A 3-D Metal-organic Framework Mn(II)Complex with Ant Net Constructed from 3-Pyridin-4-yl-benzoic Acid①
TANG Long FU Feng HOU Xiang-Yang WANG Ji-Jiang WANG Zao
(Yan'an University, Department of Chemistry and Chemical Engineering, Shaanxi Key Laboratory of Chemical Reaction Engineering, Yan'an, Shaanxi 716000, China)
1 INTRODUCTION
Current interest in coordination polymers based on the assembly of metal ions and multifunctional organic ligands is rapidly expanding, owing to their intriguing topological architectures and potential applications as functional solid materials[1,2]. Recently, large numbers of metal-organic frameworks(MOFs) have been obtained, and many of them exhibit versatile physical and chemical properties[3,4].Nevertheless, the rational design and synthesis of MOFs with unique structure and specific function still remains a long-term challenge. The topological analysis of MOFs has been a topical research area,and is not only an important tool for simplifying complicated compounds but also plays an instructive role in the rational design of functional materials.The topological types found in three-dimensional(3D) MOFs are commonly defined by the vertices(metal ions and/or ligands) and edges (links between vertices), among which three-, four-, five-, six-,seven- and eight-connected topologies are observed in many reported polymers[4,5]. Although some MOFs with mixed-connected topologies have been reported, such as Pt3O4, boracite, twisted boracite,PtS, rutile, pyrite, anatase, etc. Up to now, MOFs with high-mixed connectivity, like (3,6)-, (3,9)-,(4,8)- and (6,8)-connected architectures, are still quite scarce[6,7].
The key steps in building MOFs are to rationally design appropriate ligands and to choose metal ions with suitable coordination geometries[8]. In the synthetic design of MOFs, the asymmetrical bridging ligands (pyridine carboxylic acids) containing N- or/and O-donors have been used widely[9]. In our previous work, several compounds with pyridine carboxylic acids have been reported[10-13]. To continue our research, a multidentate pyridine-benzoate acid, 3-pyridin-4-yl-benzoic acid (3,4-Hpybz), was employed in the self-assembly. Herein, we report a new 3D coordination polymer, 1, which shows a 3D(3,6)-connected ant net with (42.6)2(44.62.88.10)topology. Moreover, the thermogravimetric analysis and magnetic properties of 1 were also investigated.
2 EXPERIMENTAL
2.1 Materials and methods
All commercially available solvents and starting materials were used as received without further purification. The FT-IR spectra were recorded from KBr pellets in the range of 4000~400 cm–1on a Bruker EQUINOX-55 spectrometer. Elemental analysis was determined with an Elementar Vario EL III elemental analyzer. Thermogravimetric analyses(TGA) were performed under nitrogen using a NETZSCH STA 449C thermogravimetric analyzer at a heating rate of 10 ℃·min–1. X-ray powder diffraction (XRPD) was carried out on a Shimadzu XRD-7000 analyzer. Variable temperature magnetic susceptibility was measured on an Oxford Maglab 2000 magnetometer with an applied field of 10 kOe.Diamagnetic correction was estimated from Pascal’s constants.
2.2 Computational details
All calculations have been processed in Gaussian 03(version 6.1) package[14]. The geometrical optimization was carried out with the hybrid DFT method on the basis of B3LYP functional[15]. The magnetic isotropic shielding tensors were also calculated using the B3LYP/6-31G(d) approach. The experimentally determined geometries for the complete structure of complex 1 were used for the calculation of magnetic exchange coupling constants. Neither variation of the geometrical parameters nor the geometry optimization[16]was attempted in this calculation because a small variation in the geometry can have a big effect on the calculated magnetic interaction parameters.
2.3 Synthesis of [Mn(3,4-pybz)2]·3(H2O) (1)
An aqueous solution (10 mL) containing 3-pyridin-4-yl-benzoic acid (0.10 mmol, 0.020 g) and MnCl2·4H2O (0.10 mmol, 0.020 g) was placed in a Parr Teflon-lined stainless steel vessel (25 mL)under autogenous pressure, which was heated to 160℃ for 5 d and subsequently cooled to room temperature at a rate of 5 ℃/h. Colorless block crystalline products were obtained (yield: 26% based on 3,4-Hpybz). Elemental analysis. calcd for C24H22N2O7Mn: C, 57.04, H, 4.38; N, 5.54%. Found:C, 57.56; H, 4.36; N, 5.82%. IR (KBr pellet, cm–1):3484 b, 1626 s, 1432 s, 1378 vs, 1199 m, 1046 m,814 m, 776 vs, 684 vs, 576 m.
