Potential risk of mitomycin C at high concentrations on peripheral nerve structure
Tao Sui, Jinhong Zhang, Shihao Du, Changhui Su, Jun Que, Xiaojian Cao
1 Department of Orthopedics, the First Af fi liated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
2 Department of Orthopedics, the Second Hospital of Nanjing, Af fi liated to Southeast University, Nanjing, Jiangsu Province, China
3 Department of Orthopedics, Af fi liated Hospital of Taishan Medical College, Taishan, Shandong Province, China
4 Department of Intensive Care Unit, the First Af fi liated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
Potential risk of mitomycin C at high concentrations on peripheral nerve structure
Tao Sui1, Jinhong Zhang2, Shihao Du1, Changhui Su3, Jun Que4, Xiaojian Cao1
1 Department of Orthopedics, the First Af fi liated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
2 Department of Orthopedics, the Second Hospital of Nanjing, Af fi liated to Southeast University, Nanjing, Jiangsu Province, China
3 Department of Orthopedics, Af fi liated Hospital of Taishan Medical College, Taishan, Shandong Province, China
4 Department of Intensive Care Unit, the First Af fi liated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
Tao Sui and Jinhong Zhang contributed equally to this work.
Although the local application of mitomycin C may prevent epidural adhesion after laminectomy, mitomycin C can induce neurotoxicity in optic and acoustic nerves at high concentrations. To determine the safe concentration range for mitomycin C, cotton pads soaked with mitomycin C at different concentrations (0.1, 0.3, 0.5, and 0.7 mg/mL) were immediately applied for 5 minutes to the operation area of rats that had undergone laminectomy at L1. Rat sciatic nerves, instead of dorsal nerves, were used in this study. The results showed that mitomycin C at 0.1-0.5 mg/mL did not damage the structure and function of the sciatic nerve, while at 0.7 mg/mL, mitomycin C signi fi cantly reduced the thickness of the sciatic nerve myelin sheath compared with lower concentrations, though no functional change was found. These experimental fi ndings indicate that the local application of mitomycin C at low concentrations is safe to prevent scar adhesion following laminectomy, but that mitomycin C at high concentrations (> 0.7 mg/mL) has potential safety risks to peripheral nerve structures.
nerve regeneration; peripheral nerve injury; mitomycin C; myelin sheath; laminectomy; electrophysiology; nerve function; NSFC grant; neural regeneration
Funding: This work was supported by the National Natural Science Foundation of China, No. 81171694, 81201374, 81371968, 81371969; a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions; and the Nature Science Foundation of Jiangsu Province No. BK2012718, BK2011844.
Sui T, Zhang JH, Du SH, Su CH, Que J, Cao XJ. Potential risk of mitomycin C at high concentrations on peripheral nerve structure. Neural Regen Res. 2014;9(8):821-827.
Introduction
Epidural scar adhesion is understood to be a main contributing factor to undesirable postoperative symptoms following laminectomy. The scar that forms constrains nerve roots and impedes their normal motion in the vertebral column, causing the refractory leg and back pain that comprise the key features of ‘failed back surgery syndrome’ (Cauchoix et al., 1978; Aldrete, 1995; Guyer et al., 2006). Epidural scar adhesion also makes any subsequent operations in the same area dangerous and technically difficult (Chandler and Cappello, 2006; Jou et al., 2007). As a result, many surgical techniques and antiadhesion agents have been developed to minimize postoperative scarring (Ozer et al., 2006; Jou et al., 2007; Cemil et al., 2009; Rabb, 2010; Kasimcan et al., 2011). Mitomycin C, a classical chemotherapeutic drug, is isolated from Streptomyces caespitosus or Streptomyces lavendulae. It is mainly used to treat upper (Keane et al., 1985; Wolf et al., 2010) and lower (Cummings et al., 1991; Ajani et al., 2008) gastrointestinal cancers and breast cancers (Konits et al., 1981; Vrdoljak et al., 2011) via intravenous infusion, as well as bladder tumors through bladder instillation (Mishina et al., 1975; Tolley et al., 1996). Accumulating evidence indicates that mitomycin C functions as an adjuvant therapy by preventing adhesion formation in ophthalmologic and otolaryngologic applications (Cruz, 1996; Schipper et al., 1997; Rahbar et al., 2000; Banthia and Selesnick, 2003). Recently, mitomycin C was shown to remarkably reduce epidural adhesion after laminectomy by decreasing scar formation (Lee et al., 2004, 2006a, 2006b; Yildiz et al., 2007; Liu et al., 2010). However, mitomycin C, as a chemotherapeutic agent, has inherent toxicity and other side effects. Several studies have demonstrated the side effects of mitomycin C, including decreased wound strength (Porter et al., 2006) and delayed wound healing (Ando et al., 1992; Demir et al., 2003; Su et al., 2012). The topical application of mitomycin C produced substantial sensorineural hearing loss and was ototoxic to the middle ear in gerbils (Moody et al., 2006). In addition, mitomycin C also had a toxic effect on the optic nerve and ciliary body (Mietz et al., 1997; Cetinkaya et al., 2008).

