Clinical Outcome of Autologous Hematopoietic Stem Cell Infusion via Hepatic Artery or Portal Vein in Patients with End-stage Liver Diseases△
Xiao-lun Huang, Le Luo, Lan-yun Luo, Hua Xue, Ling-ling Wei, Yu-tong Yao, Hai-bo Zou, Xiao-bing Huang, Yi-fan Zhu, Tian Zhang, Ping Xie, Mao-zhu Yang, and Shao-ping Deng*
1Center for Cell Transplantation (Seventh Unit of General Surgery Department), Institute of Organ Transplantation, 2Department of Blood Medicine,
3Department of Radiology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu 610072, China
LIVER transplantation hitherto remains the only possible cure for end-stage liver diseases (ESLD), which, however, is hampered by social and economic concerns and ethical restraints, as well as a limited source of donor organs.1,2Hepatocyte trans- plantation offers a promising alternative, but its long-term efficacy remains unclear.3In addition, the treatment is associated with many problems and obstacles such as poor survival after transplantation4and lack of adequate cell sources.
Bone marrow mesenchymal stem cells (MSCs) and bone marrow-derived hematopoietic stem cells (HSCs) have been studied extensively as alternatives to hepatocyte transplantation for the treatment of ESLD.5MSCs have been shown to promote the regeneration of hepatocytes and inhibit their apoptosis via paracrine signaling and also be involved in tissue repair after liver injury by modifying the expression of macromolecules.6HSCs promote recovery from hepatic injury under strong positive selection pressure when normal mechanisms of regeneration are either blocked or inadequate, whereas other cell types fail to exhibit these effects.7,8
In this prospective study, we investigated the clinical outcome of transplantation using bone marrow-derived CD34+HSCs infused via the hepatic artery or portal vein in patients with ESLD, aiming to clarify the efficacy of this therapy and, if it exists, to identify the better route of infusion.
PATIENTS AND METHODS
Patient selection
We prospectively recruited patients with hepatic decompensation who sought treatment at Sichuan Province People's Hospital from September 2010 to September 2012. Hepatic decompensation was diagnosed according to the 2010 Diagnostic Criteria of the Chinese Society of Hepatology and Chinese Society of Infectious Diseases9regardless of the primary diseases. Patients who met the following criteria were enrolled into this study: (1) Child-Pugh grade B or C; (2) serum albumin levels <35 g/L with an A/G ratio <1.0; (3) significant jaundice (bilirubin >35 μmol/L); (4) alanine transaminase (ALT) level >40 U/L and aspartate transaminase (AST) level >40 U/L; (5) prothrombin activity < 60% and prothrombin time (PT) >12.6 seconds, but ≤ 17.6 seconds. And patients were excluded if: (1) they had bacterial peritonitis, human immunodeficiency virus (HIV) infection, sepsis, systemic fungal infection or any other life-threatening infectious diseases; (2) they were diagnosed with any form of cancer; (3) they had tension ascites that did not relieve with adequate diuretic therapy, or reversed by diuretic therapy but resulted in abdominal compartment syndrome; (4) they were in the active phase of gastrointestinal hemorrhage; (5) the portal vein trunk was blocked from thrombosis, compensated formation of sponge blood vessels, superior mesenteric vein occlusion, or severe intestinal congestion; (6) they had a history of allergy to granulocyte-colony stimulating factor (G-CSF); (7) they developed ascites, hepatic encephalopathy, esophageal and gastric varices or bleeding caused by portal hypertension and hypersplenism; (8) the patients were pregnant or lactating.
The study protocol was approved by the Institutional Ethics Committee of Sichuan Academy of Medical Sciences and Sichuan Provincia People's Hospital. Written informed consents were obtained from all the participants or their legal surrogates.