2.4 Crystal structure determination
A single crystal with dimensions of 0.36mm ×0.24mm × 0.16mm was mounted on a glass fiber and the data were collected on a Bruker SMART APEX II CCD diffractometer equipped with a graphite-monochromatic MoKα radiation (λ =0.71075 Å) at 296(2) K by using an ω-φ scan mode.Absorption corrections were applied by using multi-scan program SADABS[17]. The structure was solved by direct methods with SHELXS-97 and refined with full-matrix least-squares technique using the SHELXL program package[18,19]. Anisotropic thermal parameters were applied to all of the non-hydrogen atoms. The hydrogen atoms were assigned with common isotropic displacement factors and included in the final refinement by use of geometrical restrains. A total of 6829 reflections for complex 1 were collected in the range of 2.73≤θ≤27.100 (–15≤h≤24, –19≤k≤17, –10≤l≤10) and 2588 were independent with Rint= 0.0114, of which 2426 with I > 2σ(I) (refinement on F2) were obser-ved and used in the succeeding structure calculation.The final R = 0.0275, wR = 0.0817 (w = 1/[σ2(Fo2) +(0.0550P)2+ 1.5000P], where P = (Fo2+ 2Fc2)/3), S= 1.006, (Δ/σ)max= 0.001, (Δρ)max= 0.288 and(Δρ)min= –0.284 e/Å3. Selected bond distances and bond angles are listed in Table 1.

Table 1. Selected Bond Lengths (Ǻ) and Bond Angles (°) for Complex 1
3 RESULTS AND DISCUSSION
3.1 Crystal structure of complex 1
Single-crystal X-ray diffraction analysis suggests that the asymmetric unit of complex 1 consists of one Mn(II) ion, two 3,4-pybz anions and three free water molecules. Each Mn(II) center is six-coordinated by two pyridyl nitrogen donors (Mn–N =2.3278(11) Å) and four carboxylate oxygen atoms coming from different 3,4-pybz ligands (Mn–O =2.1404(10) and 2.1613(9) Å), forming a distorted MnN2O4octahedral geometry (Fig. 1). The Mn–O(carboxylate)and Mn–N(bipy)bond lengths are in agreement with those in carboxylate- and bipy-containing manganese(II) complexes[20]. The O/N–Mn–O/N bond angles are in the range of 83.74(4)~173.89(4)°. In this structure, the 3,4-pybz ligand adopts a μ3-unidentate (Npy)/bidentate bridging(OCOO-) coordination mode (μ3-κ1-N:κ1-O:κ1-O see Scheme 1). The phenyl and pyridyl rings are not coplanar, with the dihedral angle of 36.39° in the 3,4-pybz ligand. In virtue of the bridging roles of carboxylate, two adjacent Mn(II) centers are combined to constitute an eight-membered ring, with the M···Mn separation of 4.888 Å (Fig. 2). Two different directional eight-membered rings are alternated with each other, leading to a 1D eight-membered ring chain along the c axis. The adjacent chains are interlinked by 3,4-pybz ligands to result in a 3D metal-organic framework (Fig. 3a). From the pers- pective of net topology, the 3,4-pybz ligands in this 3D structure serve as a three-connected node,and each Mn(II) center acts as a six-connected node,linking to six 3-connected 3,4-pybz. Thus, a binodal(3,6)-connected ant network with the Schläfli symbol of (42.6)2(44.62.88.10) is constituted[21](Fig. 3b).

