Association of C(-106)T Polymorphism in Aldose Reductase Gene with Diabetic Retinopathy in Chinese Patients with Type 2 Diabetes Mellitus△
Yu Deng, Xiu-fen Yang, Hong Gu, Apiradee Lim, Munkhtulga Ulziibat, Torkel Snellingen, Jun Xu, Kai Ma, and Ning-pu Liu*
1Department of Ophthalmology, Fu Xing Hospital, Capital Medical University, Beijing 100038, China
2Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology and Visual Sciences Key Laboratory, Beijing 100730, China
3Department of Mathematics and Computer Science, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani 94000, Thailand
4Sekwa Eye Hospital, Beijing 100088, China
DIABETIC retinopathy (DR) is the leading cause of legal blindness in working-age adults, accounting for 8% of the legal blindness in the United States1and in China.2In recent years, there has been considerable evidence suggesting that DR is influenced not only by the duration of diabetes and blood glucose level but also by genetic predisposition.3-7
Aldose reductase (ALR), the first and the rate-limiting enzyme in the polyol pathway, catalyzes the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reduction of glucose to sorbitol. Experimental studies suggest that sorbitol accumulate excessively in various tissues of the diabetic rats8or in the rat glomerular mesangial cells cultured under high glucose conditions.9Moreover, both increased enzyme activity and gene expression of ALR have been reported in patients with diabetic microvascular complications.10ALR has thus been proposed to be an important factor in the pathogenesis of diabetic microangiopathy. The C(-106)T polymorphism located at the promoter of the ALR gene has been reported to be associated with DR in Caucasian and Asian patients with type 1 and 2 diabetes mellitus.11-19However, results of various studies regarding the relationship between DR and the C(-106)T polymorphism are conflicting and inconclusive. While several studies suggested an association between DR and the C(-106)T polymorphism,4,7,20,21some other studies did not support this observation.22,23In this study, we aimed to verify the possible association of the C(-106)T polymorphism of the ALR gene with the risk of DR with a cohort of Chinese patients with type 2 diabetes mellitus (T2DM).
SUBJECTS AND METHODS
Study participants and clinical evaluation
Between November 2009 and September 2010, patients with T2DM were recruited from the Desheng Community of downtown Beijing. Written informed consent was obtained from each participant before enrollment. Patients were enrolled into the study if: (1) they reported physician- diagnosed T2DM treated with insulin, oral hypoglycemic agents or healthy diet only; (2) or had a fasting plasma glucose ≥7.0 mmol/L (126 mg/dl) in at least two previous examinations or a random plasma glucose ≥11.1 mmol/L (200 mg/dL). All the participants underwent a standardized evaluation consisting of a questionnaire assessing demographic information and medical history, anthropometric examinations including body mass index (BMI) and waist-hip ratio, and a comprehensive ophthalmological examination including best corrected visual acuity, slit lamp biomicroscopy, fundus examination, and fundus photography. Seven-field 30-degree stereoscopic fundus color photographs of optic disc and macula were taken through dilated pupils for all the patients using a digital fundus camera (Zeiss, Oberkochen, Germany). Fasting blood samples (15 mL) were collected to measure the fasting plasma glucose, glycosylated hemoglobin A1c (HbA1c), creatinine, uric acid, and lipid profiles [levels of total cholesterol, triglycerides, high-density and low-density lipoprotein cholesterol (HDL-C and LDL-C)]. Fasting urine samples were obtained to detect microalbuminuria.
Two ophthalmologists independently graded the fundus photographs and the final diagnosis for each patient was determined based on the higher grading of the worse eye.24The duration of diabetes was defined as the interval between the initial diagnosis by a physician and the time of enrollment into the present study. Based on the duration of diabetes and the grading of fundus photographs, patients were assigned into the diabetic without retinopathy (DWR) group if they had a duration of T2DM ≥ 10 years with no signs of DR (microaneurysms, hemorrhages or exudates) or if they had a duration of T2DM ≥ 15 years with fewer than 5 microaneurysms.4,25Patients with 5 or more microaneurysms in at least one eye were assigned into the DR group.25Patients who did not meet the criteria of either DWR or DR were excluded from the study.
The Ethics Committee of Beijing Tongren Hospital approved the study protocol and the procedures were performed in accordance with the Declaration of Helsinki.
