Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
Yan Yu, Changchun Zeng, Siyun Shu, Xuemei Liu, Chuhua Li
1 MOE Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou, Guangdong Province, China
2 Institute of Cognitive Neuroscience, South China Normal University, Guangzhou, Guangdong Province, China
3 School of Life Science, South China Normal University, Guangzhou, Guangdong Province, China
Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
Yan Yu1, Changchun Zeng1, Siyun Shu2, Xuemei Liu1, Chuhua Li3
1 MOE Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou, Guangdong Province, China
2 Institute of Cognitive Neuroscience, South China Normal University, Guangzhou, Guangdong Province, China
3 School of Life Science, South China Normal University, Guangzhou, Guangdong Province, China
Changchun Zeng, Ph.D., MOE Key
Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou 510631,
Guangdong Province, China,
gzzysys@scnu.edu.cn.
Substance P is an endogenous neurokinin that is present in the central and peripheral nervous systems. The neuropeptide substance P and its high-af fi nity receptor neurokinin 1 receptor are known to play an important role in the central nervous system in in fl ammation, blood pressure, motor behavior and anxiety. The effects of substance P in the hippocampus and the marginal division of the striatum on memory remain poorly understood. Compared with the hippocampus as a control, immuno fl uorescence showed high expression of the substance P receptor, neurokinin 1, in the marginal division of the striatum of normal rats. Unilateral or bilateral injection of an antisense oligonucleotide against neurokinin 1 receptor mRNA in the rat hippocampus or marginal division of the striatum effectively reduced neurokinin 1 receptor expression. Independent of injection site, rats that received this antisense oligonucleotide showed obviously increased footshock times in a Y-maze test. These results indicate that the marginal division of the striatum plays a similar function in learning and memory to the hippocampus, which is a valuable addition to our mechanistic understanding of the learning and memory functions of the marginal division of the striatum.
nerve regeneration; cognition; substance P; neurokinin 1 receptor; hippocampus; marginal division of the striatum; learning and memory; neostriatum; NSFC grant; neural generation
Funding: This project was supported by the National Natural Science Foundation of China, No. 30600797, 30873238.
Yu Y, Zeng CC, Shu SY, Liu XM, Li CH. Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division. Neural Regen Res. 2014;9(8):857-863.
Introduction
Substance P is an endogenous neurokinin that is present in the central and peripheral nervous systems[1-2]. The neuropeptide substance P and its high-af fi nity receptor neurokinin 1 receptor are known to play an important role in the central nervous system in inflammation, blood pressure, motor behavior and anxiety. There is also increasing evidence that substance P and neurokinin 1 are involved in learning and memory[2-3]. Many studies have so far identified that substance P has excitatory effects in the hippocampus and that it is able to facilitate long-term potentiation via activation of neurokinin 1 receptor in the hippocampus[4]. The recent discovery of the effectiveness of neurokinin 1 receptor antagonists in animal models of anxiety and depression[5]has given a new dimension to studies on the functional roles of central neurokinin 1 receptors. Furthermore, neurokinin 1 receptor is a G-protein-coupled receptor and functions via the IP3-signaling system[6]. Two signal responses of the IP3-signaling pathway are DG-PKC and IP3-Ca2+, both of which are associated with important phenomena in learning and memory, such as long-term potentiation and long-term depression[7].
The hippocampus is well known to be involved in many functional processes including regulation of emotions and learning and memory[8-9]. It is generally believed that the hippocampal structure is strongly associated with spatial cognition, and the learning abilities of normal rats in a shuttle box avoidance paradigm are correlated with hippocampal synaptic plasticity[10]. Furthermore, after damage to the hippocampus, rats may produce physiochemical alternations to early senile dementia[11]and defects of learning and memory will be manifested in animals[12-13]. The hippocampus is innervated by substance P-containing axon terminals and has a high density of substance P-containing fibers, which derive from intrinsic and extrinsic origins[14]. Peptides of the tachykinin family can powerfully excite hippocampal interneurons[15-16], an action which is mediated by neurokinin 1 receptors. Neurokinin 1, neurokinin 2 and neurokinin 3 are localized in the hippocampus[17-18].
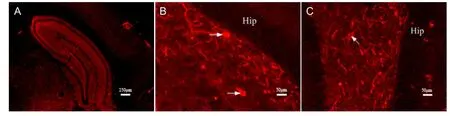
Figure 1 Neurokinin 1 receptor-positive neurons in the hippocampus as shown by immuno fl uorescence staining.
