Synthesis, Crystal Structure and Catalytic Properties of a New 2D Nickel(II) Coordination Polymer Based on Flexible Bis(benzimidazole)①
YANG Rui GE Ming VAN HECKE Kristof CUI Gung-Hu②
a (College of Chemical Engineering, Hebei United University, Tangshan, Hebei 063009, China)
b (Department of Inorganic and Physical Chemistry, Ghent University,Krijgslaan 281 S3, B-9000 Ghent, Belgium)
1 INTRODUCTION
Metal Organic Framworks (MOFs) have been of great interest in the field of supramolecular chemistry and crystal engineering because of their large numbers of promising applications in many areas,such as luminescence, catalysis, gas storage, ion exchange and so on, as well as for their diverse architectures and topologies[1-4]. However, it is still a challenge to predict the desirable coordination architecture. Some factors, such as the geometrical and electronic properties of organic ligands and central metal ions, temperature, pH value of solution,solvent, hydrogen bonding and π-π stacking interactions also have an efficient effect on assembling the final structure[5-7]. The selection of suitable linkers is the key step because the configuration, length and substituent groups of organic ligands may influence the structures and properties of the resulting MOFs[8-11]. Complexes based on benzimidazole ligands have attracted increasing attention in recent years. In this context, flexible bis(benzimidazole)derivatives are excellent building blocks, owing to their interesting characteristics that include strong coordination ability, rich coordination modes,different organic skeletons, π-π stacking interactions,and diverse conformations according to the restrictions imposed by the coordination geometry of metal ions[12,13]. However, MOFs based on flexible bis-(benzimidazole) with thiocyanate as co-ligands have rarely been reported when searching the Cambridge Structural Database (version 5.35, Feb 2014)[14]. As part of our ongoing work, herein we report the synthesis, structures and characterizations of a first Ni(II) coordination polymers constructed based on flexible bis(benzimidazole) and thiocya- nate ligands,[Ni(L)2(SCN)2]n. Furthermore, the fluorescence,thermal gravimetry and catalytic properties of the title complex are discussed in detail.
2 EXPERIMENTAL
2.1 Materials and physical measurements
All the reagents and solvents for synthesis were obtained from commercial sources and used directly without further purification. The ligand L was prepared according to the previously reported literature method[15]. Elemental analyses of C, H and N were obtained on a Perkin-Elmer 240C automatic analyzer.IR spectra were recorded on an Avatar 360 (Nicolet)spectrophotometer in the 4000~400 cm-1region using KBr pellets with a resolution of 2 cm-1. The TG-DTA measurements were performed on a NETZSCH TG 209 thermal analyzer from room temperature to 800 ℃ under N2atmosphere at a heating rate of 10 ℃/min. The luminescence spectra for the powdered solid samples were performed with a Hitachi F-7000 spectrophotometer at room temperature.
2.2 Synthesis of [Ni(L)2(SCN)2]n
A mixture of NiCl2·6H2O (0.1 mmol, 23.7 mg), L ligand (0.1 mmol, 29.0 mg) and KSCN (0.1 mmol,9.7 mg) was dissolved in 10 mL of distilled water and stirred for 0.5 h. The resulting mixture was sealed in a Teflon-lined stainless steel vessel and heated to 140 ℃ for 3 d under autogenous pressure,and then cooled down to room temperature at a rate of 10 ℃/h. Light green block crystals of the complex were obtained by filtration, washed with distilled water, and dried at ambient temperature in the yield of 54% based on NiCl2·6H2O. Anal. Calcd.(%) for C38H36N10NiS2: C, 60.41; H, 4.80; N, 18.54.Found (%): C, 60.11; H, 4.58; N, 18.72. IR (KBr,cm–1): 3106w, 2937m, 2860w, 2081s, 1615m, 1513s,1462m, 1386m, 1335m, 1293m, 1191m, 937w, 861w,751s, 623w.