Genotyping for ALR polymorphism
Blood samples (2 mL each) were collected from all participants and stored at -80°C before DNA extraction. Genomic DNA was isolated from venous blood leukocytes using a genomic DNA extraction and purification kit (TIANamp Swab DNA Kit, Tiangen Biotech, Beijing, China). The C(-106)T polymorphism (rs759853) in the promoter region of ALR gene was genotyped using the Sequenom MassALRRAY technology (Sequenom MassALRRAY, Bioyong Technologies Inc., Beijing, China).26,27
Statistical analysis
Statistical analysis was performed with the R statistical analysis packages. Baseline characteristics of the DR and DWR groups were compared using t-test for continuous variables or Chi-square test for categorical variables. Results are expressed as means ± SD or median (range). Genotype frequencies of the ALR C(-106)T polymorphism were checked for Hardy-Weinberg equilibrium using Chi-square test. Chi-square test was also used to analyze the distribution of genotypes and alleles. Associations between the ALR C(-106)T polymorphism and the presence of DR were tested by calculating the odds ratio (OR) and the 95% confidence interval (CI) using multivariate logistic regression. P<0.05 was considered statistically significant.
The power to detect a significant association between the T allele of the ALR C(-106)T polymorphism and DR was calculated under the condition of different hypothetical OR values. To detect a significantly protective effect of the T allele, the sample size in this study had 82.4% power if the T allele carriers had an OR of 0.5 and 30.4% power if the T allele carriers had an OR of 0.7. To detect a significant risk effect of the T allele, the sample size in this study had 81.7% power if the T allele carriers had an OR of 2.0 and 37.6% power if the T allele carriers had an OR of 1.5.
RESULTS