Pre-transplantation preparation
The patients were administered with polyene phosphatidyl choline [0.3-0.4 g/(kg·d)] and magnesium isoglycyrrhizinate [3-4 mg/(kg·d)] for protection of liver function, α1-thymosin (1.6 mg/d) for enhancing immunity, somatostatin (24-hour continuous infusion at 250 mg/h) and propranlolum (10-20 mg/d) for reduction of portal vein pressure. The patients with hepatitis B virus deoxyribonucleic acid (HBV-DNA) load ≥ 104U/mL received antiviral (Telbivudine, Novartis International AG, Basel, Switzerland) treatment at least one year before enrolling in this study.
Transplantation
The patients received G-CSF [5 μg/(kg·d)] for 3-5 days.10Mobilize the patients who suffer from abnormal liver function during the process (ALT≥100 U/L and/or total bilirubin ≥ 2 mg/L and/or ascites increased significantly) to stop the injection of G-CSF and adopt some methods for liver-protecting, jaundice-relieving, diuretic, and supportive treatment. Transplantation should be done after the liver function was significantly improved. When the white blood cell (WBC) count exceeded 20×109/L, patients underwent femoral vein catheterization. 100-200 mL mononuclear cells were collected from 8000-12000 mL total circulating blood. All the collected cells were transplanted back into the same patient. The anticoagulant acid citrate dextrose solution (ACD-A) was administered at a ratio of 9∶1-11∶1 (whole blood to anticoagulant). CD34+HSCs were counted by flow cytometry. The range of number was 1.13×106- 2.45×106CD34+cells /kg body weight. Collected cells were transplanted on the next day either via the hepatic artery with Seldinger technique and ultra-election hepatic artery angiography through the celiac artery or via a catheter placed in the portal vein trunk through the right gastroepiploic vein. 80 cases were thereby divided into two groups: the portal vein group (n=36) and the hepatic artery group (n=44). Patients were given anticoagulant therapy [low molecular weight heparin, 60-100 U/(kg·d)] to prevent portal vein thrombosis on the third day after transplantation.
Pathological analysis
Liver biopsies were conducted at three different time points: 2 weeks before stem cell transplantation, 6 months and 12 months after transplantation. The liver biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut at 4 μm thickness and stained with hematoxylin and eosin (HE) and Gomori methenamine silver (GMS). The results were read by two experienced pathologists independently.
Post-transplantation follow-up
The patients were followed up at 3, 6, and 12 months after transplantation, and laboratory examinations included blood routine test, liver function test (ALT, AST, albumin and total bilirubin), coagulation time analyses [prothrombin time (PT) and activated partial thromboplastin time (aPPT)], immunological examination and tumor marker tests. Contrast-enhanced ultrasound, computed tomography (CT) scan and vascular angiography were carried out to exclude malignant tumors. Liver biopsy was guided by ultrasound or during surgery. Liver inflammation and fibrosis were scored using the Knodell scoring system. Ascites was scored as: 0, absent; 1, slight; 2, moderate; or 3, severe.11
Statistical analysis
Statistical analysis was carried out using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). All data were expressed as means ± SD. ALT level, albumin level, total bilirubin, PT, portal vein diameter and Knodell scores were analyzed with one-way analysis of variance (ANOVA) followed by a Least Significance Difference (LSD) test for multiple comparisons and one-sample t test. Ascites scores were analyzed with Mann-Whitney U test and generalized estimating equation (GEE). P<0.05 was considered statistically significance.
RESULTS
Patient demographic and disease characteristics
Eighty patients (58 males and 22 females) were eligible and enrolled into this study. The median age was 52 years (range, 32-72 years). The mean body weight was 61.7 kg. The cause of liver cirrhosis was hepatitis B in 63 cases (received antiretroviral treatment over one year, HBV copy number <103copies/ml and liver function were not improved), alcoholic liver cirrhosis in 13 patients (alcohol 200 g/per day in the last 20 years), autoimmune hepatitis in 3 cases, and Wilson disease in 1 case. The Child-Pugh score was grade B in 69 cases and grade C in the remaining 11 cases. Three patients had portal vein thrombosis. HSC transplantation was carried out via the portal vein (n=36, 45%) or the hepatic artery (n=44, 55%).