Scheme 1. Coordination modes of 3,4-pybz ligands (μ3-κ1-N:κ1-O:κ1-O)

Fig. 1. Coordination environment of Mn(II) in complex 1 with 50% thermal ellipsoids.All hydrogen atoms and free water molecules are omitted for clarity

Fig. 2. 1D loop chain of complex 1 along the c axis
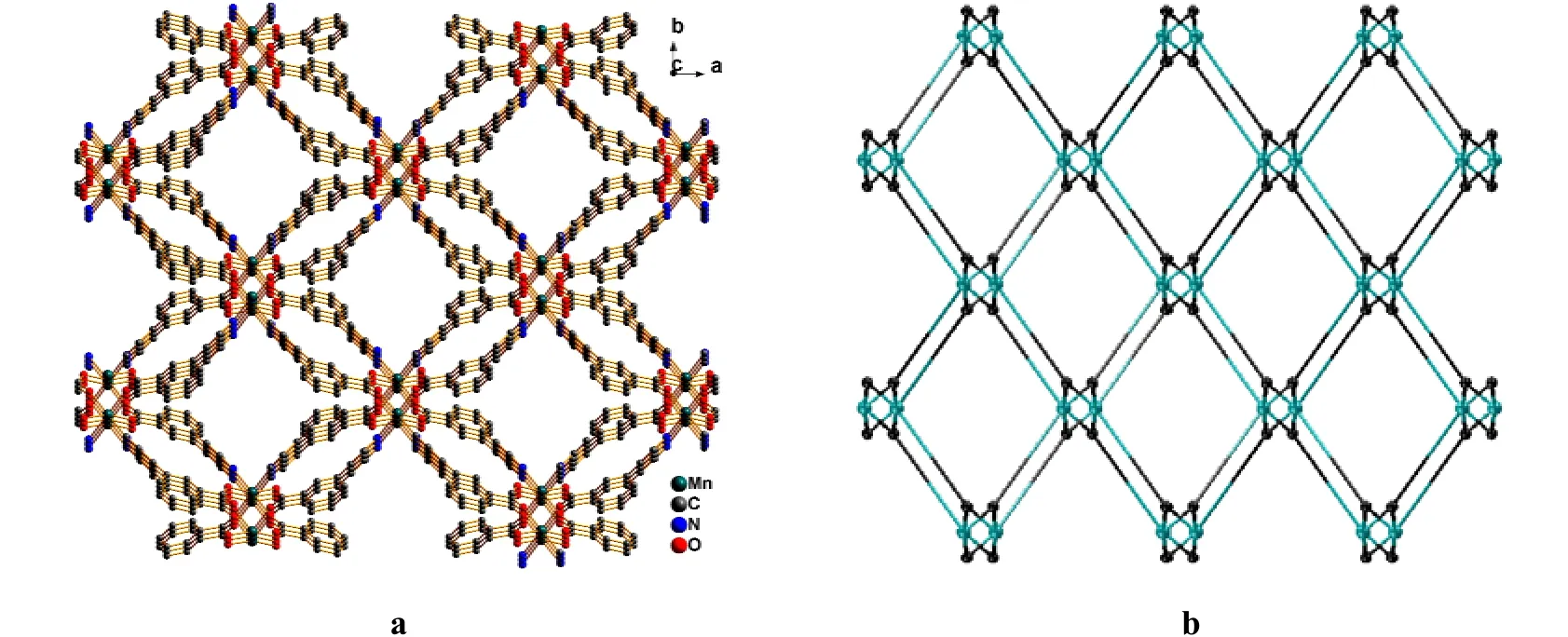
Fig. 3. 3D metal-organic framework (3a) and (3,6)-connected ant network (3b) of complex 1
There were some pyridine carboxylic acid complexes reported with structures based on 3-pyridin-4-yl-benzoic acid and the transition metal cations(Cd(II), Zn(II), Co(II), Ni(II), Cu(II), etc)[10-12]. Five different coordination polymers show interesting supramolecular patterns of unique double-stranded clasp for Cd(II), (3,5,6)-connected 2D helical tubular double layer for Zn(II), 2-fold interpenetrating cds 3D network for Co(II)/Ni(II), and 2D 2-fold interpenetrated framework for Cu(II), respectively.However, complex 1 exhibits a 3D binodal (3,6)-connected ant network in this text. Their structural difference should be ascribed to the transition metal ions used in the assembled processes.
3.2 PXRD and thermogravimetric analysis
X-ray powder diffraction (PXRD) was used to confirm the phase purity of bulk materials of 1 at room temperature (Fig. 4). Although the experimental patterns show several slightly broadened diffraction peaks in comparison to those simulated from the single-crystal data, it can still be regarded that the bulk as-synthesized materials represent the pure phases of complex 1. To examine the thermal stability of complex 1, thermal gravimetric (TG)analyses were carried out for 1 between 20 and 700℃ (Fig. 5). The samples were heated up under a static air atmosphere with a heating rate of 10℃·min–1. The TG curve indicates that the weight loss of the complex can be divided into two steps.The first weight loss is 10.1% from 120 to 160 ℃,corresponding to the removal of two water molecules in the complex (calcd. 10.69%). The second weight loss occurs between 270 to 480 ℃, giving manganese oxides as the final decomposition product which constitutes 14.2% (Calcd. 13.93 %). The residue of MnO was confirmed by X-ray powder diffraction analysis.