Figure 1 Effect of mitomycin C on the structure of sciatic nerve.
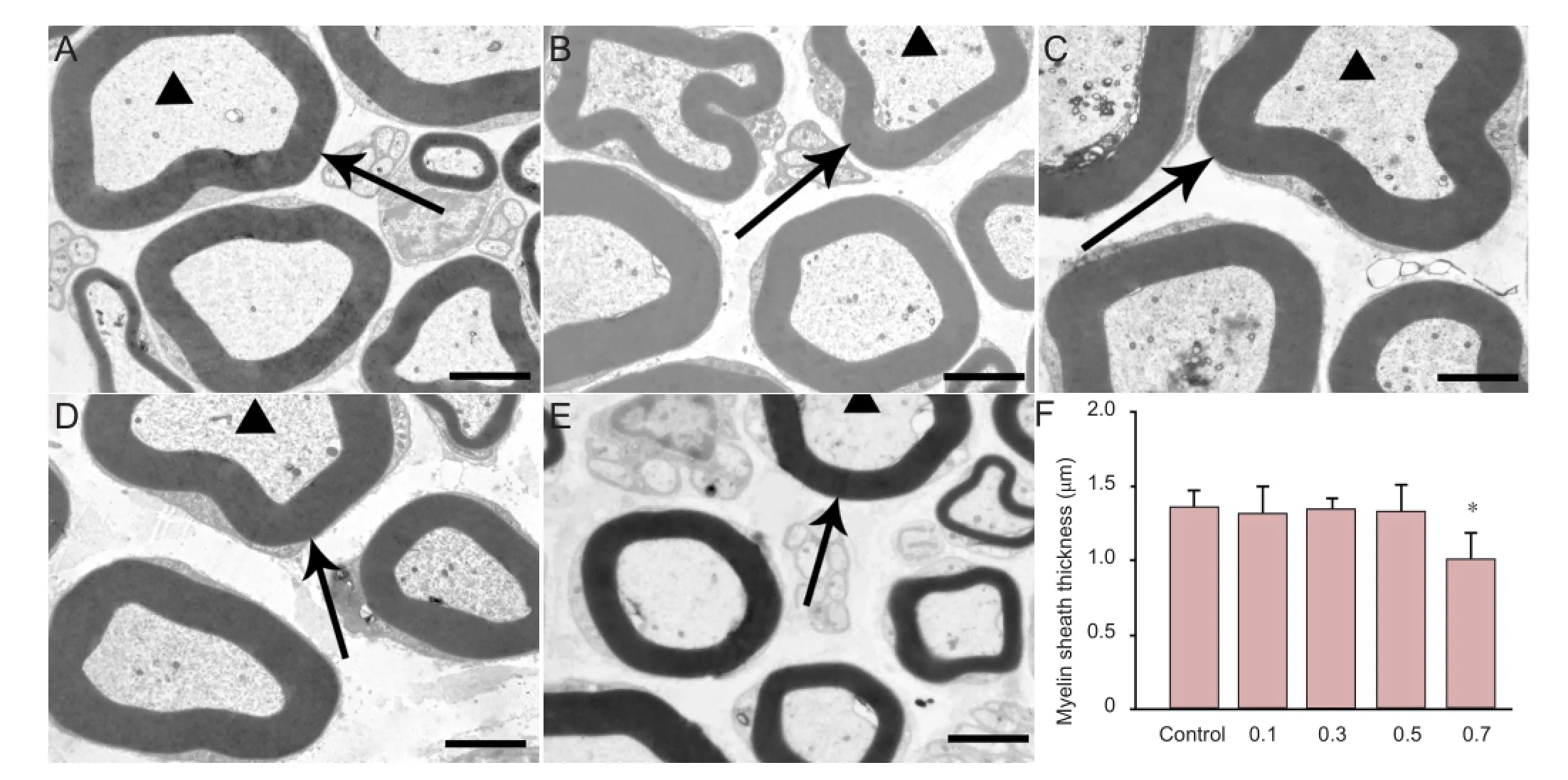
Figure 2 Effect of mitomycin C on the myelin sheath and axons of the sciatic nerve.