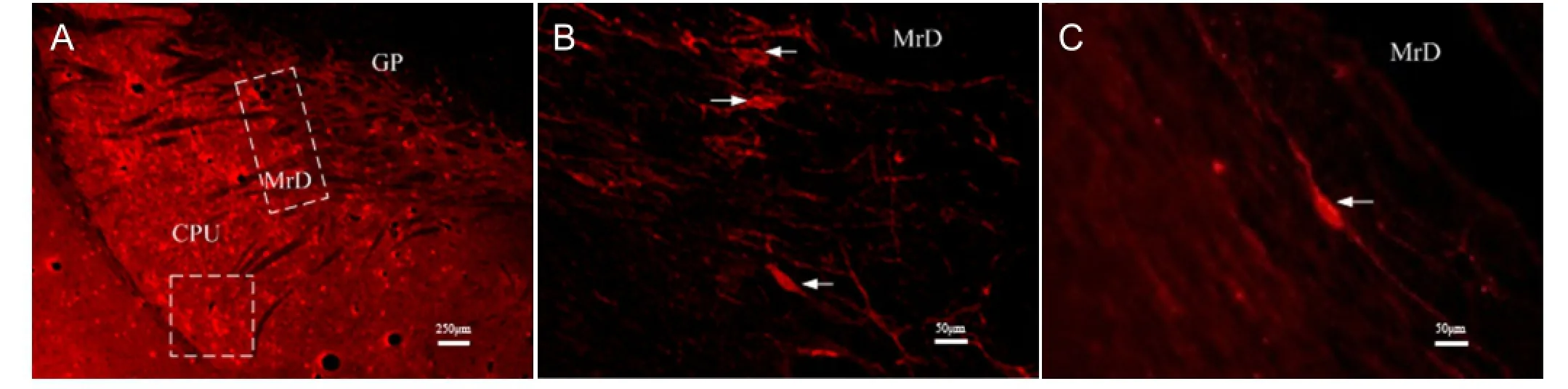
Figure 2 Neurokinin 1 receptor-positive neurons in the neostriatum (CPU, GP, MrD) as shown by immuno fl uorescence staining.
The marginal division, discovered at the caudal-most edge of the neostriatum in the brain of rats[19], intensely expresses a variety of neuropeptides and monoamines that are different from other parts of neostriatum. Chudler et al.[20]found that nociceptive neurons are exclusively localized in the marginal division of the rat striatum using methods of neurophysiology. The marginal division was shown to be involved in learning and memory by the Y-maze and Morris water-maze tests, patch clamping, long-term potentiation, and functional magnetic resonance image studies[21-23].
The neuropeptide substance P and its high af fi nity receptor neurokinin 1 receptor in the central nervous system are known to be involved in learning and memory. The in fl uence of substance P in the marginal division of the striatum in learning and memory is not yet known. In the present study, we determined the effects of injection of an neurokinin 1 receptor mRNA antisense nucleotide in the hippocampus and the marginal division of striatum on performance in the Y-maze test, and the effects of gene blockade in the hippocampus was compared with that in the marginal division.
Results
Quantitative analysis of experimental animals
A total of 40 rats were randomly and equally divided into five groups: unilateral marginal division injection group, bilateral marginal division injection group, unilateral hippocampus injection group, bilateral hippocampus injection group, and normal control group. The first four groups received unilateral (left) or bilateral injections of neurokinin 1 receptor mRNA antisense strand in the striatal marginal division and hippocampus. Te normal control group received saline in the bilateral marginal division and hippocampus. All rats were included in the fi nal analysis.
Neurokinin 1 receptor expression in the hippocampus of normal rats
The expression of neurokinin 1 receptor in the hippocampus was detected by immunofluorescence microscopy. Neurokinin 1 receptor-positive neurons in the hippocampus are multipolar that have round, oval, or triangular shapes and lightly stained compared with non-stained areas (Figure 1).
Neurokinin 1 receptor expression in the striatal marginal division of normal rats
Using immuno fl uorescence microscopy, we identi fi ed a stippled pattern of neurokinin 1 receptor-positive neurons in the neostriatum. This expression was not only observed in the medial striatum, but also more caudally in the dorsolateral part of the striatum (Figure 2A). Neurokinin 1 receptor-positive fusiform neurons with their dendrites projecteddorsoventrally in the marginal division (Figure 2B). Neurokinin 1 receptor-positive fusiform neurons in the marginal division were moderate in size and had two spiny primary dendrites emerging dorsoventrally from the two poles of cell bodies (Figure 2C).