2.3 Catalysis experiments
The catalytic activity was studied according to our previous reported literature method[16]. The catalytic experiments were carried out using a 100 mL round-bottomed flask with 0.5 mL H2O2(30%) and 15 mg of the title complex, in which the temperature was held constant at 40 ℃. At a given interval, 0.5 mL of the reaction solution was taken out and measured by using a Shanghai Jingke 722N visible spectrophotometer with an absorption wavelength of 496 nm. Control experiments without any catalyst but with an equal molar amount of NiCl2·6H2O with Ni(II) contained in the coordination framework as catalyst have been investigated under the same conditions. The degradation efficiency of Congo red was evaluated based on the following formula[17]:
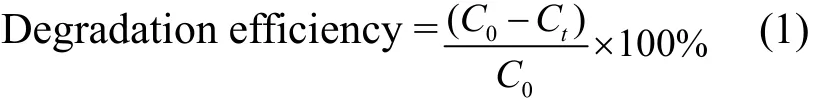
where C0(mg/L) is the initial concentration of Congo red, and Ct(mg/L) is the concentration of Congo red azo dye at reaction time t (min).
2.4 X-ray data collection and crystal structure determination
A suitable single crystal with dimensions of 0.22mm × 0.20mm× 0.19mm was collected on the top of a glass fiber with epoxy cement for the X-ray measurement. Crystallographic data for the title complex were collected at 293 K on a Bruker Smart 1000 CCD diffractometer equipped with a graphitemonochromatic Mo-Kα radiation (λ = 0.71073 Å)using an ω-2θ scan mode in the range of 2.82 < θ <27.10º. The structure was solved by direct methods using the SHELXS-97 program[18]and refined on F2by full-matrix least-squares methods with the SHELXL-97 program[19]. The non-hydrogen atoms were refined anisotropically and the hydrogen atoms were geometrically included in the final refinement. The final R = 0.0486, wR = 0.1062 (w = 1/[σ2(Fo2) +(0.0355P)2+ 9.1235P], where P = (Fo2+ 2Fc2)/3), S= 0.942, (Δρ)max= 0.748, (Δρ)min= –0.661 e·Å–3and(Δ/σ)max= 0.000. Table 1 lists the selected bond distances and bond angles.
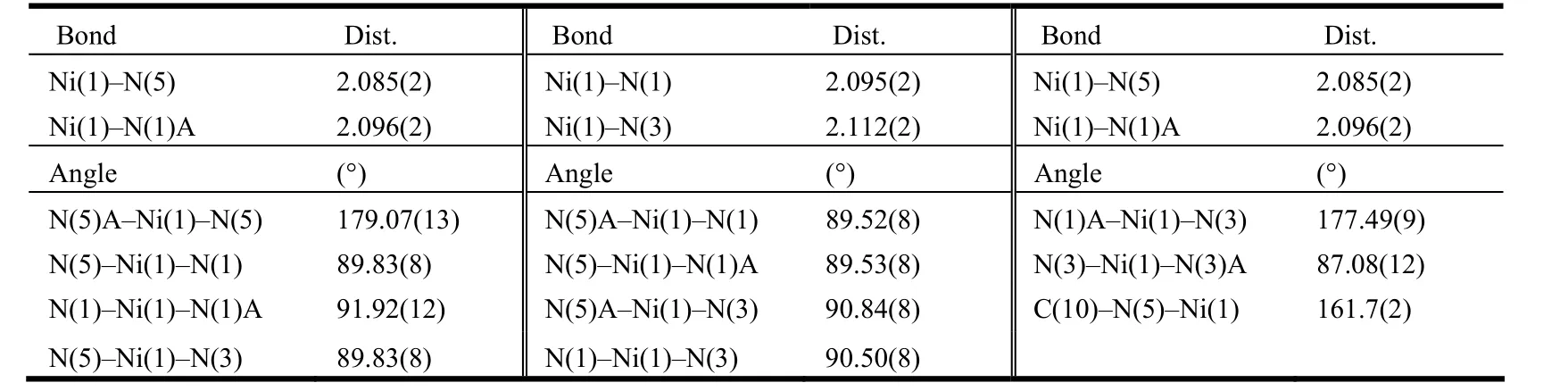
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
3 RESULTS AND DISCUSSION
3.1 Crystal structure description
X-ray crystallographic analysis revealed that the complex crystallizes in monoclinic, space group C2/c. The asymmetric unit of the complex contains one half of a Ni(II) ion, one L ligand and one SCN-counterion. As shown in Fig. 1, each Ni atom is six-coordinated, exhibiting a slightly distorted octahedral geometry, in which the equatorial plane is formed by the four N atoms (N(1), N(1A), N(3),N(3A)) from two different L ligands and the axial positions are occupied by two N atoms (N(5), N(5A))from two separated SCN–anions (symmetry code: A:1 – x, y, –z + 0.5). Remarkably, the N atoms from the SCN–ligands are nearly perpendicular to the equatorial plane, whereas the two linear SCN–anions are bent, with the angles of N(5)–Ni(1)–N(5A) and C(10)–N(5)–Ni(1) to be 179.07(13) and 161.7(2)°, respectively. The length of Ni–N(CS)(Ni(1)–N(5) = 2.085(2) Å) is shorter than those of Ni–N(L) (Ni–N = 2.095(2)~2.112(2) Å), which are comparable to those of similar nickel complexes[20].