During topical application, mitomycin C soaks the dura mater, spinal nerve, and surrounding soft tissues directly. However, an intact blood-brain barrier in the brain and spinal cord prevented mitomycin C from producing adverse effects in the central nervous system (Schwartz and Philips, 1961; Lee et al., 2006b). Within the spinal canal, spinal nerves and the spinal cord are connected by the ventral and dorsal roots. The spinal nerve is part of the peripheral nervous system and is not protected from mitomycin C by the blood-brain barrier. Little is known about the safety risks of local application of mitomycin C on the function and morphology of peripheral nerves after laminectomy. In the present study, the spinal nerve and sciatic nerve of rats following laminectomy were used to investigate the safety risks of local application of mitomycin C on the function and morphology of peripheral nerves in rats. We aimed to assess the potential safety problems of topical application of mitomycin C after laminectomy.
Materials and Methods
Animals

Figure 3 Effects of topical application of mitomycin C (0.1, 0.3, 0.5, and 0.7 mg/mL) on motor nerve conduction latency (A), amplitude of the compound muscle action potential (CMAP) (B) and paw withdrawal thermal latency (PWTL) (C) before and after surgery.
One hundred and twenty adult, clean level Sprague-Dawley rats weighing 170-200 g were provided by the Experimental Animal Center of Nanjing Medical University, China (license No. SYXK (Su) 2008-0007). The animals were maintained under standard laboratory conditions (12-hour light/dark cycle, 18-26°C, 40-70% humidity). All rats were housed in a conventional animal facility and were randomly divided into fi ve groups: a control group and 0.1, 0.3, 0.5, and 0.7 mg/mL mitomycin C treatment groups. All experimental procedures conformed to the Guideline for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and were approved by the Ethics Committee of Nanjing Medical University, China.
Rat models of laminectomy and sciatic nerve exposure
The rat laminectomy model was performed essentially as described previously (Lee et al., 2004). In brief, rats were anesthetized with pentobarbital (50 mg/kg) via intraperitoneal injection. After the hair around L4-5was shaved, the skin was sterilized with an iodine solution. A dorsal skin incision was made and continued deep to the spinous process. The paraspinal muscles were stripped away from the lamina and spinous process. Left laminectomies of L4-5were carried out using rongeurs. Next, a laminectomy defect was created, leaving the dura mater clean and fully exposed. This model was used for the paw withdrawal thermal latency assay.
The left sciatic nerve exposure rat model was also performed essentially as described previously (Ilbay et al., 2005). In brief, after anesthesia, the rat sciatic nerve was exposed and isolated from the surrounding tissues, using aseptic technique to bluntly separate the peroneal and tibial components back toward the sciatic foramen. This model was used for the neural electrophysiological recordings and histological evaluations.
Topical application of mitomycin C
After hemostasis was achieved, pieces of cotton wool (1 cm × 1 cm) were soaked with 0.1, 0.3, 0.5, and 0.7 mg/mL mitomycin C (powder purity: 100%, dissolved in saline; Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) at previously reported concentrations (Su et al., 2010; Lee et al., 2006a, b) or saline. After soaking for 1 minute, the impregnated cotton wool pieces were taken out of solution and allowed to drip untilno further water droplets formed. The surgeons then placed the impregnated cotton wool around the surgical area for 5 minutes. Next, the soaked cotton pads were removed and the laminectomy area or the exposed sciatic nerve was washed with saline to rinse away any leftover reagent. The wound was closed layer by layer in all rats using the same suture material. No prophylactic antibiotics were used.
Histological evaluation
Hematoxylin-eosin staining
The sciatic nerve exposure model rats were used for histological assessment of the 0.1, 0.3, 0.5, and 0.7 mg/mL mitomycin C groups and the control group by blinded surgical dissection. At 5 days after the topical application of mitomycin C or saline to the sciatic nerve, all rats were killed by intraperitoneal injection of an overdose of pentobarbital (60 mg/kg). The separated sciatic nerve was fi xed with 10% formalin and embedded in paraf fi n for sectioning. Nine successive transversal sections of 5 μm thickness were obtained from the sciatic nerve and stained with hematoxylin-eosin. The sciatic nerve structure and morphology were evaluated under a light microscope (FDX-35, Nikon, Tokyo, Japan).
Transmission electron microscope
For electron microscopy (JEM-1010, JEOL Ltd., Tokyo, Japan), samples were excised, rinsed three times in buffer, and then fi xed in 5% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.3) overnight at 4°C. The samples were then post fi xed in 1% osmium tetroxide for 2 hours at 4°C. After dehydration and embedding in spurr resin, thick sections (50 μm thickness) were stained with 1% toluidine blue in 1% borax at 60°C. Finally, thin sections (50 to 80 nm thickness) were cut and stained in uranyl acetate and lead citrate.