Effect of HSC transplantation on liver function
Compared with pre-transplantation, ALT levels decreased significantly at 6 and 12 months after HSC transplantation in both groups (P<0.05), but not at 3 months after transplantation (P>0.05) (Table 1). There were no significant differences between portal vein group and hepatic artery group either before or after transplantation (P>0.05). Compared with pre-transplantation, serum albumin levels increased significantly at 6 and 12 months after HSC transplantation in both groups (P<0.05), but not at 3 months (P>0.05) (Table 2). No significant differences were observed between the two groups either before or after transplantation (P>0.05). Compared with pre-transplantation, total bilirubin levels decreased significantly at 3, 6, and 12 months after HSC transplantation in both groups (P<0.05) (Table 3). No significant differences were observed between the two groups either before or after transplantation (P>0.05). In addition, compared with pre-transplantation, PT decreased at 12 months after HSC transplantation in both groups (P<0.05) (Table 4). At 6 months, the decreased level of PT had significant difference in portal vein group but not in hepatic artery group. No significant differences were observed between the two groups either before or after transplantation (P>0.05).
Outcome of HSC transplantation
Portal vein thrombosis was observed in one patient (1.25%) at 6 months post-transplantation. Upper gastrointestinal bleeding was observed in 3 patients (3.75%) at 3 months post-transplantation. Two patients (2.50%) developed level І hepatic encephalopathy at 3 months post-transplantation, who eventually recovered and were discharged. The