Fig. 4. PXRD pattern of complex 1

Fig. 5. TG curve of complex 1
3.3 IR spectra
The significant bands in IR spectra of complex 1 show broad peaks at 3484 cm–1which could be attributed to OH of water. The characteristic bands of carboxyl groups at 1626 and 1432 cm–1are assigned to the carboxylate group asymmetric and symmetric stretching vibrations. The separation value between vasym(CO2) and vsym(CO2) indicates that the carboxylate group coordinates in a bis-monodentate (194 cm–1) fashion[22], which is confirmed by the X-ray analysis.
3.4 Magnetic properties
The magnetic properties of complex 1 were investigated over the temperature range of 3~300 K in a field of 10 kOe. The magnetic susceptibilities χMand χMT versus T plots are shown in Fig. 6. For complex 1, the experimental χMT value at 300 K is 8.799 cm3·K·mol–1, slightly larger than the spin-only value(8.750 cm3·K·mol–1) expected for the spin-only Mn(II) ion (S = 5/2). The χMT value of 1 remains almost constant from 300 to 75 K, and then decreases on further cooling, reaching a value of 1.582 cm3·K·mol–1at 3 K. This behavior indicates a dominant antiferromagnetic interaction between the Mn(II) ions in the structure. The temperature dependence of the reciprocal susceptibilities (1/χM) obeys the Curie-Weiss law above 3 K with θ = – 9.24 K, C= 7.56 and R = 1.241 × 10–5. The values of θ for 1 indicate weak antiferromagnetic interactions between the adjacent Mn(II) ions.
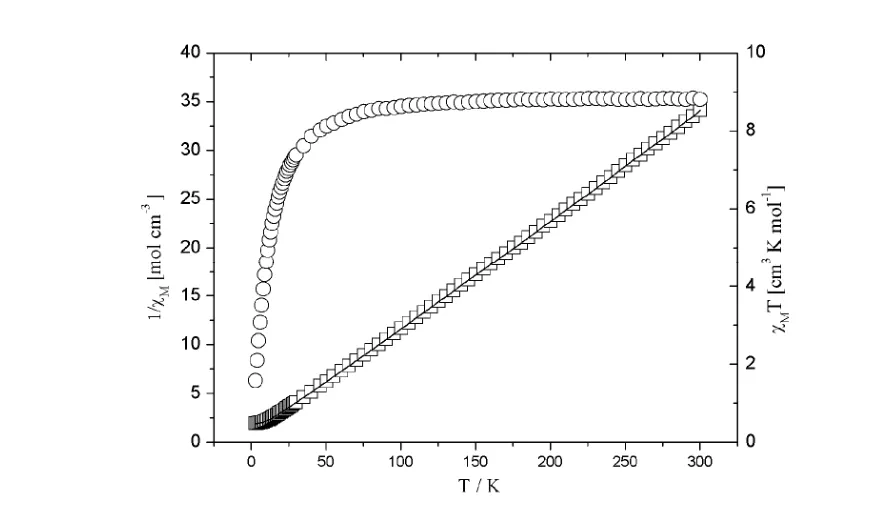
Fig. 6. Thermal variation of χMT and 1/ χM for complex 1 (○, χMT experimental values; □, 1/ χM experimentalvalues and solid lines, theoretical values)
3.5 Theoretical calculation
3. 5. 1 Optimized geometry structure
Complex 1 was a 3D structure, and theoretical calculations would be a difficult task for such a large periodical system. Here, the asymmetric unit Mn-(3,4-pybz)2(3,4-Hpybz)4of 1 was intercepted, then the geometry of the unit was optimized by the DFT method with the B3LYP functional. The main bond lengths and bond angles for 1 in the opti- mization structure are shown in Table 1. The Mn–O and Mn–N bond lengths calculated are slightly longer than those in experiment, and the Δcal.-exp.(the difference between the calculated and experimental values) of the bond lengths is ranging from 0.001 to 0.005 Å. The highest deviation is about 0.55° for the bond angles in complex 1. There is a little deviation between the calculated and experimental values probably due to the following reasons: the approximation of calculation methods and basis set, the neglect of anionic effect in the course of calculation and the chemical environmental difference of the complex. The deviation can be accepted in theoretical calculation for a big system.