Fig. 1. Coordination environment around the Ni atom in the complex(Symmetry codes: A: –x+1, y, –z+1/2; B: –x, y, –z+1/2; F: –x+1, –y+1, –z+1/2)
In the structure of the complex, the two SCN–groups act as terminal ligands to link the Ni(II)centers. The L ligands adopt two types of coordination conformations, namely cis- and trans-,in which the dihedral angles between the mean planes of the two benzimidazole rings are 180° and 70.08(4)°, respectively. The trans-conformation L ligands bridge the adjacent two hexa-coordinate Ni(II) centers to form a 1D zigzag chain of Ni(Ltrans)2along the c axis; Simultaneously, each cis-conformation L links these 1D chains together with a linear mode toward the a direction, generating 2D (4,4) sheets (Fig. 2). The distances between the Ni centers are 9.4760(3) Å (Ni1–NiE) and 12.9140(4)Å (Ni(1)–Ni(1D)) across the trans-conformation and cis-conformation L (symmetry codes: D = 2–x, 1–y,1–z, E = 1 + x, y, z), respectively. The angle of Ni(1C)···Ni(1)···Ni(1D) is 125.441(0)° (symmetry code: C = –x, 1–y, –z). In addition, the 2D network is further extended into a 3D supramolecular network by face-to-face π-π stacking interactions between the neighboring benzimidazole rings of the L ligands(Fig. 3), which features the centroid-to-centroid distance of 3.797(2) Å, and an inter-planar angle α is 0°.