one-year survival rate was 100%. Portal vein diameter decreased significantly at all three time points after HSC transplantation (P<0.05), except at 3 months after HSC transplantation in the hepatic artery group (Table 5). No significant differences were observed between the two groups either before or after transplantation (P>0.05). Ascites decreased significantly after HSC transplantation in both groups (P<0.05) (Table 6). No significant difference was observed between the two groups at either time points (P>0.05).

Table 1. Alanine transaminase (ALT) levels before and after hematopoietic stem cell (HSC) transplantation via the hepatic artery or portal vein in patients with end-stage liver diseases (U/L) §

Table 2. Albumin levels before and after HSC transplantation in patients with end-stage liver diseases (g/L) §

Table 3. Total bilirubin levels before and after HSC transplantation in patients with end-stage liver diseases (μmol/L) §

Table 4. Prothrombin time (PT) prior to and after HSC transplantation in patients with end-stage liver diseases (s) §
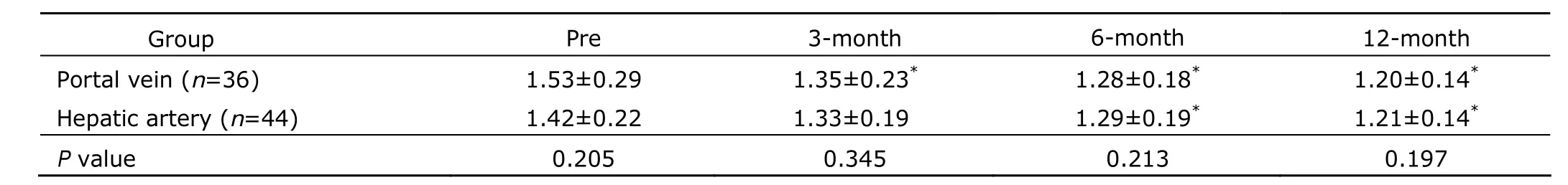
Table 5. Portal vein diameter before and after HSC transplantation in patients with end-stage liver diseases (cm) §
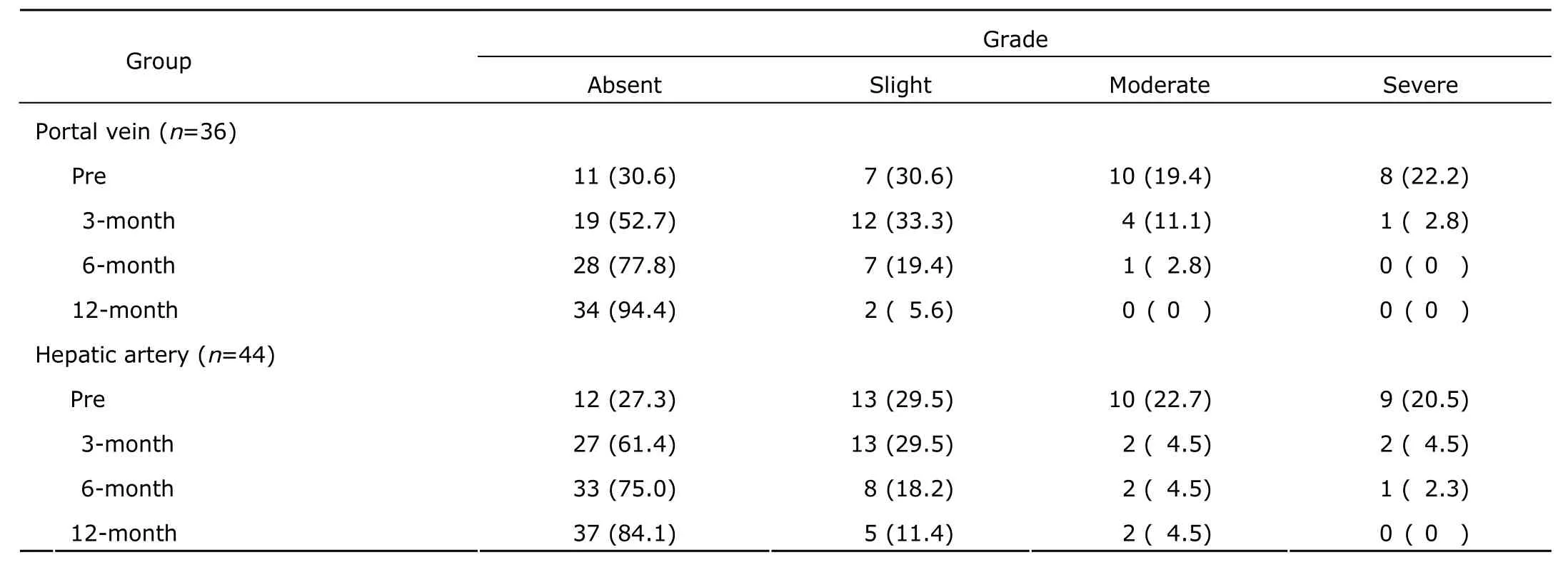
Table 6. Ascites grade before and after HSC transplantation in patients with end-stage liver disease [n (%)]

Figure 1. Histological examination of the liver of patients with end-stage liver disease (ESLD) before and after HSC transplantation. HE staining and Gomori reticular fiber staining of the liver biopsies showed marked improvement after HSC transplantation (A-D: HE, 400×; E-H: Gomori reticular fiber staining, 400×; scale bar: 50 μm).