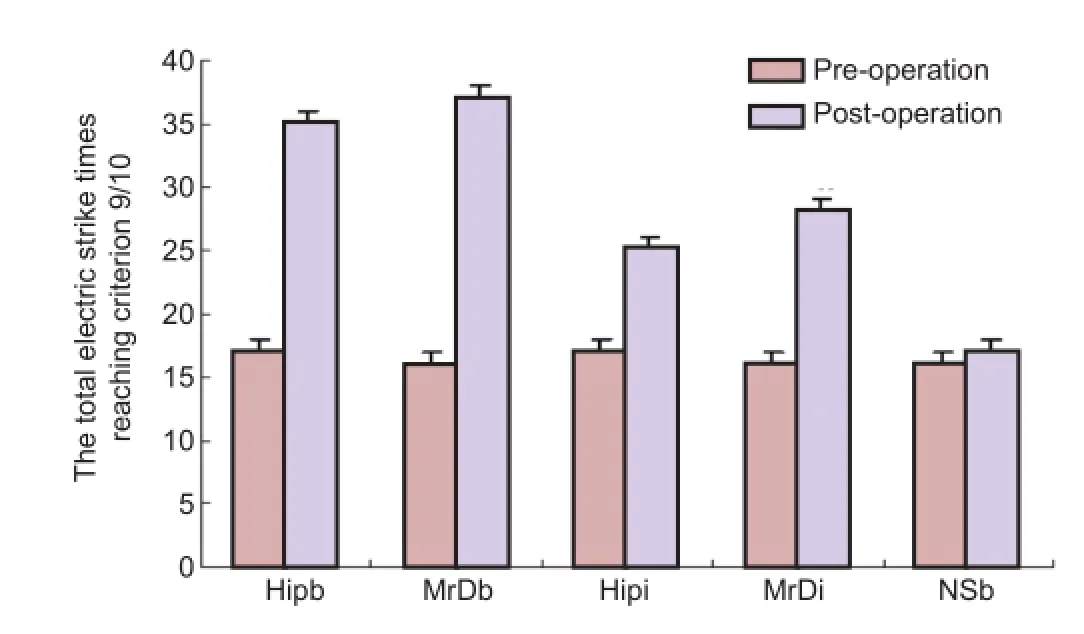
Figure 3 Comparison of learning and memory in a Y-maze after blockade of neurokinin 1 receptor mRNA.
Effects of substance P receptor expression on memory in rats
Before microinjection of the antisense oligonucleotide of neurokinin 1 receptor mRNA into the hippocampus and marginal division, there were no significant differences among the fi ve groups (P > 0.05). Additionally, there was no signi fi cant difference between pre-injection and post-injection in the normal control group (P > 0.05). However, footshock times in rats injected either unilaterally or bilaterally with the antisense oligonucleotide of neurokinin 1 receptor mRNA in the hippocampus and marginal division increased signi fi cantly compared with the corresponding group before the microinjection (P < 0.05 for unilateral and P < 0.01 for bilateral). Moreover, footshock times in the bilateral hippocampus and marginal division injection group increased signi fi cantly after the microinjection compared with the unilateral hippocampus and marginal division injection groups (P < 0.05). However, there was no signi fi cant difference between the hippocampus and marginal division groups after microinjection of antisense oligonucleotides of neurokinin 1 receptor mRNA in either the bilateral injection or in the unilateral injection groups (Figure 3).
Discussion
Substance P (H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2) is known to be involved in processes related to fear, anxiety, stress, and learning and memory[2]. As a member of the tachykinin peptide family, it can have neurotrophic as well as memory-promoting effects upon direct application into the nucleus basalis magnocellularis[24]. Huston et al.[25]showed that substance P plays a part in learning and memory when injected into the medial septum, lateral hypothalamus, ventral pallidum, and after systemic administration in rats. Kertes et al.[26]demonstrated that substance P facilitates passive avoidance learning when injected either into the globus pallidus or into the amygdaloid body, and also showed that substance P and neurokinin 1 receptors play important roles in pallidal positive reinforcing mechanisms[27]. Substance P receptor (neurokinin 1)-positive cells are distributed throughout the brain, including in the basal ganglia, hippocampus, hypothalamus, midbrain, and medulla oblongata[17-18]. Neurokinin 1 receptor, a G-protein-coupled receptor, functions via the IP3-signaling system[6]. The IP3-signaling pathway has two signal responses, IP3-Ca2+and DG-PKC, both of which are associated with mechanism regulating learning and memory, such as long-term potentiation and long-term depression[7]. It has recently been demonstrated that the endogenous ligands of the neurokinin 1 and neurokinin 2 receptors, substance P and neurokinin A (H-His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu-Met-NH2), respectively, have both high affinity for neurokinin 1 receptor[28-32]. Neurokinin 1 receptor, the well-documented site of action for substance P, is an integral membrane protein belonging to the rhodopsin-type family of G-protein coupled receptors[33-35]. Neurokinin 1 receptors may be important mediators of substance P evoked excitation in the ventral pallidum and affect the postsynaptic excitability of the cholinergic neurons in this brain region[36]. It has been suggested that substance P has excitatory effects in the hippocampus and is able to facilitate long-term potentiation via activation of the neurokinin 1 receptor[4].