A total of 268 patients, including 129 with DR and 139 with DWR, were enrolled in this study. Clinical features of the patients are displayed in Table 1. The mean age of diabetic onset was 48.68 ± 9.58 years in the DR group and 50.45 ± 8.00 years in the DWR group (P=0.10). Percentage of males was 44.2% in the DR group and 41.7% in the DWR group (P=0.78). Compared with the DWR group, the DR group had higher levels of creatinine (P=0.02), fasting plasma glucose (P=0.01), HbA1c (P<0.001), and higher incidence of microalbuminuria (P<0.001). The DR group also had a higher percentage of patients taking insulin therapy compared with the DWR group (62.8% vs. 33.8%, P<0.001). No statistically significant difference was observed between the DR and the DWR groups in the duration of diabetes (P=0.25), BMI (P=0.29), blood pressure (systolic blood pressure, P=0.58; diastolic blood pressure, P=0.34), serum levels of uric acid (P= 0.84), and lipid profiles (total cholesterol, P=0.70; triglycerides, P=0.75; HDL-C, P=0.98; LDL-C, P=0.98) (Table 1).
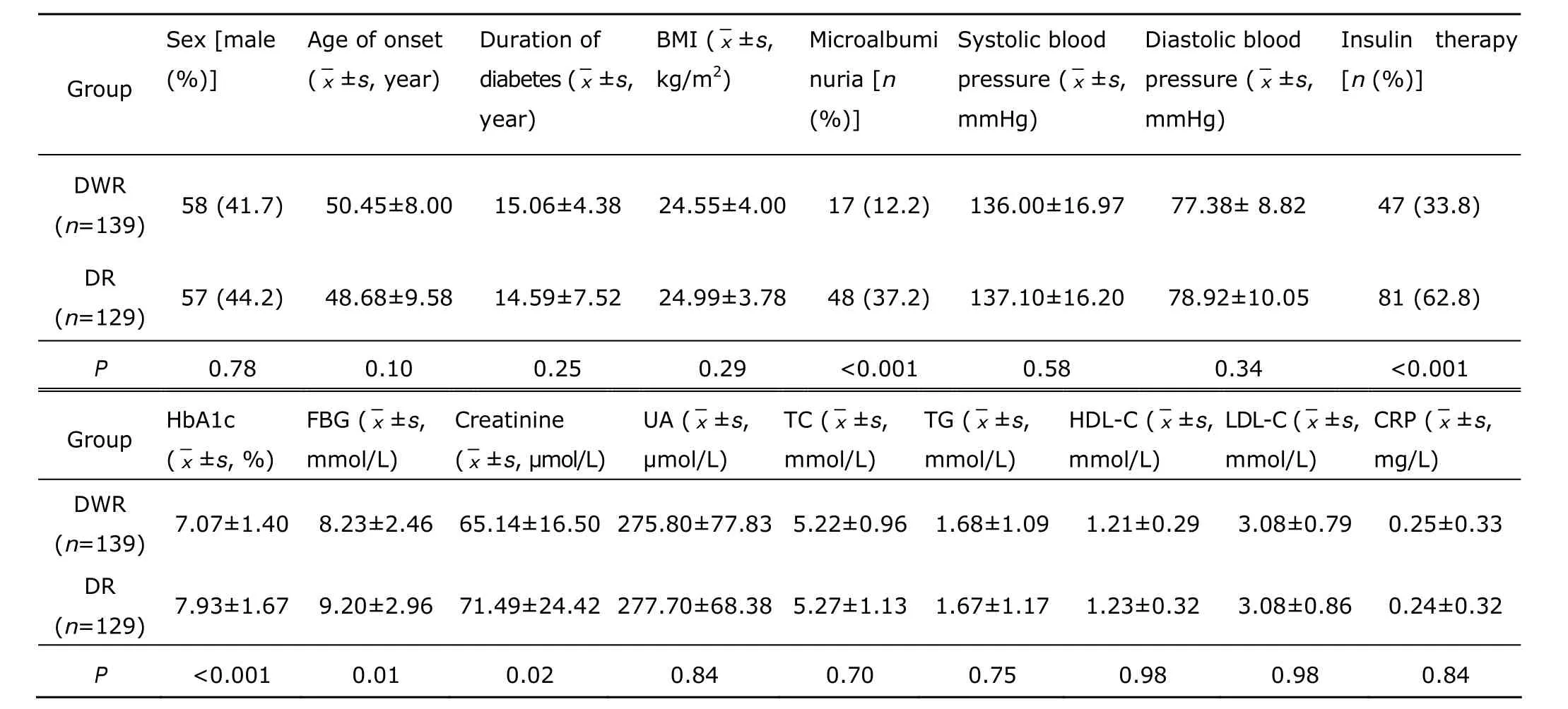
Table 1. Clinical characteristics of the 268 patients with type 2 diabetes mellitus§
The genotype and allele frequencies of the ALR C(-106)T polymorphism in the DR and DWR groups are listed in Table 2. The success rate of genotyping for the patients was 99.6% (267/268). Frequencies of the ALR C(-106)T genotypes were under Hardy-Weinberg equilibrium in the DR (P=0.20) and the DWR group (P=1.00). The frequency of the minor T allele was 16.0% (41/256) in the DR group and 19.4% (54/278) in the DWR group (P=0.36). No significant difference in genotype distribution was observed between the DR and DWR groups (P=0.40). Compared with the wild type CC genotype, the OR for the risk of DR was 0.7 (95% CI: 0.38-1.3) for the heterozygous CT genotype and 0.76 (95% CI: 0.18-3.25) for the homozygous TT genotype after adjustment for the age of diabetic onset, duration of diabetes, BMI, HbA1c, fasting plasma glucose, creatinine level, microalbuminuria, and insulin therapy.
Significant positive association of DR was observed with microalbuminuria (adjusted OR=4.61, 95% CI: 2.34-9.05; P<0.001) and insulin therapy (adjusted OR=3.43, 95% CI: 1.94-6.09; P<0.001) (Table 3).