Neuroelectrophysiological recording
Electrophysiological evaluation, including motor nerve conduction latency (Snooks et al., 1985; Tankisi et al., 2007) and measurement of compound muscle action potential (Krarup et al., 2002; Laughlin et al., 2011) was used to assess neurological function before mitomycin C treatment, and at 1 and 5 days after mitomycin C or saline treatment in the sciatic nerve exposure model rats. A bipolar stimulating probe (Medtronic, Jacksonville, FL, USA) connected to a constant current stimulator that delivered monophasic square wave pulses was selected to perform the electrical stimulation. The stimulating electrode was placed in the sciatic nerve with a 2-mm separation between the electrodes proximally at the spine. The motor nerve conduction latency and compound muscle action potential were recorded using similar bipolar probes placed in the belly of the gastrocnemius. To assure supramaximal stimulation at the beginning of the experiment, the electrical stimulation was in the range of 2-15 mA. The duration of each stimulus was 0.1 ms.
Paw withdrawal thermal latency assay
The laminectomy model rats from each group were used for the paw withdrawal thermal latency assay to assess neurological function before the operation and during the acute phase at 1, 2, 3, and 5 days after mitomycin C or saline treatment.
The paw withdrawal thermal latency assay was performed as previously described (Li and Chen, 2004). In brief, the rats were placed in a transparent plexiglass chamber to measure their heat sensitivity using a plantar test apparatus (IITC Life Science Inc., Los Angeles, CA, USA). Stimuli were repeated four or fi ve times to calculate the mean paw withdrawal thermal latency for each rat. The inter-stimulus interval was more than 10 minutes. If the latency exceeded 25 seconds, the stimulus was stopped to avoid excessive tissue injury, and the region was considered to have had no response.
Statistical analysis
Data are expressed as the median and statistical range of the H-score for each group. All other results are expressed as mean ± SD. Differences between the groups were evaluated using two-tailed Student’s t-tests. The statistical analysis was performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). P-values less than 0.05 were considered to be statistically signi fi cant.
Results
Effect of mitomycin C on general conditions of laminectomy model rats
No remarkable differences were found in the signs of hind limb neurological function after saline or mitomycin C treatment, and no treated rats exhibited a full-thickness wound dehiscence.
Effect of mitomycin C on sciatic nerve morphology of laminectomy model rats
Hematoxylin-eosin staining revealed no damage to the sciatic nerve structure or morphology in the control and 0.7 mg/mL mitomycin C-treated group rats. The epineurium remained intact, and the diameter and axonal density of the sciatic nerve were similar in the control and all mitomycin C-treated groups during the fi rst 5 days after the operation (Figure 1). By electron microscopy, the axons were arranged in a regular fashion in both the saline and mitomycin C-treated groups. The astrocytes displayed normal morphology. The small and large vessels presented a regular appearance with a smooth, thin basement membrane. The collagen surrounding the vessels was distributed and the pial septa did not show consistent differences between the treated and control nerves. The thickness of the myelin sheaths in the 0.7 mg/ mL mitomycin C-treated group was signi fi cantly lower than those of the other mitomycin C-treated groups and the control group 5 days after treatment (P < 0.05; Figure 2).
Effects of mitomycin C on the behavior of laminectomy model rats
Motor nerve conduction latency
Square wave pulse stimulation was applied to the injured (left) sciatic nerve in the 0.1 mg/mL treatment group before and at 1 and 5 days after mitomycin C treatment. The motor nerve conduction latencies on the right side were similar to those on the left side (P > 0.05). For pulse stimulation to the left and right sciatic nerve in the 0.3, 0.5, and 0.7 mg/ mL groups before and at 1 and 5 days after mitomycin Ctreatment, the motor nerve conduction latency recorded at the gastrocnemius was similar in all groups (P > 0.05). The motor nerve conduction latency on both the injured and control sides was also similar in all groups before and at 1 and 5 days after the operation (P > 0.05; Figure 3A).
Compound muscle action potential amplitude
After stimulation to the injured (left) sciatic nerve in the 0.1, 0.3, 0.5, and 0.7 mg/mL mitomycin C-treated groups before and at 1 and 5 days after the operation, the amplitudes of the compound muscle action potential at the gastrocnemius on the injured side were not significantly different among the groups (P > 0.05). During stimulation to the right side, the mean amplitude of the compound muscle action potential recorded was similar to those of the injured side (P > 0.05; Figure 3B).
Paw withdrawal thermal latency
As shown in Figure 3C, the paw withdrawal thermal latencies were all within the normal range (Dirig et al., 1997) before and at 1, 2, 3, and 5 days after saline or mitomycin C treatment. The paw withdrawal thermal latency showed no signi fi cant differences in any groups before and at 1, 2, 3, and 5 days after saline or mitomycin C treatment (P > 0.05).