The hippocampus is an important component of the cerebral marginal system. It contains two main regions, Ammon’s horn[37]and the dentate gyrus. There are two competing ideas regarding hippocampal function: inhibition and memory. The behavioral inhibition theory was popular up to the 1960s[38]. Animals with hippocampal damage often have difficulty learning to inhibit responses that they have previously been taught, especially if the response requires remaining quiet as in a passive avoidance test. Gray et al.[39]developed the role of the hippocampus in anxiety. The second idea regarding of hippocampal function is that it relates to memory. This idea derived its main impetus from a famous report by Scoville and Milner[40]describing the results of surgical destruction of the hippocampus in Squire[41]. The hippocampus clearly plays an important role in memory; however, the precise nature of this role remains widely debated[42]. Substance P-related projections to the hippocampus originate from several brain regions including the medial septum and supramammilary area[43-44]. In particular, these projections to the hippocampus are related to learning and memory, especially to spatial cognitive function. After hippocampal damage, rats may produce physiochemical alternation to early senile dementia[11]and manifest defects in learning and memory[12-13].
The striatum, a major nucleus of the basal ganglia, is involved in the orchestration of complex behaviors and extensive evidence has shown that it also plays a role in learningand memory. In particular, the striatum has been implicated in the acquisition of instrumental responses, habit formation, and various motor learning tasks[45]. In the marginal division, which is at the caudomedial edge of the caudate putamen and rostrolateral to the globus pallidus, the distribution of neurokinin 1 receptor-positive neurons has been observed to be similar to that in the ‘‘patch’’ compartment. This observation raises the possibility that the marginal division is involved in learning and memory and is presumed to be a new component of the limbic system. The marginal division discovered by Shu et al.[46]in mammals, a part of the striatum, located between nucleus caudatus putamen and globus pallidus, is the band structure composed of spindle cells that are distributed dorsoventrally and has also been shown to be involved in learning and memory in the electric Y-maze test with a rather complicated conditional reflex behavior[19,47]. The marginal division is involved in learning and memory, and has intense structural and functional connections with other memory-related brain regions[48]. The marginal division plays an important role in the learning and memory circuit[49-50]. The marginal division contains a large amount of neurotransmitters relevant to learning and memory functions and has extensive fibrous connection with globus pallidus, black substance, thalamus, amygdaloid nucleus and the basal nucleus of Meynert[22,51].
This study reports that (1) neurokinin 1 receptor-positive neurons in the hippocampus are multipolar, round, oval or triangular shapes and show light staining and (2) neurokinin 1 receptor-positive neurons in the marginal division are spindles with two dendrites extending dorsoventrally over a long distance from two poles of neuronal cell bodies.
Different mazes are used to speci fi cally examine different aspects of learning and memory. The Y-maze can determine memory rapidly and is sensitive to various parameters of behavior and effective spatial memory[52]. Therefore, the Y-maze has been applied extensively to verify differential learning, spatial alternative performance, and working and reference memory[53-54]. To study the role of substance P in the hippocampus and marginal division in learning and memory, we previously used a selective neurokinin 1 receptor antagonist to investigate whether blockade of neurokinin 1 receptors in these regions impairs learning and memory[36]. In the current study using the Y-maze, footshock times in the unilateral hippocampus or marginal division injection groups and in the bilateral hippocampus or marginal division injection groups increased significantly compared with the corresponding group before microinjection of antisense oligonucleotides against neurokinin 1 receptor mRNA. Moreover, footshock times in the bilateral injection groups increased significantly after the microinjection compared with the unilateral injection groups in the hippocampus and marginal division. These findings indicate that substance P in the hippocampus and marginal division is involved in learning and memory through the neurokinin 1 receptor. Furthermore, there were no signi fi cant differences in the bilateral or unilateral injection groups between the hippocampus and the marginal division, which demonstrates that learning and memory was inhibited while neurokinin 1 receptor was blocked in the hippocampus and marginal division.