DISCUSSION
In this study, we investigated the association of ALR C(-106)T polymorphism with susceptibility to DR. Consistent with studies in Brazilian,23French,28and Australian patients20with type 1 diabetes, and Euro-Brazilian patients with type 2 diabetes,22the present study found no association between the ALR C(-106)T polymorphism and the risk of DR in this Chinese cohort of T2DM. However, a Japanese study11and a Chilean study21reported a higher risk of DR with the CC genotype of the C(-106)T polymorphism as compared with the CT or TT genotype. A previous study on Chinese patients with T2DM also reported a higher risk of DR with the CC genotype of the C(-106)T polymorphism.7The discrepancy among different studies could be due to the different genetic background, sampling or experimental bias, or the presence of confounding factors. Indeed, the CC genotype frequency of the C(-106)T polymorphism has been reported to be higher in Asian29,30than in Caucasian20,23,31and Chilean populations.21Moreover, the relatively small sample size inthe Chilean study (53 subjects)21or the previous study with Chinese T2DM patients (40 with DR and 105 with DWR)7could potentially lead to a selection bias. In addition, many environmental and genetic factors could contribute to the pathogenesis of DR, which could bias the results. Other factors possibly interfering with the study outcomes include the diagnostic criteria of DR and DWR, which are not the same in different studies, and the recruitment methods of the study participants. For example, most studies recruited participants from hospitals whereas some studies employed population- or community-based recruitment procedures. It should be noted that the sample size in the present study had sufficient statistical power to detect only a strong association between the ALR C(-106)T polymorphism and DR. We therefore cannot exclude the possibility of a weak association between the ALR C(-106)T polymorphism and DR in Chinese population.

Table 2. Frequencies of the C(-106)T polymorphism genotypes and alleles in the ALR gene
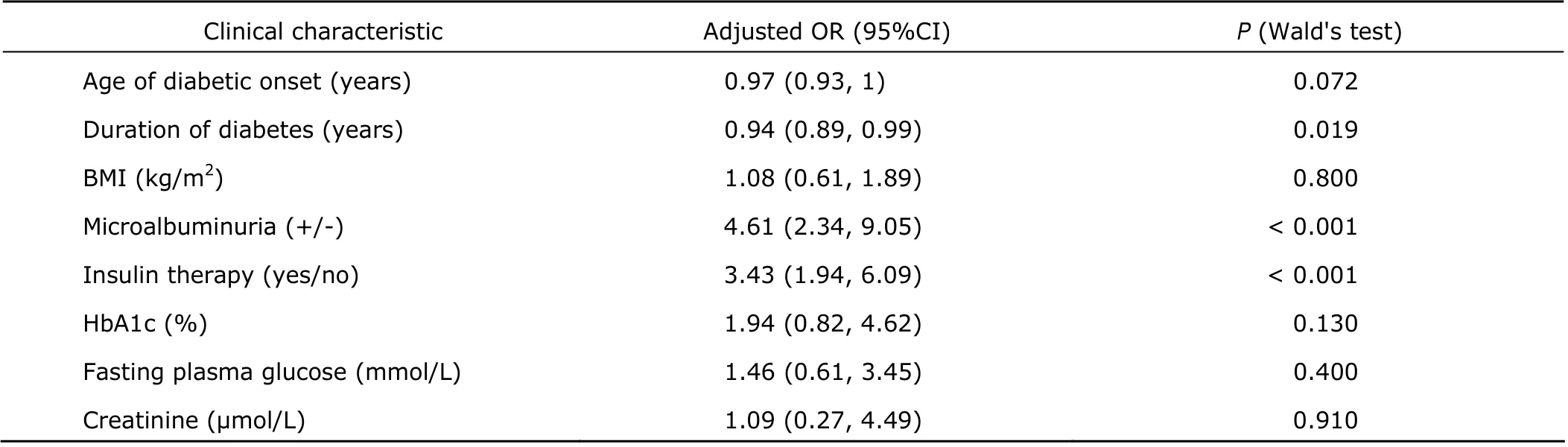
Table 3. Clinical characteristics and the risk of DR by multivariate logistic regression
Poor glycemic control and higher glycosylated hemog- lobin levels are the conventional risk factors for the development or progression of DR.31-34In the present study, we confirmed that these two factors are associated with the risk of DR. Consistent with previous findings,35,36we also found that the use of insulin was associated with a higher risk of DR. One possible explanation is that patients using insulin are more likely to have a poor glycemic control or to be at the late stage of the disease.37Poor glycemic control with oral agents is usually the primary reason to begin insulin treatment. The patients with insulin use are therefore at a higher risk of having DR. In the present study, 37.2% of the patients in the DR group had microalbuminuria. The association between DR and microalbu- minuria observed in the present study may be explained by the hypothesis that microalbuminuria might represent a state of generalized vascular dysfunction.38Furthermore, microalbu- minuria and DR may share common determinants.