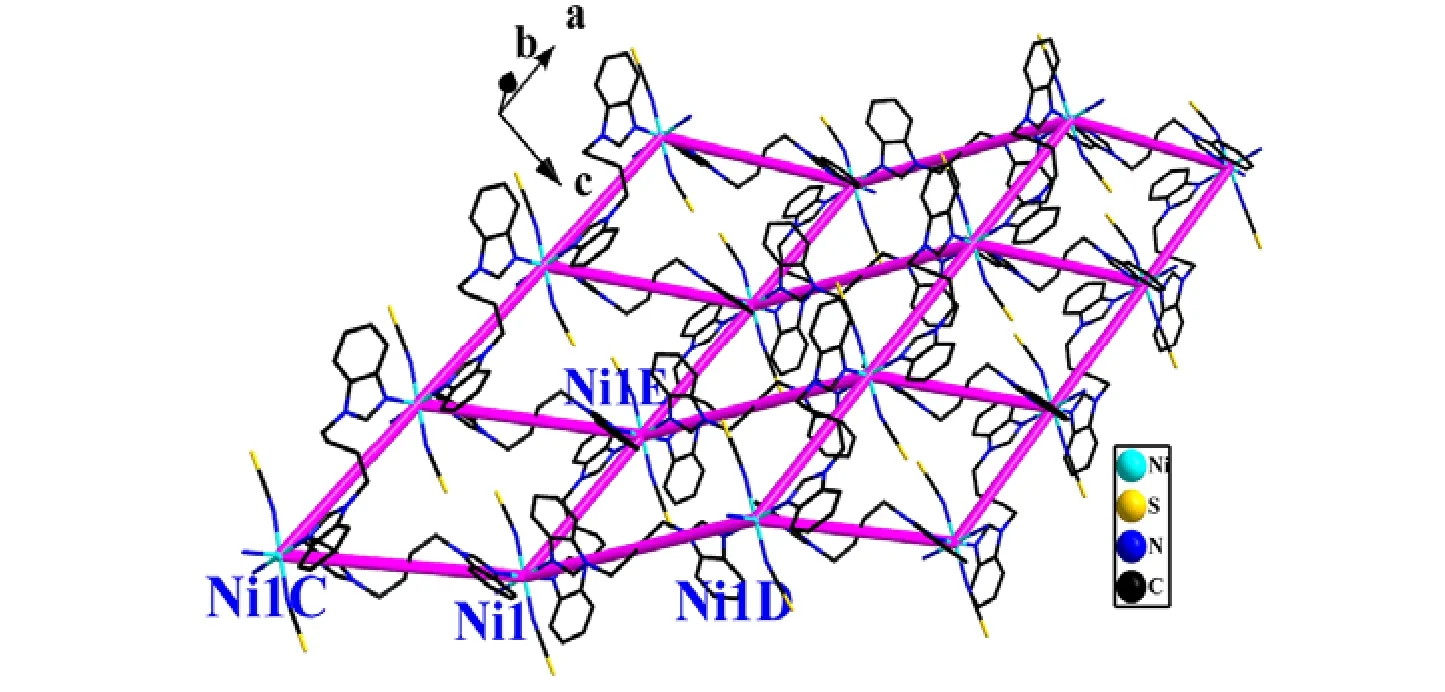
Fig. 2. 2D sheet of the complex
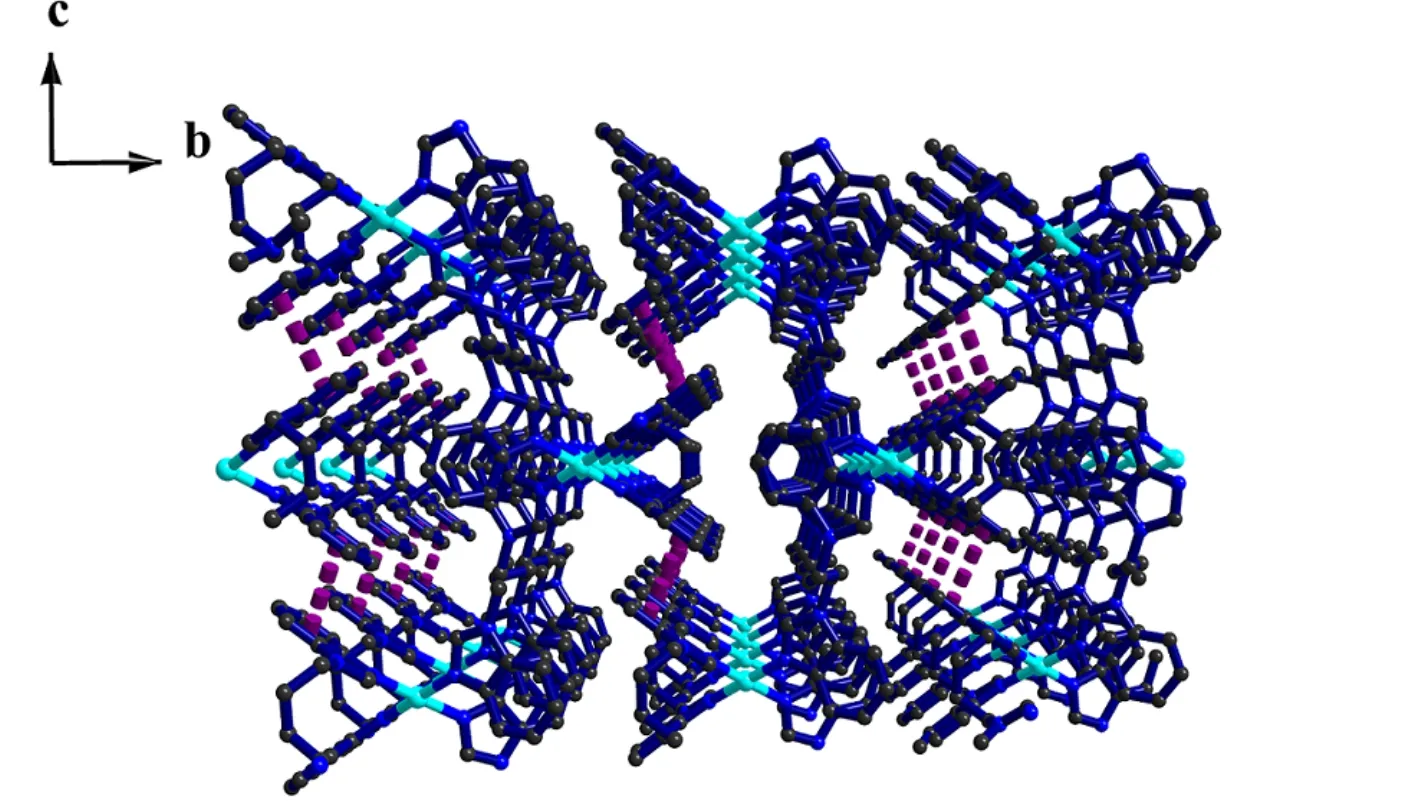
Fig. 3. 3D supramolecular network formed by π-π (dashed lines) stacking interactions of the title complex
3.2 IR spectra of the complex
In the IR spectra of the coordination polymer, the strong absorption band at 2081 cm-1is assigned to the CN stretching frequency[21]. The peak at 1513 cm-1may be attributed to ν(C=N)absorption in the imidazole ring of the L ligand. The bands at 3106 and 2937 cm-1for the title compound could be associated with Ar–H stretching vibration and CH2stretching vibration of the L ligand, respectively.
3.3 XRPD analysis and thermal analysis
In order to confirm the phase purity of the bulk materials, an X-ray powder diffraction (XRPD)experiment was performed for the title complex. It is apparent that the corresponding simulated patterns calculated from the single-crystal X-ray diffraction data and as-synthesized patterns of the complex are in good agreement (Fig. 4). There are a few unindexed diffraction differences between the measured and simulated patterns, which may be related to the different orientation of the crystals in the powdered samples.