Discussion
One of the major problems in failed back surgery syndrome is epidural scar formation (Burton et al., 1981). The epidural scars that form can lead to intractable pain and sensory and motor deficits because of compression and/or tethering of the nerve roots. Many studies have attempted to prevent epidural scar formation by applying various drugs and materials, such as recombinant tissue-plasminogen activator gel, bioelastic polymers, hyaluronan, and Adcon-L (Henderson et al., 1993; Alkalay et al., 2003; Ganzer et al., 2003; Massie et al., 2005).
Since it was first reported that mitomycin C prevented epidural fi brosis after laminectomy (Henderson et al., 1993), a number of studies have shown its ability to decrease fi broblast proliferation and induce fibroblast apoptosis, which leads to the reduction of scar formation (Henderson et al., 1993; Dogulu et al., 2003; Massie et al., 2005; Lee et al., 2006a, b). With accumulating experience, however, several adverse effects of mitomycin C treatment have appeared, such as delays in wound healing and neurotoxicity (Rubinfeld et al., 1992; Moody et al., 2006; Su et al., 2012). Still, the safety of local application of mitomycin C on peripheral nerves after laminectomy is not completely understood. In the present study, a rat model was used to investigate the impact of topical mitomycin C application on peripheral nerves.
The sciatic nerve is a large nerve in both humans and animals that is derived from the L4-5spinal nerves and runs cross the buttocks and down the lower limb. It contains both the anterior and posterior fi bers of the lumbosacral plexus (Schmalbruch, 1986; Rigaud et al., 2008). The sciatic nerve is widely used to evaluate the effects of various agents on nerve function. In the present study, sciatic nerve was used to perform neural electrophysiological tests and histological observations to evaluate the effects of the local application of mitomycin C on peripheral nerves.
These results confirm that the local application of mitomycin C at less than 0.5 mg/mL was safe for rat peripheral nerve. The wound healing was complete in all groups, and no muscle cavity was found in any of the mitomycin C treatment groups. The gross anatomy and hematoxylin-eosin staining showed that mitomycin C did not damage the structure or epineurium of the sciatic nerve. However, the electron microscopy of the sciatic nerve after mitomycin C treatment demonstrated that the thickness of the myelin sheath was signi fi cantly decreased in the 0.7 mg/mL group compared with the control and other concentrations groups. It is well known that the essential parameters for nerve conduction ef fi ciency include the myelin sheath length and diameter (Waxman, 1997). In addition, numerous peripheral neuropathies can affect myelin sheath thickness and cause compact myelin deposition (Suter and Scherer, 2003). To determine whether this decrease affected the function of the sciatic nerve, we conducted the neural electrophysiological test and paw withdrawal thermal latency assay.
Peripheral nerve and nerve root injuries that increase the excitability of neurons and lead to central sensitization in the spinal dorsal horn are thought to constitute the conditions of allodynia and hyperalgesia (Wagner et al., 1998). Hyperalgesia results in notably shorter paw withdrawal latencies compared with normal conditions (Hargreaves et al., 1988; Zhang et al., 2008). In the present study, the laminectomy model rat was used for the paw withdrawal thermal latency assay. The assay results showed no significant differences between any groups either before or at 1 or 5 days after the operation. The paw withdrawal thermal latency assay results suggest that hyperalgia was not an effect of the topical application of mitomycin C on spinal nerve function.
Alterations to motor nerve conduction latency and compound muscle action potential amplitude re fl ect the function of the myelin sheath and the axons of motor neurons during acute nerve injury (Sun et al., 2007). The values of these neural electrophysiological parameters measured in the present study on sciatic nerve did not show any changes with increasing concentration of mitomycin C. The local application of mitomycin C at concentrations below 0.7 mg/mL does not appear to harm myelin sheath function or the axons of the sciatic nerve in the short term.