In conclusion, we confirmed that patients with DR have a higher percentage of microalbuminuria (P<0.001) and are more likely to use insulin (P<0.001) compared with DWR patients. However, no evidence was observed to support a significant association of the C(-106)T polymorphism with DR in this study, suggesting that the C(-106)T polymorphism of the ALR gene might not be a major susceptibility factor of DR in Chinese patients with T2DM. Whether there is a weak association between the ALR C(-106)T polymorphism and DR in Chinese population needs to be confirmed with a larger-size sample in future studies.
1. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003; 290: 2057-60.
2. Xie X, Xu L, Yang H, et al. Frequency of diabetic retinopathy in the adult population in China: the Beijing Eye Study 2001. Int Ophthalmol 2009; 29: 485-93.
3. Hanis CL, Hallman D. Genetics of diabetic retinopathy. Curr Diab Rep 2006; 6: 155-61.
4. Demaine A, Cross D, Millward A. Polymorphisms of the aldose reductase gene and susceptibility to retinopathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci 2000; 41: 4064-8.
5. Arar NH, Freedman BI, Adler SG, et al. Heritability of the severity of diabetic retinopathy: the find-eye study. Invest Ophthalmol Vis Sci 2008; 49: 3839-5.
6. Olmos P, Futers S, Acosta AM, et al. (AC)23 [Z-2] polymorphism of the aldose reductase gene and fast progression of retinopathy in Chilean type 2 diabetics. Diabetes Res Clin Pract 2000; 47: 169-76.
7. Li Q, Xie P, Huang J, et al. Polymorphisms and functions of the aldose reductase gene 5' regulatory region in Chinese patients with type 2 diabetes mellitus. Chin Med J (Engl) 2002; 115: 209-13.
8. Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoino- sitides, and sodium-potassium-atpase in the pathogenesis of diabetic complications. N Engl J Med 1987; 316: 599-606.
9. Lewko B, Latawiec E, Maryn A, et al. Osmolarity and glucose differentially regulate aldose reductase activity in cultured mouse podocytes. Exp Diabetes Res 2011: 278963.
10. Abhary S, Burdon KP, Laurie KJ, et al. Aldose reductase gene polymorphisms and diabetic retinopathy susceptibility. Diabetes Care 2010; 33: 1834-6.
11. Katakami N, Kaneto H, Takahara M, et al. Aldose reductase c-106t gene polymorphism is associated with diabetic retinopathy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2011; 92: e57-60.
12. Ng DPK. Human genetics of diabetic retinopathy: current perspectives. J Ophthalmol 2010 [cited 2012 Jul 13]. Available from: http://www.hindawi.com/journals/joph/2010/1725 93/
13. Moczulski DK, Scott L, Antonellis A, et al. Aldose reductase gene polymorphisms and susceptibility to diabetic nephropathy in type 1 diabetes mellitus. Diabet Med 2000; 17: 111-8.
14. Neamat-Allah M, Feeney SA, Savage DA, et al. Analysis of the association between diabetic nephropathy and polymorphisms in the aldose reductase gene in type 1 and type 2 diabetes mellitus. Diabet Med 2001; 18: 906-14.
15. Wang Y, Ng MC, Lee SC, et al. Phenotypic heterogeneity and associations of two aldose reductase gene polymorphisms with nephropathy and retinopathy in type 2 diabetes. Diabetes Care 2003; 26: 2410-5.
16. Makiishi T, Araki S, Koya D, et al. C-106T polymorphism of AKR1B1 is associated with diabetic nephropathy and erythrocyte aldose reductase content in Japanese subjects with type 2 diabetes mellitus. Am J Kidney Dis 2003; 42: 943-51.
17. Sivenius K, Niskanen L, Voutilainen-Kaunisto R, et al. Aldose reductase gene polymorphisms and susceptibility to microvascular complications in type 2 diabetes. Diabet Med 2004; 21: 1325-33.
18. Sivenius K, Pihlajamäki J, Partanen J, et al. Aldose reductase gene polymorphisms and peripheral nerve function in patients with type 2 diabetes. Diabetes Care 2004; 27: 2021-6.
19. Gosek K, Moczulski D, Zukowska-Szczechowska E, et al. C-106t polymorphism in promoter of aldose reductase gene is a risk factor for diabetic nephropathy in type 2 diabetes patients with poor glycaemic control. Nephron Exp Nephrol 2005; 99: e63-7.