3. 5. 2 Magnetic properties of DFT calculations
The DFT calculations have been widely proved to be one of the most efficient tools to investigate the magnetic structure of transition metal complexes[23]. The magnetic interaction between two paramagnetic centers with local spin operatorscan be written in a spin Hamiltonian suggested originally by Heisenberg et al[24,25].
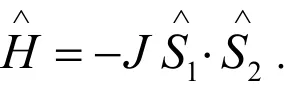
In this study, the broken symmetry (BS) formalism, proposed by Noodlemann et al[26], was used. For the present systems with two unpaired electrons on the magnetic centers (MnIIMnII, where S1= S2= 5/2), the coupling constant (J) can be defined by[27]

where EBSand EHSare the energy of the broken symmetry state and the high-spin state (HS) (EBS=–6117.359153 a.u. and EHS= –6117.359869 a.u.).We have used approach via Eq. (1) to estimate the magnetic coupling constants (J = –6.29 cm-1) of complex 1. The computed J values (J < 0) of complex 1 predict antiferromagnetic ground state with a BS-HS splitting, and the results agreed with the experimental data (θ).
4 CONCLUSION
Using the 3-pyridin-4-yl-benzoic acid, a new coordination polymer [Mn(3,4-pybz)2]n·3(H2O) (1),was prepared and characterized crystallographically and magnetically. Complex 1 exhibits a 3D metalorganic framework with (3,6)-connected ant net topology. Furthermore, theoretical calculation results also demonstrate the rationality of crystal structures,and the magnetic behavior of 1 was analyzed by experiments and calculations, which show all the antiferromagnetic behaviors.
ACKNOWLEDGEMENT The authors thank Professor Wang Wen-Liang of Shaanxi Normal University for providing Gauss calculation Software.
(1) Li, D. S.; Zhao, J.; Wu, Y. P.; Liu, B.; Bai, L.; Zou, K.; Du, M. Co5/Co8-cluster-based coordination polymers showing high-connected self-penetrating networks: syntheses, crystal structures, and magnetic properties. Inorg. Chem. 2013, 52, 8091-8091.
(2) Ma, L. F.; Han, M. L.; Qin, J. H.; Wang, L. Y.; Du, M. MnIIcoordination polymers based on bi-, tri-, and tetranuclear and polymeric chain building units: crystal structures and magnetic properties. Inorg. Chem. 2012, 51, 9431-9442.
(3) Li, J. R.; Kuppler, R. J.; Zhou, H. C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477-1504.
(4) Li, D. S.; Wu, Y. P.; Zhao, J.; Zhang, J.; Lu, J. Y. Metal-organic frameworks based upon non-zeotype 4-connected topology. Coord. Chem. Rev. 2014,261, 1-27.
(5) Zhang, Y. B.; Zhang, W. X.; Feng, F. Y.; Zhang, J. P.; Chen, X. M. A highly connected porous coordination polymer with unusual channel structure and sorption properties. Angew. Chem. Int. Ed. 2009, 48, 5287-5290.
(6) Jiang, X. J.; Du, M.; Sun, Y.; Guo, J. H.; Li, J. S. Three-dimensional (3-D) metal-organic frameworks with 3-pyridin-4-yl-benzoate defining new (3,6)-connected net topologies. J. Solid State Chem. 2009, 182, 3211-3214.
(7) Zhang, X. M.; Zheng, Y. Z.; Li, C. R.; Zhang, W. X.; Chen, X. M. Unprecedented (3,9)-connected (42.6)3(46.621.89) net constructed by trinuclear mixed-valence cobalt clusters. Cryst. Growth Des. 2007, 7, 980-983.
(8) Zeng, M. H.; Wang, Q. X.; Tan, Y. X.; Hu, S.; Zhao, H. X.; Long, L. S.; Kurmoo. M. Rigid pillars and double walls in a porous metal-organic framework: single-crystal to single-crystal, controlled uptake and release of iodine and electrical conductivity. J. Am. Chem. Soc. 2010, 132,2561-2563.
(9) Fu, F.; Li, D. S.; Wu, Y. P.; Gao, X. M.; Du, M.; Tang, L.; Zhang, X. N.; Meng, C. X. A versatile V-shaped tetracarboxylate building block for constructing mixed-ligand Co(II) and Mn(II) complexes incorporating various N-donor co-ligands. CrystEngComm. 2010, 12, 1227-1237.