To investigate the thermal stability of the complex,thermogravimetric analysis was carried out on a temperature gradient from room temperature to 800℃ at a heating rate of 10 ℃/min under N2atmosphere. As shown in Fig. 5, the complex remains stable up to 298 ℃ and exhibits a mass loss of 77.92% (calcd.: 76.76%) between 299 and 580 ℃,which can be assigned to the decomposition of the L ligands, and the final residual weight is 22.08%(calcd.: 23.24%), corresponding to Ni(SCN)2as the final product.

Fig. 4. X-ray powder diffraction patterns of the title compound
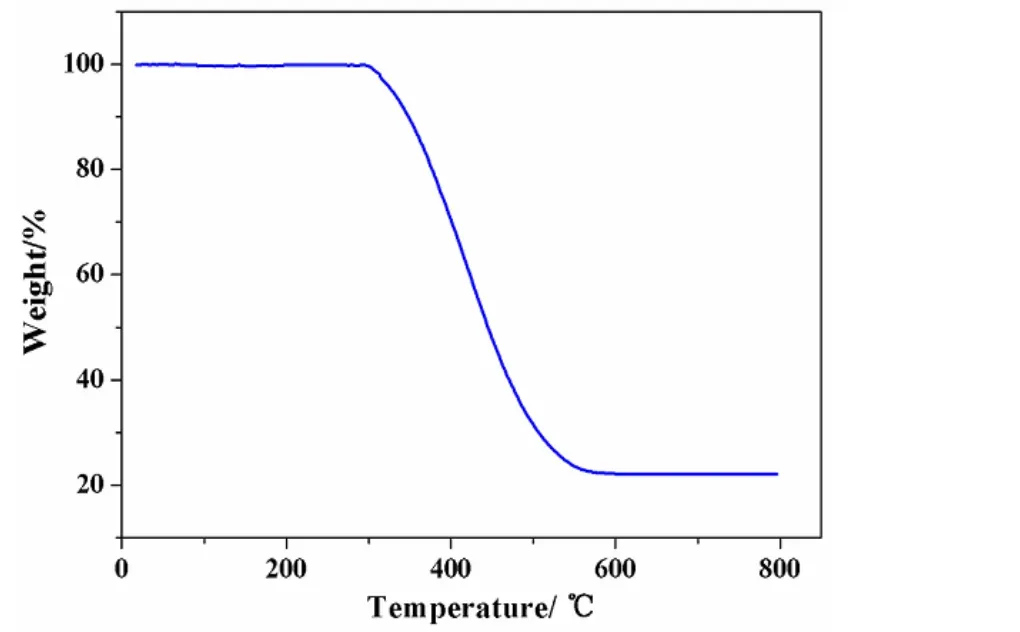
Fig. 5. TG curve of the complex
3.4 Fluorescence properties
The emission spectra of the compound as well as the free L ligand are shown in Fig. 6. The free ligand exhibits an emission peak at 405 nm upon excitation at 350 nm. It can be presumed that the peak comes from the π → π* transition[22]. For the complex, the maximal emission peak is are observed at 480 nm(λex= 349 nm). When compared with the free ligand,the emission of the coordination polymer is 75 nm red-shifted, which may be assigned to metal-toligand charge transfer[23].