20. Kao YL, Donaghue K, Chan A, et al. A novel polymorphism in the aldose reductase gene promoter region is strongly associated with diabetic retinopathy in adolescents with type 1 diabetes. Diabetes 1999; 48: 1338-40.
21. Olmos P, Bastías MJ, Vollrath V, et al. C(-106)T polymorphism of the aldose reductase gene and the progression rate of diabetic retinopathy. Diabetes Res Clin Pract 2006; 74: 175-82.
22. Santos KG, Tschiedel B, Schneider J, et al. Diabetic retinopathy in Euro-Brazilian type 2 diabetic patients: relationship with polymorphisms in the aldose reductase, the plasminogen activator inhibitor-1 and the methylene- tetrahydrofolate reductase genes. Diabetes Res Clin Pract 2003; 61: 133-6.
23. Richeti F, Noronha RM, Waetge RT, et al. Evaluation of AC(n) and C(-106)T polymorphisms of the aldose reductase gene in Brazilian patients with dm1 and susceptibility to diabetic retinopathy. Mol Vis 2007; 13: 740-5.
24. Chaturvedi N, Sjoelie AK, Svensson A, et al. The Diabetic Retinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. J Renin Angiotensin Aldosterone Syst 2002; 3: 255-61.
25. Suganthalakshmi B, Anand R, Kim R, et al. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis 2006; 12: 336-41.
26. Abel K, Reneland R, Kammerer S, et al. Genome-wide snp association: identification of susceptibility alleles for osteoarthritis. Autoimmun Rev 2006; 5: 258-63.
27. Liu S, Chen HD, Makarevitch I, et al. High-throughput genetic mapping of mutants via quantitative single nucleotide polymorphism typing. Genetics 2010; 184: 19-26.
28. Fanelli A, Hadjadj S, Gallois Y, et al. Polymorphism of aldose reductase gene and susceptibility to retinopathy and nephropathy in caucasians with type 1 diabetes. Arch Mal Coeur Vaiss 2002; 95: 701-8.
29. Watarai A, Nakashima E, Hamada Y, et al. Aldose reductase gene is associated with diabetic macroangiopathy in Japanese type 2 diabetic patients. Diabet Med 2006; 23: 894-9.
30. So WY, Wang Y, Ng MC, et al. Aldose reductase genotypes and cardiorenal complications: an 8-year prospective analysis of 1,074 type 2 diabetic patients. Diabetes Care 2008; 31: 2148-53.
31. dos Santos KG, Canani LH, Gross JL, et al. The -106CC genotype of the aldose reductase gene is associated with an increased risk of proliferative diabetic retinopathy in Caucasian-Brazilians with type 2 diabetes. Mol Genet Metab 2006; 88: 280-4.
32. Looker HC, Krakoff J, Knowler WC, et al. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in pima indians. Diabetes Care 2003; 26: 320-6.
33. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001; 44: 156-63.
34. van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol 2003; 121: 245-51.
35. Chorny A, Lifshits T, Kratz A, et al. Prevalence and risk factors for diabetic retinopathy in type 2 diabetes patients in Jewish and Bedouin populations in southern Israel. Harefuah 2011; 150: 906-10, 935.
36. Thomas RL, Dunstan F, Luzio SD, et al. Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for wales: retrospective analysis. BMJ 2012; 344: e874.
37. U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249-58.
38. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32: 219-26.
 Chinese Medical Sciences Journal2014年1期
Chinese Medical Sciences Journal2014年1期
- Chinese Medical Sciences Journal的其它文章
- Sorafenib in Liver Function Impaired Advanced Hepatocellular Carcinoma
- Isolated Pancreatic Tuberculosis in Non-immunocompromised Patient Treated by Whipple’s Procedure: a Case Report
- Reversible Posterior Leukoencephalopathy Syndrome in Children with Nephrotic Syndrome: a Case Report
- Laryngo-tracheobronchial Amyloidosis: a Case Report and Review of Literature
- Comparison of the Outcomes of Monopolar and Bipolar Radiofrequency Ablation in Surgical Treatment of Atrial Fibrillation
- Bloodstream Infection with Carbapenem-resistant Klebsiella Pneumoniae and Multidrug-resistant Acinetobacter Baumannii: a Case Report