(10) Li, D. S.; Tang, L.; Fu, F.; Du, M.; Zhao, J.; Wang, N.; Zhang, P. Coordination assemblies of CdII/ZnII/CoIIwith the 3-(pyridin-4-yl) benzoate tecton:structural diversity and properties. Inorg. Chem. Commun. 2010, 13, 1126-1130.
(11) Tang, L.; Fu, F.; Gao, L. J.; Wu, Y. P.; Liu, Q. R.; Gao, X. M. A 3D nickel(II) coordination polymer with cds nets constructed from 3-pyridin-4-yl-benzoic acid. Z. Anorg. Allg. Chem. 2011, 637, 608-612.
(12) Tang, L.; Fu, F.; Gao, L. J.; Wei, Q. B.; Zhang, Z. L.; Liu, Q. R. Synthesis, crystal structure, and magnetic properties of a new 2D twofold interpenetrated coordination polymer [Cu(3,4-pybz)2]n.. Z. Anorg. Allg. Chem. 2013, 639, 918-921.
(13) Tang, L.; Wu, Y. P.; Fu, F.; Zhang, P.; Wang, N.; Gao, L. F. A 3-D cobalt(II)-coordination polymer with mixed-connected network topology constructed from 4-pyridin-3-yl-benzoic acid. J. Coord. Chem. 2010, 63, 1873-1881.
(14) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, Jr. J. A.; Vreven, T.; Kudin, K. N.; Burant,J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada,M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.;Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.;Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M.C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.;Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.;Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian (version 6.1), Inc., Wallingford CT 2004.
(15) Becke, A. D. Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J. Chem. Phys. 1997, 107,8554-8554.
(16) Ruiz, E.; Rodríguez-Fortea, A.; Tercero, J.; Cauchy, T.; Massobrio, C. Exchange coupling in transition-metal complexes via density-functional theory:comparison and reliability of different basis set approaches. J. Chem. Phys. 2005, 123, 074102-074102.
(17) Sheldrick, G. M. SADABS, A Program for Empirical Absorption Correction of Area Detector Data. University of Göttingen, Germany 1997.
(18) Sheldrick, G. M. SHELXS 97, Program for Crystal Structure Solution. University of Göttingen, Germany 1997.
(19) Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure. University of Göttingen, Germany 1997.
(20) Guo, F. Synthesis and crystal structures of pH-dependent Mn(II) coordination polymers with 3-pyrid-3-ylbenzoic acid. J. Coord. Chem. 2009, 62,3606-3612.
(21) Zou, J. P.; Peng, Q.; Wen, Z. H.; Zeng, G. S.; Xing, Q. J.; Guo, G. C. Two novel metal-organic frameworks (MOFs) with (3,6)-connected net topologies: syntheses, crystal structures, third-order nonlinear optical and luminescent properties. Cryst. Growth Des. 2010, 10, 2613-2619.
(22) Wu, Y. P.; Li, D. S.; Fu, F.; Dong, W. W.; Tang, L.; Wang, Y. Y. 3D PbII-coordination framework based on rod-shaped Pb–O–Pb SBUs defining a new(4,5)-connected net topology. Inorg. Chem. Commun. 2010, 13, 1005-1008.
(23) Tang, L.; Fu, F.; Wang, W. L.; Li, D. S.; Wu, Y. P.; Gao, X. M.; Yang, X. G. Two novel 3D hydrogen-bonded architectures constructed from maleic acid and N-donor ligands: structures, magnetic properties and theoretical studies. Chin. J. Chem. 2009, 27, 273-280.
(24) Dirac, P. A. M. Quantum mechanics of many-electron systems. Proc. Roy. Soc. London 1929, 123, 714-733.
(25) Van Vleck, J. H. Theory of Electric and Magnetic Susceptibilities. Oxford University Press, London 1932.
(26) Ruiz, E.; Cano, E.; Alvare, S.; Alemany, P. Broken symmetry approach to calculation of exchange coupling constants for homobinuclear and heterobinuclear transition metal complexes. J. Comp. Chem. 1999, 20, 1391-1400.
(27) Beghidja, C.; Rogez, G.; Kortus, J.; Wesolek, M.; Welter, R. Theoretical studies of magnetic interactions in Mn(II)(hfac)2{di(4-pyridyl)phenylcarbene} and Cu(II)(hfac)2{di(4-pyridyl)-phenylcarbene}. J. Am. Chem. Soc. 2006, 128 , 3140-3141.