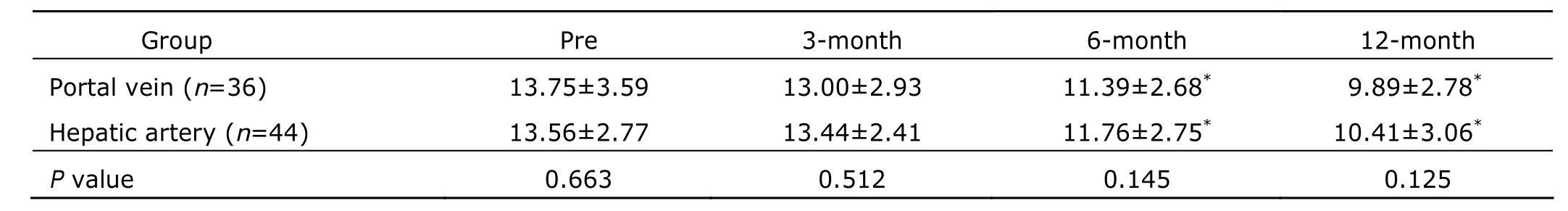
Table 7. Knodell scores before and after HSC transplantation in patients with end-stage liver diseases§
Histological examination
Histological examination of the liver biopsies showed hepatocyte swelling, acidophilic degeneration, piecemeal necrosis, apparent proliferation of fibroblasts in the portal area and portal-portal bridging hepatic necrosis before transplantation (Fig. 1A). Liver cell edema, degeneration, necrosis and inflammation were significantly relieved at 3, 6, 12 months after transplantation (Fig.1 B-H). Moreover, Knodell score decreased significantly at 6 and 12 months, but not 3 months after HSC transplantation (Table 7). There were no significant differences between the two groups either before or after transplantation (P>0.05).
DISCUSSION
The available evidence suggests that the peripheral blood may contain multipotential stem cells, and it has been suggested that these cells could be used for treating ischemic and degenerative diseases. Because HSCs can be easily isolated and handled, they have received a great deal of attention from several researchers. Zhang et al12showed that differentiation of stem cells into mature and functional hepatocytes, especially from an extra-hepatic stem cell source, would circumvent the scarcity of liver donors and human hepatocytes, and most importantly it would offer an ideal and promising source of hepatocytes for cell therapy and tissue engineering in treating liver disease. HSCs could be readily isolated from peripheral blood, and have been used successfully in animal models of many diseases.13Jang et al14showed that HSCs differentiated into mature hepatocytes and rescued mice from fatal liver damage.
CD34+, a glycoprotein that participates in cell-cell adhesion and is expressed on MSCs, cells in the umbilical cord, endothelial progenitor cells, and mature endothelial cells, is the most commonly used molecular marker for identifying adult HSCs in peripheral blood.13Circulating CD34+cells have been shown to contribute to neo-angiogenesis following tissue ischemia and organ regeneration. The number of CD34+cells increases in patients following hepatic resection.8In a preliminary study of 9 patients with alcoholic hepatic cirrhosis, transplantation of bone marrow- derived CD34+HSCs improved the Child-Pugh scores.15In addition, a randomized controlled trial of 140 patients with viral or autoimmune-induced ESLD showed that autologous HSCs improved serum albumin, bilirubin, International Normalized Ratio, and ALT levels. Wan et al16observed 30 acute-on-chronic liver failure (ACLF) patients. The mobilized CD34+cells expressed both CD34 mRNA and liver-specific markers including cytokeratin 19 and α-fetoprotein. In parallel with mobilization of bone marrow-derived CD34+cells, elevated serum levels of hepatocyte growth factor, interleukin-6, stem cell factor, G-CSF and matrix metallo- proteinase 9 were observed in ACLF patients. These findings led us to speculate that autologous CD34+and CD133+stem cell transplantation could be used as a supportive treatment for ESLD.11,17
Bone marrow mobilization by G-CSF alone has been used to treat ESLD in humans. It is reported the mobilization of bone marrow stem cells into the blood had remarkable effect, the whole process was safe and dose- dependent, but did not improve liver function,18suggesting that bone marrow stem cells need to be directed to the target organ more efficiently. Gaia et al10confirmed that G-CSF-induced mobilization of bone marrow cells that co-express markers for epithelial cells and stem cells are present in the cirrhotic liver. Furthermore, splenomegaly up to 170 mm does not prevent safe bone marrow cell mobilization following G-CSF treatment in patients with ESLD; mobilized BMC may represent an easy immature cell source potentially useful for novel approaches for liver regeneration.19All together, these findings suggest that mobilized bone marrow cells constitute a readily obtainable source of immature stem cells, and could be used to treat ESLD.19So we safely did bone marrow mobilization by G-CSF and got satisfactory effects. No patient showed severe reactions.
In the present study, HSC transplantation improved major indices of hepatic function, including serum albumin, bilirubin, PT, and ALT. Notably, improvement in most measures was still evident at 12 months after transplantation, suggesting that transplanted cells are successful in establishing themselves in the liver and in maintaining functioning in a sustainable manner. None of the patients in the current study experienced a severe adverse reaction. Upper gastrointestinal hemorrhage occurred in 3 out of 80 patients receiving HSC transplantation, which, however, most likely represents a consequence of the natural course of the underlying diseases.
Hepatic artery infusion of HSCs has been reported to improve liver function in an animal model of liver failure.20Because the hepatopetal blood flow of the portal vein system is considerably larger than that of the hepatic artery, there may be different result between the two implant ways to infuse HSCs into the liver. However, in this study, we failed to observe significant differences between the hepatic artery group and the portal vein group in liver function parameters and pathological measures such as portal vein diameter and ascites, suggesting that the two approaches equally effective in improving liver function in ESLD patients.
This study is limited for non-randomized allocation of patients to either treatment group. HSCs were infused via the portal vein if patients required splenectomy or splenectomy combined with pericardial devascularisation, otherwise infused via the hepatic artery. Under such a design, patients in the portal vein group might have more advanced underlying diseases, but a comparison of the baseline characteristics revealed no significant differences. As a result, we believe that this limitation did not produce a significant bias.
Consistent with previous reports,17,21we observed a significant reduction of ascites in both groups across all time points. Combined with the other indicator of this research, i.e. portal vein diameter consistently decreasing after transplantation, suggesting that hepatic fibrosis could be attenuated or even reversed by HSC transplantation. As we all know, liver cirrhosis represents a late stage of progressive hepatic fibrosis characterized by distortion of the hepatic architecture and formation of regenerative nodules.22Researchers summarized the goal of stem cell transplantaion as: (1) to improve regeneration and reduce scarring in liver cirrhosis by modulating the liver's own regenerative processes, (2) to down-regulate immune- mediated liver damage, (3) supplying hepatocyte-like cells (HLCs) derived from stem cells for use in extracorporeal bio-artificial liver machines, and (4) to use stem cell derived HLCs for cell transplantation to supplement or replace hepatocyte function.23Thus, anti-fibrosis and tissue reconstruction are particularly important. Epithelial progenitor cells could attenuate fibrosis in rodent models of liver cirrhosis.24However, isolation of endothelial progenitor cells in humans is not practical. Terai et al25and Thomas et al26discovered that autologous bone marrow-derived mononuclear cells could increase the proliferation of hepatocytes and decrease fibrosis in the liver. In our study, the biopsy results (Knodell score) indicated a significant reduction of inflammation and fibrosis at 6 and 12 months after transplantation in both treatment arms. Future studies using more specific methods, e.g., immunostaining, could further clarify the issue.
In conclusion, our results indicate that HSC transplantation in patients with ESLD improves liver function, and attenuates the underlying pathological changes (such as dilatation of the portal vein). Hepatic artery infusion is equally effective as portal vein infusion.
1. Dalgetty DM, Medine CN, Iredale JP, et al. Progress and future challenges in stem cell-derived liver technologies. Am J Physiol Gastrointest Liver Physiol 2009; 297: G241-8.
2. Hara H, Gridelli B, Lin YJ, et al. Liver xenografts for the treatment of acute liver failure: clinical and experimental experience and remaining immunologic barriers. Liver Transpl 2008; 14:425-34.
3. Puppi J, Strom SC, Hughes RD, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant 2012; 21:1-10.
4. Sokal EM. From hepatocytes to stem and progenitor cells for liver regenerative medicine: advances and clinical perspectives. Cell Prolif 2011; 44:39-43.
5. Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 2011; 145: 851-62.
6. Ezzat TM, Dhar DK, Newsome PN, et al. Use of hepatocyte and stemcells for treatment of post-resectional liver failure: are we there yet. Liver international 2011; 31:773-84.
7. Herencia C, Rodríguez-Ariza C, Canalejo A, et al. Differential bone marrow hematopoietic stem cells mobilization in hepatectomized patients. J Gastrointest Surg 2011; 15:1459-67.
8. Wesson RN, Cameron AM. Stem cells in acute liver failure. Adv Surg 2011; 45:117-30.
9. Chinese Society of Hepatology and Chinese Society of Infectious Diseases. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Chin J Clin Infect Dis 2011; 4:1-13.
10. Gaia S, Smedile A, Omedè P, et al. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol 2006; 45:13-9.
11. Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981; 1:431-5.
12. Zhang Z, Liu J, Liu Y et al. Generation, characterization and potential therapeutic applications of mature and functional hepatocytes from stem cells. J Cell Physiol 2013; 228:298-305.
13. Romagnani P, Lasagni L, Romagnani S. Peripheral blood as a source of stem cells for regenerative medicine. Expert Opin Biol Ther 2006; 6:193-202.
14. Jang YY, Collector MI, Baylin SB, et al. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004; 6:532-9.
15. Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow- derived CD34+cells into patients with alcoholic liver cisshosis. Am J Gastroenterol. 2008; 103:1952-8.
16. Wan Z, You S, Rong Y, et al. CD34+hematopoietic stem cells mobilization, paralleled with multiple cytokines elevated in patients with HBV-related acute-on-chronic liver failure. Dig Dis Sci 2013; 58:448-57.
17. Lorenzini S, Gitto S, Grandini E, et al. Stem cells for end stage liver disease: How far have we got? World J Gastroenterol 2008; 14: 4593-9.
18. Salama H, Zekri ARN, Bahnassy AA, et al. Autologous CD34+and CD133+stem cells transplantation in patients with end stage liver disease. World J Gastroenterol 2010; 16: 5297-305.
19. Piscaglia AC, Campanale M, Gasbarrini A, et al. Stem cell-based therapies for liver diseases: state of the art and new perspectives. Stem Cells Int 2010; 2010:259-461.
20. Alison MR, Islam S, Lim S. Stem cells in liver regene- ration, fibrosis and cancer: the good, the bad and the ugly. J Pathol 2009; 217:282-98.
21. Salama H, Zekri AR, Zern M, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant 2010; 19:1475-86.
22. Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 2009; 21:1199-205.
23. Forbes SJ, Newsome Q PN. New horizons for stem cell therapy in liver disease. J Hepatol 2012; 56:496-9.
24. Nakamura T, Torimura T, Sakamoto M, et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology 2007; 133: 91-107.
25. Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006; 24:2292-8.
26. Thomas JA, Pope C, Wojtacha D, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells 250 improving fibrosis, regeneration and function. Hepatology 2011; 53:2003-15.
 Chinese Medical Sciences Journal2014年1期
Chinese Medical Sciences Journal2014年1期
- Chinese Medical Sciences Journal的其它文章
- Reversible Posterior Leukoencephalopathy Syndrome in Children with Nephrotic Syndrome: a Case Report
- Bloodstream Infection with Carbapenem-resistant Klebsiella Pneumoniae and Multidrug-resistant Acinetobacter Baumannii: a Case Report
- Comparison of the Outcomes of Monopolar and Bipolar Radiofrequency Ablation in Surgical Treatment of Atrial Fibrillation
- Laryngo-tracheobronchial Amyloidosis: a Case Report and Review of Literature
- Factors Associated with Coronary Artery Disease in Young Population (Age≤40): Analysis with 217 Cases
- Isolated Pancreatic Tuberculosis in Non-immunocompromised Patient Treated by Whipple’s Procedure: a Case Report