3.5 Catalytic properties
Azo dyes are an important source of environmental contamination, and most of them are toxic,non-biodegradable and potentially carcinogenic in nature. Therefore, it is necessary to find an effective way to remove color from wastewater. Advanced oxidation technologies such as a Fenton-like method have drawn more attention to degrade dye wastewater[24,25]. To date, some researches have proven that the transition metal coordination complexes exhibit prominent catalytic activities in Fenton-like process[25,26]. The different catalytic performances of these metal complexes may be due to the distinct coordination environments around the metal centers or molecular structures[16]. Herein, we choose Congo red (sodium salt of benzidinediazo-bis-1-naphthlamine-4sulfonic acid) as a model dye to evaluate the catalytic effect on wastewater.
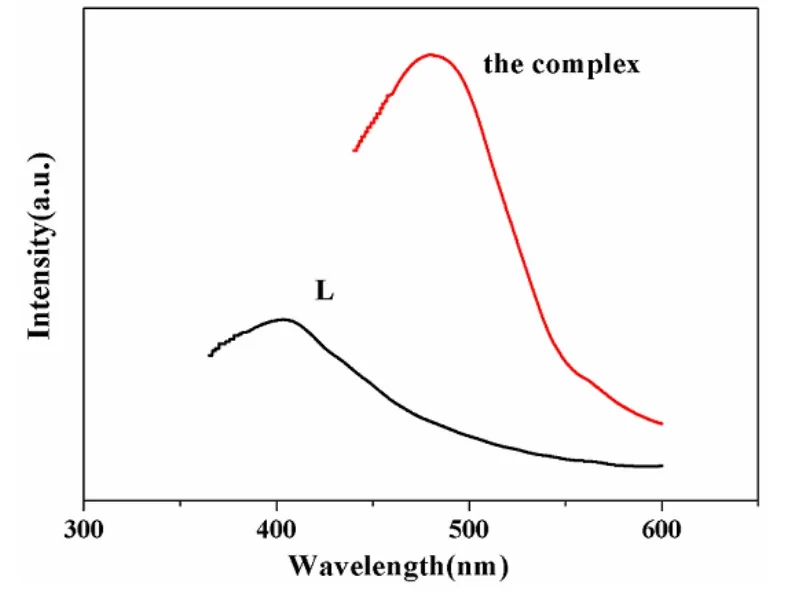
Fig. 6. Solid-state photoluminescent spectra of free L ligand and the complex
As shown in Fig. 7, when H2O2alone was added into the Congo red solution as the control experiment, clearly, there was no evident color removal of Congo red with the degradation of 12.7%, indicating that Congo red can not be effectively oxidized by hydrogen peroxide. However, when adding the title complex into the system, degradation efficiency went up to 93 % after 120 min. Since Ni(II) species may play a key role in the reaction, an experiment with an equal amount of NiCl2·6H2O with Ni(II)contained in the network as catalyst, instead of the title complex was carried out under the same condition. The degradation efficiency showed a lower overall 35 % compared to the title complex. The result revealed that the complex has a higher catalytic effect on the degradation of Congo red in the Fenton-like system. The degradation mechanism can be presented as follows (2~4):

Fig. 7. Experimental results of the catalytic degradation of Congo red
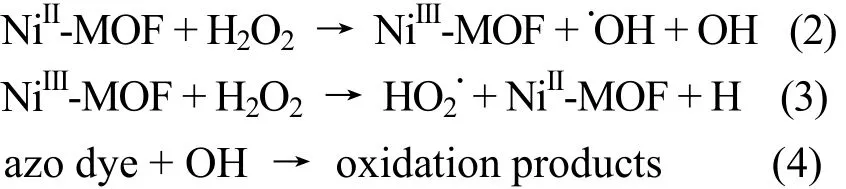
Over the past decades, several transition metal complexes were applied to catalyze the degradation of azo dyes in a Fenton-like process. Our group synthesized three-dimensional binodal (4, 10)-connected MOFs based on pentanuclear cobalt(II) clusters showing high catalytic activity for the degradation of organic dyes in the presence of Na2S2O8[26]. Li and coworkers reported a (3,5)-connected copper(II)MOF, which shows distinct catalytic activity for the degradation of Methyl orange under the condition of H2O2, and eventually the degradation efficiency was up to 97.3%[27]. In addition, our group has reported three Ag(I) complexes based on bis(imidazole) and benzenedicarboxylic acid ligands which possesses a remarkable activity for the degradation of MO (methyl orange) by persulfate in a Fenton-like process[28].
(1) O’Keeffe, M.; Yaghi, O. M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets.Chem. Rev. 2012, 112, 675–702.
(2) Dai, Y. M.; Huang, J. F.; Fang, Y. L. (2D+1D) Hydrogen bond structure constructed from dendrimer precursor. Chin. J. Struct. Chem. 2013, 32,1437–1442.
(3) Du, M.; Li, C. P.; Liu, C. S.; Fang, S. M. Design and construction of coordination polymers with mixed ligand synthetic strategy. Coord. Chem.Rev. 2013, 257, 1282–1305.
(4) Cui, G. H.; Li, J. R.; Tian, J. L.; Bu, X. H.; Batten, S. R. Multidimensional metal-organic frameworks constructed from flexible bis(imidazole)ligands. Cryst. Growth Des. 2005, 5, 1775–1780.
(5) Qin, L.; Li, Y. H.; Ma, P. J.; Cui, G. H. Exploring the effect of chain length of bridging ligands in cobalt(II) coordination polymers based on flexible bis(5,6-dimethylbenzimidazole) ligands: synthesis, crystal structures, fluorescence and catalytic properties. J. Mol. Struct. 2013, 1051,215–220.
(6) Geng, J. C.; Jiao, C. H.; Hao, J. M.; Cui, G. H. Assembly of three cadmium(II) complexes based on flexible α,ω-bis(benzimidazolyl)alkane ligands.Z. Naturforsch. 2012, 67b, 791–798.
(7) Su, Z.; Fan, J.; Okamura, T. A.; Sun, W. Y.; Ueyama, N. Ligand-directed and pH-controlled assembly of chiral 3d-3d heterometallic metal-organic frameworks. Cryst. Growth Des. 2010, 10, 3515–3521.
(8) Jiao, C. H.; He, C. H; Geng, J. C.; Cui, G. H. Syntheses, structures, and photoluminescence of three cadmium(II) coordination polymers with flexible bis(benzimidazole) ligands. J. Coord. Chem. 2012, 65, 2852–2861.
(9) Wang, X. L.; Qu, Y.; Liu, G. C.; Luan, J.; Lin, H. Y.; Kan, X. M. A series of flexible bis(imidazole)-based coordination polymers tuned by central metal ions and dicarboxylates: diverse structures and properties. Inorg Chim Acta 2014, 412, 104–113.
(10) Bu, X. H.; Hou, W. F.; Du, M.; Chen, W.; Zhang, R. H. Varying the frameworks of novel silver(I) coordination polymers with thioethers by altering the backbone or terminal groups of ligands. Cryst. Growth Des. 2002, 2, 303–307.
(11) Liu, P. P.; Wang, Y. Q.; Tian, C. Y.; Peng, H. Q.; Gao, E. Q. Nickel(II) and copper(II) coordination polymers with 1,2-bis(tetrazol-1-yl)ethane and thiocyanate: structure, supramolecular isomerism and magnetism. J. Mol. Struct. 2009, 920, 459–465.
(12) Qin, L.; Ming, C. L.; Xiao, S. L.; Ma, P. J.; Cui, G. H. Synthesis, structures, and catalytic properties of two-cobalt(II) coordination polymers based on 5-substituted isophthalate and flexible bis(benzimidazole) co-ligands. Z. Anorg. Allg. Chem. 2014, 640, 491–496.
(13) Li, L. L.; Yuan, R. U.; Liu, L. L.; Ren, Z. G.; Zheng, A. X.; Cheng, H. J.; Li, H. X.; Lang, J. P. Formation of [CuSCN]n-based topological structures via a family of flexible benzimidazolyl-based linkers with different spacer lengths. Cryst. Growth Des. 2010, 10, 1929–1938.
(14) Allen, F. H. The Cambridge structural database: a quarter of a million crystal structures and rising. Acta Cryst. 2002, B58, 380–388.
(15) Hoskins, B. F.; Robson, R.; Slizys, D. A. An infinite 2D polyrotaxane network in Ag2(bix)3(NO3)2(bix = 1,4-bis(imidazol-1-ylmethyl)benzene). J.Am. Chem. Soc. 1997, 119, 2952–2953.
(16) Geng, J. C.; Liu, L. W.; Xiao, S. L.; Cui, G. H. Two 2D cobalt(II) coordination frameworks with unusual binodal network topology: synthesis,structures, and catalytic properties. Transi. Met. Chem. 2013, 38, 143–148.
(17) Hameed, B. H.; Lee, T. W. Degradation of malachite green in aqueous solution by Fenton process. J. Hazard. Mater. 2009, 164, 468–472.
(18) Sheldrick, G. M. SHELXS 97, Program for the Solution of Crystal Structures. University of Göttingen, Germany 1997.
(19) Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structures. University of Göttingen, Germany 1997.
(20) Feng, X.; Ma, L. F.; Wang, L. Y.; Zhao, J. S. A unique tetranuclear nickel(II) complex containing pyridine-2-carboxaldehyde derivative bearing an intramolecular acetato: synthesis, crystal structure and magnetic property. Inorg. Chem. Commun. 2011, 14, 584–589.
(21) Bai, Y.; Shang, W. L.; Dang, D. B.; Gao, H.; Niu, X. F.; Guan, Y. F. Synthesis, crystal structure and luminescent properties of a thiocyanato bridged two-dimensional heteronuclear polymeric complex of cadmium(II) and copper(II). Inorg. Chem. Commun. 2008, 11, 1470–1473.
(22) Jin, C. M.; Chen, Z. F.; Mei, H. F.; Shi, X. K. Ag(I) coordination polymers with flexible bis-imidazole ligands: 2D interwoven structure and wavy layer network based on silver–silver interaction. J. Mol. Struct. 2009, 921, 58–62.
(23) Allendrof, M. D.; Bauer, C. A.; Bhakta, R. K.; Houk, R. J. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352.
(24) Ateer, B. M.; Beattie, N.; Richens, D. T. Catalytic oxidation of cyclohexene by aqueous iron(III)/H2O2in mildly acidic solution: epoxidation versus allylic oxidation. Inorg Chem Commun. 2013, 35, 284–289.
(25) Jiao, C. H.; He, C. H.; Geng, J. C.; Cui, G. H. Synthesis, crystal structures and catalytic properties of two 1D cobalt(II) coordination polymers.Transi. Met. Chem. 2012, 37, 17–23.
(26) Hao, J. M.; Wang, L. N.; Van Hecke, K.; Cui, G. H. An unprecedented binodal (4, 10)-connected metal-organic framework based on pentanuclear cobalt (II) clusters. Inorg. Chem. Commun. 2014, 41, 43–46.
(27) Li, M.; Zhao, S.; Peng, Y. F.; Li, B. L.; Li, H. Y. A polythreading array formed by a (3,5)-connected 3D anionic network and 1D cationic chains:synthesis, structure, and catalytic properties. Dalton Trans. 2013, 42, 955–964.
(28) Ming, C. L.; Li, Y. H.; Li, G. Y.; Cui, G. H. Synthesis, crystal structures, luminescence and catalytic properties of three silver(I) coordination polymers with bis(imidazole) and benzenedicarboxylic acid ligands. Transi. Met. Chem. 2014, 39, 477–485.