Our fi ndings are consistent with those of previous studies. Damage of bilateral marginal division of the striatum can remarkably affect hippocampal long-term potentiation[55]. Langosch et al.[4]demonstrated that substance P has excitatory effects in the hippocampus and is able to facilitate long-term potentiation via activation of neurokinin 1 receptor in the hippocampus. Huston et al.[25]found that when injected into the lateral hypothalamus, medial septum, ventral pallidum and after systemic administration substance P facilitates learning and memory in rats. In a study by Kertes et al.[26], the results not only demonstrated that substance P plays important roles in passive avoidance learning when injected either into the globus pallidus or into the amygdaloid body, but also showed that substance P and neurokinin 1 receptors facilitate pallidal-mediated positive reinforcement[27]. Substance P has neurotrophic and memory-promoting effects upon direct application into the nucleus basalis magnocellularis[24]. Thus, neurokinin 1 receptor may be a mediator of the effects of substance P in learning and memory.
Results from this study confirmed that neurokinin 1 receptor-positive neurons are enriched both in the hippocampus and marginal division, and hippocampus and marginal division of the striatum play important functions in learning and memory in the cerebrum. The hippocampus was the fi rst region discovered related to learning and memory function of the brain and has been widely studied since this discovery, especially its role in spatial cognitive function in animals and humans[56]. However, it is not clear whether its learning and memory functions are different from those of the marginal division of the striatum. Some researchers have proposed that the marginal division of the striatum is probably a subcortical center and have hypothesized that it is a medial pivot associated with subcortical structures, like the cortex and hippocampus[51]. The fi ndings of the present study further support this hypothesis. Whether the marginal division of the striatum is able to control the learning and memory functions of the hippocampus or there is a relationship between the hippocampus and marginal division remain poorly understood. Thus, there is a need for more studies to compare the functions of the hippocampus and marginal division of the striatum to further confirm the differences and functional importance of both in controlling learning and memory in the cerebrum. Additional behavioral assays of learning and memory, such as the Morris water maze[57], can be used to compare the functions of the hippocampus and marginal division and help elucidate the functions of various neural circuits associated with learning and memory.
In conclusion, our experiments on the expression of neurokinin 1 receptor in the hippocampus and marginal division of the striatum and the in fl uence of blockade of the neurokinin 1 receptor using oligonucleotides against neurokinin 1 receptor mRNA indicate that neurokinin 1 receptor mediates the role of substance P in learning and memory in the hippocampus and marginal division. There was nosignificant difference in neurokinin 1 receptor-dependent effects between the hippocampus and marginal division on learning and memory function in rats after blockade of neurokinin 1 receptor mRNA. That is, the marginal division plays a similar function in learning and memory to the hippocampus, establishing a role of the marginal division in learning and memory.
Materials and Methods
Design
A randomized controlled animal study.
Time and setting
Experiments were performed in the MOE Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, China from May 2011 to March 2012.
Materials
A total of 50 young male Sprague-Dawley rats, weighing 200-250 g and aged 2-3 months, were purchased from Guangdong Provincial Medicine Laboratory Animal Center (License No. SCXK (Yue) 2008-0002). All rats were housed under controlled conditions at 22 ± 2°C in a 12-hour light/ dark cycle. The animals had free access to food and water. All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[58]and the study was approved by the Animals Ethics Committee, South China Normal University in China.
Methods
Stereotaxic coordinates of brain microinjections
The rats were anesthetized with 10% chloral hydrate and set on a stereotaxic apparatus (RWD Life Science Co., Ltd., Shenzhen, Guangdong Province, China). A hole was drilled on the craniums of the rats with an electric drill. All injections were administered through a micro-glass-tube (20 μm in a diameter) at 1 μL/10 minutes. The coordinates for the marginal division and hippocampus were[59]the marginal division: anteroposterior = bregma -1.5 mm, mediolateral = ± 4.1 mm, height = 5.5 mm; hippocampus: anteroposterior = bregma -2.0 mm, mediolateral = ± 2.2 mm, height = 3.5 mm. The bilateral marginal division or hippocampus injection groups received 2 μL neurokinin 1 receptor antisense nucleotide (1 mg/mL, Chemicon, Temecula, CA, USA) injections. The normal control group received 2 μL normal saline as a placebo both in the marginal division and hippocampus. The unilateral marginal division or hippocampus injection group received injections in the left hemisphere. The two oligonucleotides fragment sequences of rat neurokinin 1 receptor gene (Academia Sinica Shanghai Biochemistry Institute, Shanghai, China), nucleotides 559-606 and 1,075-1,140, were 5′-GCA TCC CAA CAG GAC TTA TGA GAA AAG CGT ACC-3′ and 5′-CCA CTG TGG TGG GAG CCC ATG AGA AGC-3′. The ratio of the two kinds of nucleotide chains was 1:1. Five days after the brain microinjections, Y-maze tests were carried out again to check the memory ability of the rats. The data were recorded by criterion 9/10. Representation of criterion 9/10 was the total footshock times, which the rats needed to run correctly 9 times among the 10 tests[60].
Y-maze performance following gene blockade using neurokinin 1 receptor mRNA
The Y-maze was a three-armed maze with equal angles between all arms, which are 50 cm long and 16 cm wide with walls 14 cm high, enclosed with Plexiglas. There was a signal lamp at the end of each of three arms and on the bottoms of the maze was a copper shock grid (0.2 cm in diameter and 14 cm long with 1.0 cm space). When the rats were tested, only one arm had a light on (bright arm), indicating a safe area without footshock, whereas the other two had the light off (dark arm), indicating unsafe areas with footshock (1.5 mA, with 125 ms stimulus duration). The safe arm and the unsafe arms were set randomly. It is considered to be the correct response when the rats directly ran to the bright arm in 10 seconds after changing the safe and unsafe arms in the Y-maze. In our experiments, the rats were pretested 30 times with the Y-maze at 1 and 3 days. Only the rats running correctly more than 15 times among the 30 tests were chosen for further experiments. All tests were carried out in a dark and quiet small room[61].
Preparation of rat brain tissue section
After the behavioral test, rats were anesthetized with 10% chloral hydrate (0.4 mL/kg) and then perfused through the heart/ ascending aorta with 150 mL 0.9% normal saline, followed by 500 mL 4% paraformaldehyde dissolved in 0.01 mol/L PBS with pH 7.2-7.4 for 2 hours. The brains of the rats were removed quickly and stored in 4% paraformaldehyde at 4°C overnight to post fi x, and then equilibrated in 0.01 mol/L PBS (pH 7.4) containing 10%, 20%, 30% sucrose at 4°C, until the brains sank to the bottom of the container. The brains were sectioned into 30 μm-thick slices using a cryostat microtome (Lecia CM1950, Jena, Germany). Sections were collected in 0.01 mol/L PBS (pH 7.4) and processed free- fl oating for immuno fl uorescence.
Immunofluorescence methods for the location and cytoarchitectural characteristics of the hippocampus and marginal division
Sections in the hippocampus and marginal division collected by the process described above were rinsed with 0.01 mol/L PBS (pH 7.4) on a rocking bed (60 r/min, 10 minutes × 3 times), and then non-speci fi c binding was blocked with 0.3% Triton X-100 and 3% normal goat serum in 0.01 mol/L PBS (pH 7.4) for 0.5 hour at 37°C. The sections were then incubated in guinea pig anti-neurokinin 1 receptor polyclonal antibodies (1:1,000; Chemicon) diluted in 0.01 mol/L PBS (pH 7.4) with 1% bovine serum albumin, 0.3% TritonX-100 and 0.05% sodium azide (NaN3) for 36-48 hours at 4°C. On the following day, after being thoroughly washed with 0.01 mol/L PBS (pH 7.4), the sections were incubated in a Cy3-goat anti-guinea pig IgG (1:300; Chemicon) at room temperature for 3 hours. After being washed three times with PBS, sections were then mounted onto gelatine-coated slides and coverslipped with liquid paraffin and then pho-tographed using a fl uorescence microscope (Lecia). Images were recorded through a CoolSNAP CF2 Color camera (Roper, Photometrics, San Francisco, CA, USA), analyzed and displayed in CoolSNAP software. Contrast and brightness were adjusted in Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA, USA). For the negative control tests, 0.01 mol/L PBS (pH 7.4) was used to replace the primary antibody and the corresponding secondary antibody in single immunolabeling.
Statistical analysis
All data were expressed as mean ± SD. The statistical analyses were performed using SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). The distribution of the values was checked for normality, and the comparisons between the groups were conducted by one-way analysis of variance followed by Student’s t-test. A value of P < 0.05 was considered statistically signi fi cant.
Author contributions:Yu Y participated in study concept and design, analysis and interpretation of data, drafting of the manuscript. Zeng CC was in charge of study design, analysis and data interpretation. Shu SY was responsible for study design, and stereotaxic coordinates of brain microinjections. Liu XM performed the operation and immunofluorescence detection. Li CH was in charge of image processing and analysis. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:Comparing the distribution of neurokinin 1 receptor between the hippocampus and striatal marginal division in learning and memory using a series of experimental methods, found that substance P has played an important role in learning and memory, mediated by neurokinin 1 receptor in the hippocampus and striatal marginal division.
[1] Pennefather JN, Lecci A, Candenas ML, et al. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74(12):1445-1463.
[2] Severini C, Improta G, Falconieri-Erspamer G, et al. The tachykinin peptide family. Pharmacol Rev. 2002;54(2):285-322.
[3] Carvalho MC, Masson S, Brandão ML, et al. Anxiolytic-like effects of substance P administration into the dorsal, but not ventral, hippocampus and its influence on serotonin. Peptides. 2008;29(7):1191-1200.
[4] Langosch JM, Kupferschmid S, Heinen M, et al. Effects of substance P and its antagonist L-733060 on long term potentiation in guinea pig hippocampal slices. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):315-319.
[5] Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281(5383):1640-1645.
[6] Tau fi q AM, Fujii S, Yamazaki Y, et al. Involvement of IP3 receptors in LTP and LTD induction in guinea pig hippocampal CA1 neurons. Learn Mem. 2005;12(6):594-600.
[7] Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, et al. Spatial association of prolyl oligopeptidase, inositol 1,4,5-triphosphate type 1 receptor, substance P and its neurokinin-1 receptor in the rat brain: an immunohistochemical colocalization study. Neuroscience. 2008;153(4):1177-1189.
[8] Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41-50.
[9] Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18(5-6):365-374.
[10] Ramirez OA, Carrer HF. Correlation between threshold to induce long-term potentiation in the hippocampus and performance in a shuttle box avoidance response in rats. Neurosci Lett. 1989;104(1-2):152-156.
[11] Krügel U, Bigl V, Eschrich K, et al. Deafferentation of the septo-hippocampal pathway in rats as a model of the metabolic events in Alzheimer’s disease. Int J Dev Neurosci. 2001;19(3):263-277.
[12] Alberini CM. Genes to remember. J Exp Biol. 1999;202(Pt 21):2887-2891.
[13] Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19(7):2789-2798.
[14] Borhegyi Z, Leranth C. Substance P innervation of the rat hippocampal formation. J Comp Neurol. 1997;384(1):41-58.
[15] Deng PY, Porter JE, Shin HS, et al. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577(Pt 2):497-511.
[16] Zaninetti M, Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci. 2000;12(11):3975-3984.
[17] Maeno H, Kiyama H, Tohyama M. Distribution of the substance P receptor (NK-1 receptor) in the central nervous system. Brain Res Mol Brain Res. 1993;18(1-2):43-58.
[18] Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides. 1998;32(1):1-49.
[19] [19] Shu SY, Penny GR, Peterson GM. The ‘marginal division’: a new subdivision in the neostriatum of the rat. J Chem Neuroanat. 1988;1(3):147-163.
[20] Chudler EH, Sugiyama K, Dong WK. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. J Neurophysiol. 1993;69(6):1890-1903.
[21] Shu SY. Marginal division of the neostriatum: a subcortical memory center. J Biomed Sci. 2003;10(1):14-29.
[22] Shu SY, Bao XM, Zhang C, et al. A new subdivision, marginal division, in the neostriatum of the monkey brain. Neurochem Res. 2000;25(2):231-237.
[23] Zeng J, Shu SY, Bao X, et al. Properties of acetylcholine receptor ion channels in the acutely dissociated neurons of the marginal division in the rat striatum. Neurochem Res. 1999;24(12):1571-1575.
[24] Huston JP, Hasenöhrl RU. The role of neuropeptides in learning: focus on the neurokinin substance P. Behav Brain Res. 1995;66(1-2):117-127.
[25] Huston JP, Oitzl MS. The relationship between reinforcement and memory: parallels in the rewarding and mnemonic effects of the neuropeptide substance P. Neurosci Biobehav Rev. 1989;13(2-3):171-180.
[26] Kertes E, László K, Berta B, et al. Effects of substance P microinjections into the globus pallidus and central nucleus of amygdala on passive avoidance learning in rats. Behav Brain Res. 2009;198(2):397-403.
[27] Kertes E, László K, Berta B, et al. Positive reinforcing effects of substance P in the rat globus pallidus revealed by conditioned place preference. Behav Brain Res. 2010;215(1):152-155.
[28] Hastrup H, Schwartz TW. Septide and neurokinin A are high-af fi nity ligands on the NK-1 receptor: evidence from homologous versus heterologous binding analysis. FEBS Lett. 1996;399(3):264-266.
[29] Sagan S, Beaujouan JC, Torrens Y, et al. High af fi nity binding of [3H]propionyl-[Met(O2)11]substance P(7-11), a tritiated septide-like peptide, in Chinese hamster ovary cells expressing human neurokinin-1 receptors and in rat submandibular glands. Mol Pharmacol. 1997;52(1):120-127.
[30] Beaujouan JC, Saffroy M, Torrens Y, et al. Pharmacological characterization of tachykinin septide-sensitive binding sites in the rat submaxillary gland. Peptides. 1999;20(11):1347-1352.
[31] Beaujouan JC, Saffroy M, Torrens Y, et al. Different subtypes of tachykinin NK(1) receptor binding sites are present in the rat brain. J Neurochem. 2000;75(3):1015-1026.
[32] Torrens Y, Beaujouan JC, Saffroy M, et al. Further evidence for the presence of “septide-sensitive” tachykinin binding sites in tissues possessing solely NK(1) tachykinin receptors. Biochem Biophys Res Commun. 2000;270(2):668-672.
[33] Yokota Y, Sasai Y, Tanaka K, et al. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989; 264(30):17649-17652.
[34] Hershey AD, Krause JE. Molecular characterization of a functional cDNA encoding the rat substance P receptor. Science. 1990;247(4945):958-962.
[35] Takeda Y, Chou KB, Takeda J, et al. Molecular cloning, structural characterization and functional expression of the human substance P receptor. Biochem Biophys Res Commun. 1991; 179(3):1232-1240.
[36] Singewald N, Chicchi GG, Thurner CC, et al. Modulation of basal and stress-induced amygdaloid substance P release by the potent and selective NK1 receptor antagonist L-822429. J Neurochem. 2008;106(6):2476-2488.
[37] Portavella M, Vargas JP, Torres B, et al. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in gold fi sh. Brain Res Bull. 2002;57(3-4):397-399.
[38] Nadel L, O’Keefe J, Black A. Slam on the brakes: a critique of Altman, Brunner, and Bayer’s response-inhibition model of hippocampal function. Behav Biol. 1975;14(2):151-162.
[39] Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press. 2000.
[40] Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11-21.
[41] Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009;61(1):6-9.
[42] Squire LR. Memory and the hippocampus: a synthesis from fi ndings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195-231.
[43] Peterson GM, Shurlow CL. Morphological evidence for a substance P projection from medial septum to hippocampus. Peptides. 1992;13(3):509-517.
[44] Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Exp Brain Res. 1997;115(2):369-374.
[45] Dang MT, Yokoi F, Yin HH, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci U S A. 2006;103(41):15254-15259.
[46] Shu SY, Bao XM, Ning Q, et al. New component of the limbic system: Marginal division of the neostriatum that links the limbic system to the basal nucleus of Meynert. J Neurosci Res. 2003;71(5):751-757.
[47] Jiang G, Shu SY, Bao XM, et al. Comparison of escape learning and memory function between marginal division of the striatum and hippocampus. Zhongguo Linchuang Kangfu. 2005;9(32):254-256.
[48] Liu XM, Shu SY, Zeng CC, et al. The role of substance P in the marginal division of the neostriatum in learning and memory is mediated through the neurokinin 1 receptor in rats. Neurochem Res. 2011;36(10):1896-1902.
[49] Shu SY, Bao XM, Ning Q, et al. New component of the limbic system: Marginal division of the neostriatum that links the limbic system to the basal nucleus of Meynert. J Neurosci Res. 2003; 71(5):751-757.
[50] Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007; 8(11):872-883.
[51] Shu SY, Wu YM, Bao XM, et al. A new area in the human brain associated with learning and memory: immunohistochemical and functional MRI analysis. Mol Psychiatry. 2002;7(9):1018-1022.
[52] Conrad CD, Lupien SJ, Thanasoulis LC, et al. The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res. 1997;759(1):76-83.
[53] Biggan SL, Beninger RJ, Cockhill J, et al. Quisqualate lesions of rat NBM: selective effects on working memory in a double Y-maze. Brain Res Bull. 1991;26(4):613-616.
[54] Riedel G, Wetzel W, Reymann KG. Computer-assisted shock-reinforced Y-maze training: a method for studying spatial alternation behaviour. Neuroreport. 1994;5(16):2061-2064.
[55] Shu SY, Bao XM, Wu YM, et al. Hippocampal long-term potentiation attenuated by lesions in the marginal division of neostriatum. Neurochem Res. 2003;28(5):743-747.
[56] Astur RS, Taylor LB, Mamelak AN, et al. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132(1):77-84.
[57] D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001; 36(1):60-90.
[58] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[59] Bao XM, Shu SY. The Stereotaxic Atlas of the Rat Brain. Beijing: People’s Medica Publishing House.1991.
[60] Fletcher PJ. Tryptamine impairs the acquisition of a one-way active avoidance task. Pharmacol Biochem Behav. 1989;32(1):317-321.
[61] Spain JW, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology (Berl). 1991;105(1):101-106.
Copyedited by Murnane K, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.131603
http://www.nrronline.org/
Accepted: 2014-02-08
- 中国神经再生研究(英文版)的其它文章
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Apoptosis is an obstacle to the differentiation of adipose-derived stromal cells into astrocytes
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases

