) Differentiation factors that influence neuronal markers expression in vitro from human amniotic epithelial cells. Eur Cell Mater 19:22-29.
Okawa H, Okuda O, Arai H, Sakuragawa N, Sato K (2001) Amniotic epithelial cells transform into neuron-like cells in the ischemic brain. Neuroreport 12:4003-4007.
Peng CG, Zhang Q, Yang Q, Zhu QS (2012) Strain and stress variations in the human amniotic membrane and fresh corpse autologous sciatic nerve anastomosis in a model of sciatic nerve injury. Neural Regen Res 7:1779-1785.
Sakuragawa N, Kakinuma K, Kikuchi A, Okano H, Uchida S, Kamo I, Kobayashi M, Yokoyama Y (2004) Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res 78:208-214.
Sankar V, Muthusamy R (2003) Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 118:11-17.
Shen Z, Sun CJ, Feng TJ, Ma HS (2010) Tensile mechanical properties of the brachial plexus of experimental animals. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 14:3730-3733.
Sibinski M, Wo niakowski B, Drobniewski M, Synder M (2010) Secondary gleno-humeral joint dysplasia in children with persistent obstetric brachial plexus palsy. Int Orthop 34:863-867.
Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y (2007) Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell 20:77-84.
Terzis JK, Papakonstantinou KC (2000) The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg 106:1097-1124.
Terzis JK, Barmpitsioti A (2012) Our experience with triceps nerve reconstruction in patients with brachial plexus injury. J Plast Reconstr Aesthet Surg 65:590-600.
Vekris MD, Pa fi las D, Lykissas MG, Soucacos PN, Beris AE (2010) Correction of elbow fl exion contracture in late obstetric brachial plexus palsy through arthrodiatasis of the elbow (Ioannina method). Tech Hand Up Extrem Surg 14:14-20.
Wan P, Wang X, Ma P, Gao N, Ge J, Mou Y, Wang Z (2011) Cell delivery with fi xed amniotic membrane reconstructs corneal epithelium in rabbits with limbal stem cell de fi ciency. Invest Ophth Vis Sci 52:724-730.
Xia J, Xu YF, Xu YW, Wan AG, Ding J (2011) Repair of human amniotic epithelial cells transplantation on ulnar injury. Shiyong Linchuang Yixue 12:44-45, 51.
Xu DH, Li DY, Li XY, Ding HY (2013) Stress relaxation properties of fetal umbilical cord vein grafts. Zhongguo Zuzhi Gongcheng Yanjiu 17:2956-2959.
Xu LQ, Ding DF, Li XF, Wang YJ, Shi Q, Zhou CJ (2011) Embryonic stem cell transplantation for spinal cord injury (review). Zhongguo Kangfu Lilun yu Shijian 17:51-53.
Yang JH, Li CD, Zhai YS, Wang Y (2007) Experimental study on transplantation of embryonic stem cells in treating spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21:487-491.
Yang XX (2009) The effect of transplantion of human amniotic epithelial cells into the lateral ventricle of Parkinson model rats on behavior, dopaminergic neurons and neurotransmitters. Zhongfeng yu Shenjing Jibing Zazhi 26:532-535.
Zhang Y, Liu CX, Wang L, Zhang L, Liu W, Zhao W, Li Z (2013) Human amniotic epithelial cell transplantation for repair of brachial plexus injury in rabbits. Zhongguo Zuzhi Gongcheng Yanjiu 17:9157-9162.
Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T (2005) Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation 79:528-535.
Zhao QF, Wang S, Geng DY, Yang WB (2011) MRI diagnosis and classifi cation of brachial plexus injury. Zhongguo Yixue Jisuanji Chengxiang Zazhi 17:513-516.
Zheng LL, Xiao B (2011) The pathway of regenerative spinal motoneurous after brachial plexus injury in rats. Zhongguo Kangfu Yixue Zazhi 26:120-123.
Zhou H, Kong FB (2011) MRI diagnosis and clinical application of brachial plexus injury. Jiefangjun Yixue Zazhi 36:309-312.
Zhou J, Xie SC, Lei B, Li TL, Na CY, Yang YH (2013) MRI diagnosis of adult brachial plexus injury. Shiyong Yixue Yingxiang Zazhi 14:230-232.
Copyedited by McCarty W, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.147947
Hai Jin, M.D., China-Japan Union Hospital of Jilin University, Changchun 130033, Jilin Province, China,
plyangqi1980@sina.com.
http://www.nrronline.org/
Accepted: 2014-10-20
- 中国神经再生研究(英文版)的其它文章
- Hydrogen sul fi de controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases
- Activities of nicotinic acetylcholine receptors modulate neurotransmission and synaptic architecture
- A novel arti fi cial nerve graft for repairing longdistance sciatic nerve defects: a self-assembling peptide nano fi ber scaffold-containing poly(lactic-co-glycolic acid) conduit
- The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury
- Long-term treatment with PP2 after spinal cord injury resulted in functional locomotor recovery and increased spared tissue
- Thermomineral water promotes axonal sprouting but does not reduce glial scar formation in a mouse model of spinal cord injury