The local or systemic applications of mitomycin C may cause dose-dependent complications such as in fl ammation, hypervascularization, hematoma, and necrosis due to its antiproliferative effects (Rubinfeld et al., 1992; Kureshi et al., 2006; Mearza and Aslanides, 2007). In the present study, no harmful effects of mitomycin C on the function of sciatic nerve were observed after treatment. This lack of any differences may be caused by the low mitomycin C doses, short application times, and washing of the operation site with plenty of normal saline. However, treatment with the 0.7 mg/mL mitomycin C reduced the myelin sheath thickness, though this reduction did not in fl uence the function of the sciatic nerve. The magnitude of this reduction maynot have passed the threshold necessary to effect a change in function, or the repair of the myelin sheath could have been too rapid for this change to affect nerve function. The topical use of mitomycin C below 0.5 mg/mL appears to have no effect on the spinal nerve after laminectomy in the short term because this dosage is nontoxic to the sciatic nerve in rats. However, a longer follow-up time must be assessed in future studies before drawing a fi rm conclusion. A large animal model, such as the dog laminectomy model, can help surgeons to minimize the iatrogenic spinal nerve injury during the operation. In a future study, the dog laminectomy model will be used to investigate the effects of local application of mitomycin C on the spinal nerve in the spinal canal. A better way to control the dose administered would help to improve the reproducibility of the experiment.
In conclusion, the local application of mitomycin C below 0.5 mg/mL for preventing post-laminectomy epidural scar formation was demonstrated to have no effect on the peripheral nerve. The local application of mitomycin C at concentrations over 0.7 mg/mL, however, may have potential adverse effects on the peripheral nerve by decreasing the thickness of the myelin sheath. A follow-up study using a large animal model is needed to confirm the effects of the local application of mitomycin C on the sciatic nerve.
Author contributions:Sui T and Zhang JH conducted the experiments, collected and analyzed data, and wrote the manuscript. Cao XJ was in charge of funds, guided the study, and provided technical support. Du SH, Su CH and Que J participated in data analysis and provided technical support. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:Mitomycin can prevent adhesion, the existing hypothesis that it can affect the central nervous system and peripheral nervous system has insufficient theoretical evidence. This study aimed to discuss the influence of mitomycin at different concentrations on the morphology and function of peripheral nervous system, providing clinical guidance value.
Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson 3rd AB, Thomas Jr CR, Mayer RJ, Haddock MG, Rich TA, Willett C (2008) Fluorouracil, mitomycin, and radiotherapy vs fl uorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 299:1914-1921.
Aldrete JA (1995) Epidural fi brosis after permanent catheter insertion and infusion. J Pain Symptom Manag 10:624-631.
Alkalay RN, Kim DH, Urry DW, Xu J, Parker TM, Glazer PA (2003) Prevention of postlaminectomy epidural fibrosis using bioelastic materials. Spine (Phila Pa 1976) 28:1659-1665.
Ando H, Ido T, Kawai Y, Yamamoto T, Kitazawa Y (1992) Inhibition of corneal epithelial wound healing. A comparative study of mitomycin C and 5- fl uorouracil. Ophthalmology 99:1809-1814.
Banthia V, Selesnick SH (2003) Mitomycin-C in the postsurgical ear canal. Otolaryngol Head Neck Surg 128:882-886.
Burton CV, Kirkaldy-Willis W, Yong-Hing K, Heithoff KB (1981) Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res 157:191-199.
Cauchoix J, Ficat C, Girard B (1978) Repeat surgery after disc excision. Spine (Phila Pa 1976) 3:256-259.
Cemil B, Tun K, Kaptanoglu E, Kaymaz F, Cevirgen B, Comert A, Tekdemir I (2009) Use of pimecrolimus to prevent epidural fi brosis in a postlaminectomy rat model: Laboratory investigation. J Neurosurg Spine 11:758-763.
Cetinkaya A, Akman A, Take G, Bilezikci B, Akova YA (2008) Ciliary body toxicity of subconjunctival suramin compared with mitomycin-C in the rabbit eye: determining the toxic concentration. Ophthalmic Res 41:91-97.
Chandler K, Cappello R (2006) Laminectomy membrane formation in dogs: is the answer still elusive? Vet J 172:1-2.
Cruz OA (1996) Evaluation of mitomycin to limit postoperative adhesions in strabismus surgery. J Pediatr Ophthalmol Strabismus 33:89-92.
Cummings BJ, Keane TJ, O’Sullivan B, Wong CS, Catton CN (1991) Epidermoid anal cancer: treatment by radiation alone or by radiation and 5- fl uorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys 21:1115-1121.
Demir T, Turgut B, Akyol N, Ozercan I, Ulaş F, Celiker U (2003) Effects of amniotic membrane transplantation and mitomycin C on wound healing in experimental glaucoma surgery. Ophthalmologica 216: 438-442.
Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL (1997) Characterization of variables de fi ning hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods 76:183-191.
Dogulu F, Kurt G, Emmez H, Erdem O, Memis L, Baykaner K, Ceviker N (2003) Topical mitomycin C-induced inhibition of postlaminectomy peridural fi brosis in rabbits. J Neurosurg 99:76-79.
Ganzer D, Giese K, Völker L, Pietzner U, Follak N, Merk H (2003) Twoyear results after lumbar microdiscectomy with and without prophylaxis of a peridural fi brosis using Adcon-L. Arch Orthop Trauma Surg 123:17-21.
Guyer RD, Patterson M, Ohnmeiss DD (2006) Failed back surgery syndrome: diagnostic evaluation. J Am Acad Orthop Surg 14:534-543.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77-88.
Henderson R, Weir B, Davis L, Mielke B, Grace M (1993) Attempted experimental modi fi cation of the postlaminectomy membrane by local instillation of recombinant tissue-plasminogen activator gel. Spine (Phila Pa 1976) 18:1268-1272.
Ilbay K, Etus V, Yildiz K, Ilbay G, Ceylan S (2005) Topical application of mitomycin C prevents epineural scar formation in rats. Neurosurg Rev 28:148-153.
Jou IM, Tai TW, Tsai CL, Tsai TM, Yung WS, Jung YC (2007) Spinal somatosensory evoked potential to evaluate neurophysiologic changes associated with postlaminotomy fi brosis: an experimental study. Spine (Phila Pa 1976) 32:2111-2118.
Kasimcan MO, Bakar B, Aktaş S, Alhan A, Yilmaz M (2011) Effectiveness of the biophysical barriers on the peridural fibrosis of a postlaminectomy rat model: an experimental research. Injury 42:778-781.
Keane TJ, Harwood AR, Elhakim T, Rider WD, Cummings BJ, Ginsberg RJ, Cooper JC (1985) Radical radiation therapy with 5- fl uorouracil infusion and mitomycin C for oesophageal squamous carcinoma. Radiother Oncol 4:205-210.
Konits PH, Aisner J, van Echo DA, Lichtenfeld K, Wiernik PH (1981) Mitomycin C and vinblastine chemotherapy for advanced breast cancer. Cancer 48:1295-1298.
Krarup C, Archibald SJ, Madison RD (2002) Factors that influence peripheral nerve regeneration: an electrophysiological study of the monkey median nerve. Ann Neurol 51:69-81.
Kureshi F, Kalaaji AN, Halvorson L, Pittelkow MR, Davis MD (2006) Cutaneous complications of intravesical treatments for bladder cancer: Granulomatous in fl ammation of the penis following BCG therapy and penile gangrene following mitomycin therapy. J Am Acad Dermatol 55:328-331.
Laughlin RS, Spinner RJ, Daube JR (2011) Electrophysiological testing of spinal accessory nerve in suspected cases of nerve transection. Muscle Nerve 44:715-719.
Lee JY, Stenzel W, Ebel H, Wedekind C, Ernestus RI, Klug N (2004) Mitomycin C in preventing spinal epidural fi brosis in a laminectomy model in rats. J Neurosurg 100:52-55.
Lee JY, Stenzel W, Löhr M, Stützer H, Ernestus RI, Klug N (2006a) The role of mitomycin C in reducing recurrence of epidural fi brosis after repeated operation in a laminectomy model in rats. J Neurosurg Spine 4:329-333.
Lee JY, Stenzel W, Impekoven P, Theisohn M, Stützer H, Löhr M, Reithmeier T, Ernestus RI, Ebel H, Klug N (2006b) The effect of mitomycin C in reducing epidural fi brosis after lumbar laminectomy in rats. J Neurosurg Spine 5:53-60.
Li KC, Chen J (2004) Differential roles of spinal protein kinases C and a in development of primary heat and mechanical hypersensitivity induced by subcutaneous bee venom chemical injury in the rat. Neurosignals 12:292-301.
Liu J, Ni B, Zhu L, Yang J, Cao X, Zhou W (2010) Mitomycin C-polyethylene glycol controlled-release fi lm inhibits collagen secretion and induces apoptosis of fi broblasts in the early wound of a postlaminectomy rat model. Spine J 10:441-447.
Massie JB, Schimizzi AL, Huang B, Kim CW, Garfin SR, Akeson WH (2005) Topical high molecular weight hyaluronan reduces radicular pain post laminectomy in a rat model. Spine J 5:494-502.
Mearza A, Aslanides I (2007) Uses and complications of mitomycin C in ophthalmology. Expert Opin Drug Saf 6:27-32.
Mietz H, Prager TC, Schweitzer C, Patrinely J, Valenzuela JR, Font RL (1997) Effect of mitomycin C on the optic nerve in rabbits. Br J Ophthalmol 81:584-589.
Mishina T, Oda K, Murata S, Ooe H, Mori Y (1975) Mitomycin C bladder instillation therapy for bladder tumors. J Urol 114:217-219.
Moody MW, Lang H, Spiess AC, Smythe N, Lambert PR, Schmiedt RA (2006) Topical application of mitomycin C to the middle ear is ototoxic in the gerbil. Otol Neurotol 27:1186-1192.
Ozer AF, Oktenoglu T, Sasani M, Bozkus H, Canbulat N, Karaarslan E, Sungurlu SF, Sarioglu AC (2006) Preserving the ligamentum fl avum in lumbar discectomy: a new technique that prevents scar tissue formation in the fi rst 6 months postsurgery. Neurosurgery 59:ONS126-133.
Porter GT, Gadre SA, Calhoun KH (2006) The effects of intradermal and topical mitomycin C on wound healing. Otolaryngol Head Neck Surg 135:56-60.
Rabb CH (2010) Failed back syndrome and epidural fi brosis. Spine J 10:454-455.
Rahbar R, Valdez TA, Shapshay SM (2000) Preliminary results of intraoperative mitomycin-C in the treatment and prevention of glottic and subglottic stenosis. J Voice 14:282-286.
Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH (2008) Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain 136:188-201.
Rubinfeld RS, P fi ster RR, Stein RM, Foster CS, Martin NF, Stoleru S, Talley AR, Speaker MG (1992) Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology 99:1647-1654.
Schipper I, Suppelt C, Gebbers JO (1997) Mitomycin C reduces scar formation after excimer laser (193 nm) photorefractive keratectomy in rabbits. Eye (Lond) 11:649-655.
Schmalbruch H (1986) Fiber composition of the rat sciatic nerve. Anat Rec 215:71-81.
Schwartz H, Philips F (1961) Pharmacology of mitomycin C. II. Renal excretion and metabolism by tissue hemogenates. J Pharmacol Exp Ther 133:335-342.
Snooks SJ, Badenoch DF, Tiptaft RC, Swash M (1985) Perineal nerve damage in genuine stress urinary incontinence; an electrophysiological study. Br J Urol 57:422-426.
Su C, Sui T, Zhang X, Zhang H, Cao X (2012) Effect of topical application of mitomycin-C on wound healing in a postlaminectomy rat model: an experimental study. Eur J Pharmacol 674:7-12.
Sun Y, Wang LX, Wang L, Sun SX, Cao XJ, Wang P, Feng L (2007) A comparison of the effectiveness of mitomycin C and 5- fl uorouracil in the prevention of peridural adhesion after laminectomy. J Neurosurg Spine 7:423-428.
Suter U, Scherer SS (2003) Disease mechanisms in inherited neuropathies. Nat Rev Neurosci 4:714-726.
Tankisi H, Pugdahl K, Johnsen B, Fuglsang-Frederiksen A (2007) Correlations of nerve conduction measures in axonal and demyelinating polyneuropathies. Clin Neurophysiol 118:2383-2392.
Tolley DA, Parmar MK, Grigor KM, Lallemand G, Benyon LL, Fellows J, Freedman LS, Grigor KM, Hall RR, Hargreave TB, Munson K, Newling DW, Richards B, Robinson MR, Rose MB, Smith PH, Williams JL, Whelan P (1996) The effect of intravesical mitomycin C on recurrence of newly diagnosed super fi cial bladder cancer: a further report with 7 years of follow up. J Urol 155:1233-1238.
Vrdoljak E, Boban M, Omrcen T, Hrepic D, Fridl-Vidas V, Boskovic L (2011) Combination of capecitabine and mitomycin C as fi rst-line treatment in patients with metastatic breast cancer. Neoplasma 58: 172-178.
Wagner R, Heckman HM, Myers RR (1998) Wallerian degeneration and hyperalgesia after peripheral nerve injury are glutathione-dependent. Pain 77:173-179.
Waxman SG (1997) Axon-glia interactions: building a smart nerve fiber. Curr Biol 7:R406-410.
Wolf M, Zehentmayr F, Niyazi M, Ganswindt U, Haimerl W, Schmidt M, Hölzel D, Belka C (2010) Long-term outcome of mitomycin C-and 5-FU-based primary radiochemotherapy for esophageal cancer. Strahlenther Onkol 186:374-381.
Yildiz KH, Gezen F, Is M, Cukur S, Dosoglu M (2007) Mitomycin C, 5-fl uorouracil, and cyclosporin A prevent epidural fi brosis in an experimental laminectomy model. Eur Spine J 16:1525-1530.
Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L (2008) IL-1ra alleviates in fl ammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 135:232-239.
Copyedited by MaCarty W, Norman C, Feng SQ, Deng LX, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.131598
Xiaojian Cao, Ph.D., Department of
Orthopedics, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China,
xiaojiancao@gmail.com.
http://www.nrronline.org/
Accepted: 2014-03-21
- 中国神经再生研究(英文版)的其它文章
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases

