Partial characterization of superoxide dismutase activity in the Barber pole worm-Haemonchus contortus infecting Capra hircus and abomasal tissue extracts
Sadia Rashid, Malik Irshadullah,2*
1Section of Parasitology, Department of Zoology, Aligarh Muslim University, Aligarh-202002, U.P, India
2Faculty of Applied Medical Sciences, Jazan University, PO Box 114, Jazan-45142, Kingdom of Saudi Arabia
Partial characterization of superoxide dismutase activity in the Barber pole worm-Haemonchus contortus infecting Capra hircus and abomasal tissue extracts
Sadia Rashid1, Malik Irshadullah1,2*
1Section of Parasitology, Department of Zoology, Aligarh Muslim University, Aligarh-202002, U.P, India
2Faculty of Applied Medical Sciences, Jazan University, PO Box 114, Jazan-45142, Kingdom of Saudi Arabia
ARTICLE INFO
Article history:
Received 25 Feb 2014
Received in revised form 20 Mar 2014
Accepted 10 Jun 2014
Available online 2 Jul 2014
Superoxide dismutase
Haemonchus contortus
Polyacrylamide gel electrophoresis
Cu/Zn
3-D densitogram
Objective:To determine the activity of superoxide dismutase (SOD) in the male and female haematophagous caprine worms, Haemonchus contortus infecting Capra hircus, and their E/S products and also to analyse the effect of Haemonchus infection on the level of host SOD.
1. Introduction
Haemonchus contortus(H. contortus), a highly pathogenic gastrointestinal nematode parasite of sheep and goat causes great production losses in these animals due to its bloodfeeding behaviour. Both adult and fourth stage larvae are haematophagous and thereby responsible for severe anaemia, loss in weight, milk, meat and wool production and frequent death particularly in young animals[1-3]. Average blood loss due toH. contortusinfection has been reported to be 0.03 mL per day per worm[3]. The adult parasites cause oedema and hemorhages to abomasal mucosa due to continuous attachment[4].
Parasites are exposed to the reactive oxygen species (ROS) such as superoxide anion (O2-), hydroxyl radicals(.OH) andhydrogen peroxide (H2O2), produced during normal cellular metabolic processes[5,6]. The production of ROS generated by macrophagous, neutrophils and eosinophils increases considerably in response to parasitic infection, which is thought to play a role in killing or expulsion of parasite from the host and thereby prevent the establishment of infection[7-10]. ROS are deleterious and can damage various biomolecules by oxidation of protein, lipid peroxidation, depolymerization of polysaccharide and nucleic acids[6,11]. To counter these destructive processes, parasites have developed several protective mechanisms, including the production of antioxidant enzymes which are used to neutralize the free radicals generated by the host and repair the ROS derived damage[6]. In addition to this, some parasites induce alteration in host metabolism in such a way that reduces the production of ROS particularly in their microhabitat[6, 9,12].
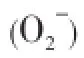
The SOD activity has been detected in many parasitic nematodes species such asTrichinella spiralis(T. spiralis),Trichinella pseudospiralisandTrichinella nelsoni[17],Trichostrongylus colubriformis,Trichostrongylus vitrinus,Nematodirus battus,Nippostrongylus brasiliensis(N. brasiliensis), Teladorsagia circumcinctaandH. contortus[18,19],Dirofilaria immitis[20],Litomosoides cariniiandSetaria cervi(S. cervi)[21],Dictyocaulus viviparous[22],N. brasiliensisandNematospiroides dubius[7]. In these studies the sex of the parasite was not taken into consideration, however, few studies have been carried out on male and female worms ofBrugia malayi(B. malayi)[14],S. cerviandAscaris suum[23,24], but there is paucity of information on SOD activity of male and femaleH. contortus. Furthermore, there is no study of SOD available inH. contortusin the Asian continent as Yinet al.[4] have found high level of genetic differentiation amongH. contortusfrom different continents.
The present study was, therefore, undertaken to characterize the SOD in male and femaleH. contortus, its excretory/ secretory (E/S) forms and in the host tissue. The effect ofH. contortuson the level of host SOD which may be helpful in understanding the host parasite relationship was also investigated.
2. Materials and methods
2.1. Collection and preparation of parasite host tissues extracts
In the present study, male and femaleH. contortus, (ovigerous and non-ovigerous mature), uninfected, low (<100 worms), mild (100-500 worms) and heavily (>1 000 worms) infected host tissue (abomasum) and E/S products of parasites were used for the estimation of SOD activity. All specimens were collected from the naturally infected goats (Capra hircus), slaughtered at the local abattoir (Aligarh, India), washed several times with Hanks balanced salt solution premaintained at (37±2) °C to remove all the debris. Isolated parasites and small pieces of host tissues were blotted and homogenized separately in a Potter Elvehjm homogenizer in 0.1 mol/L ice cold phosphate buffer (pH 7.4) at 4 °C. The homogenate were sonicated (Ultrasonic processor-5 mm probe) on an ice bath for 3×1 min with 30 seconds interval and then centrifuged at 10 000 r/min for 15 min at 4 °C in microfuge (Hitachi, Japan). After centrifugation, the supernatants were collected and stored at -20 °C in the form of aliquots for further use.
2.2. Collection ofE/Sproducts
In order to obtain E/S products, equal numbers (150) of males and females were incubated separately in 5 mL RPMI-1640 medium (Hi media, AT028) in water bath premaintained at (37±2) °C for 6 h. After incubation, the worms were removed and medium was centrifuged at 10 000 r/min for 5 min and then concentrated by dialysis, using cellulose tubing (Sigma-Aldrich, D9777).
2.3. Enzyme assay
The activity of SOD was measured by the inhibition of pyrogallol autoxidation procedure of Marklund and Marklund[25], with minor modifications. A total of 0.05 mL sample solution was added to 2.85 mL of 50 mmol/L triscacodylate buffer (pH 8.5), containing 1 mmol/L diethylene-triaminepentaacetic acid and 1 mmol/L ethylene diamine tetraacetic acid, thoroughly mixed and incubated at 25 °C for 10 min. Purified bovine liver Cu/Zn SOD (Sigma Chemical Co., USA) preparation was also run simultaneously to standardize the assay. After incubation, the reaction was initiated by the addition of 0.1 mL of freshly prepared 2.6 mmol/L pyrogallol solution to attain a final concentration of 0.13 mmol/L in the assay mixture. The assay mixture was transferred to a 3.5 mL cuvette and the rate of increase in the absorbance at 420 nm was recorded for 3 min after an initial lag period of 30 seconds in a UV/Vis spectrophotometer (Systronics, India). The lag period of 30 seconds was allowed for steady state of autoxidation of pyrogallol to be attained which is important for reproducibility of results. The activity of SOD was presented as unit/mg protein. One unit of SOD is described as the amount of enzyme required to cause 50% inhibition of pyrogallol autoxidation under specified assay condition. Protein concentration in the samples was determined by the dye binding method of Spector[26], using bovine serum albumin standard.
2.4. Determination of metallic cofactor ofSOD
To determine the types of SOD, crude homogenates of male and female parasite, their E/S products and host tissues (both infected and non-infected) were separately mixed with 0.5, 1.0, 3.0 and 5.0 mmol/L concentration of potassium cyanide (KCN), 1, 3, 5 and 10 mmol/L concentration of sodium azide (NaN3), and hydrogen peroxide (H2O2) and incubated at 37 °C for 10 min. After incubation, the SOD activity in each sample was measured as described above. Based on per cent inhibition, the presence of metallic cofactor in the active site of SOD was identified. Inhibition studies in the gels were also performed by using only the final concentrations of above mentioned inhibitors.
2.5. Electrophoretic fractionation ofSOD
The parasites and host SODs were fractionationated on 12.5% polyacrylamide gel under native conditions by using discontinuous buffer system of Laemmli[27], without sodium dodecyl sulfonate and 2 mercaptoethanol. The sample solutions and purified Cu/Zn SOD from bovine liver (Sigma Chemical Co., USA) were separately incubated in Laemmli’s sample buffer (without sodium dodecyl sulfonate) in 3:1 ratio (v/v) for 15 min at room temperature and then loaded onto the gel. After loading 40 µg protein samples and 2 µg purified SOD, the electrophoresis was carried out at 100 V for 2 h in refrigerator, using Mini Protean dual slab 3 cell system (Biorad Ltd., USA). After electrophoresis, the staining of the gels for SOD activity was performed according to the methods of Beauchamp and Fridovich[28]. In brief, the gels were soaked in a solution containing 0.2% nitroblue tetrazolium, 0.028 mol/L N,N,N”,N”-tetramethylenediamine and 2.8×10-5mol/L riboflavin in 50 mmol/L potassium phosphate buffer (pH 7.8) for 1 h at room temperature in the dark and shaken at constant intervals. After incubation, the gel was rinsed thoroughly with double distilled water and placed under a fluorescent light until achromatic zones indicating SOD activity were clearly visible in the blue background. For inhibition studies, inhibitors were added in incubation medium at a final concentration of 5 mmol/L in case of KCN and 10 mmol/L for NaN3and H2O2.
2.6. Documentation and gel analysis
Stained gels were scanned on all in one HP Deskjet (F2235) computer assembly and densitogram was then prepared using Image J (1.46 r) software (National Institute of Health, USA).
2.7. Statistical analysis
Statistical analysis was performed by using One way ANOVA followed by thepost hocTukeys HSD multiple comparisons test using the statistical software R (2.15.1 version, Austria)[29]. Confidence level was held at 95% andP<0.05 was considered as significant.
3. Results
3.1. SOD activity in parasites and host tissues
Specific SOD activity measured in soluble extracts of parasites and host tissues and E/S products of parasites are presented in Table 1. Ovigerous females showed significantly higher values than non ovigerous adult females and males, whereas, the differences between male and non ovigerous adult female were insignificant (Figure 1a). Maximum specific SOD activity was observed in the non-infected host tissue which declined in the infected tissue and was related to the worm burdens (Table 1). Statistical analysis revealed that heavily infected host tissue had significantly low level of SOD than low, mild and non-infected tissues. However, differences between low and mild as well as between low and non-infected host tissues were insignificant (Figure 1b). The amount of SOD secreted by adult females wassignificantly higher than males (Figure 1a). The worms secrete approximately 6 times more SOD to the specific activity determined in their respective homogenates and the female worms secrete comparatively higher level of SOD than their male counterparts.
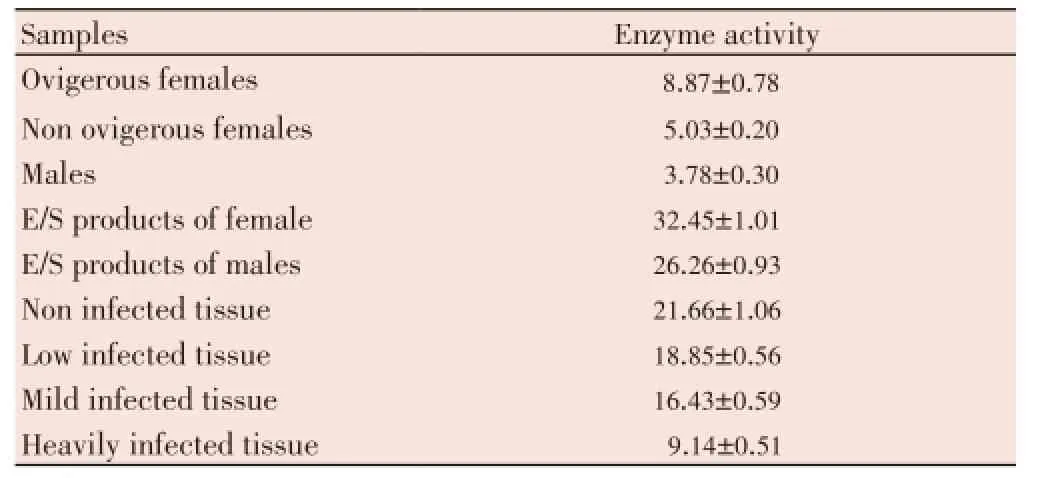
Table 1 Specfic activity of SOD in crude homogenates of H. contortus, E/S products and host tissues.
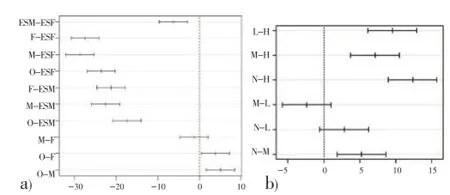
Figure 1. Post hoc Tukey tests for group wise comparisons of significant SOD activity.Each horizontal bar represents the differences between two groups with 95% confidence interval. If the interval excludes 0 then the difference is considered as significant for that pair wise comparison.a) O: Ovigerous female, F: Non ovigerous adult female, M: Male, ESF: E/S products of female , ESM: E/S products of male.b) N: Non-infected tissue, L: Low infected tissue, M: Mild infected tissue, H: Heavily infected tissue.
3.2. Electrophoretic analysis ofSOD
Analysis of SOD activity as determined in worm extracts by native polyacrylamide gel electrophoresis followed by specific enzyme staining by riboflavin-nitro blue tetrazolium method revealed 3 and 4 activity bands in male and female worms respectively, while, infected and non-infected host tissue presented only one activity band (Figure 2). The 2nd and 3rd activity bands of male and female parasites respectively on the gels (Figure 2, lanes 2 and 3) were present at the same level of host tissue (Figure 2, lanes 5 and 6), indicating that these bands may be of host origin. The E/ S products of adult worms also presented one activity band at the same level of fastest migrating band of the somatic extracts of parasites (Figure 2a). The 3D densitograms prepared from gel scans also demonstrate noticeable differences in the SOD activity bands in the parasite and host tissue (Figure 2b).
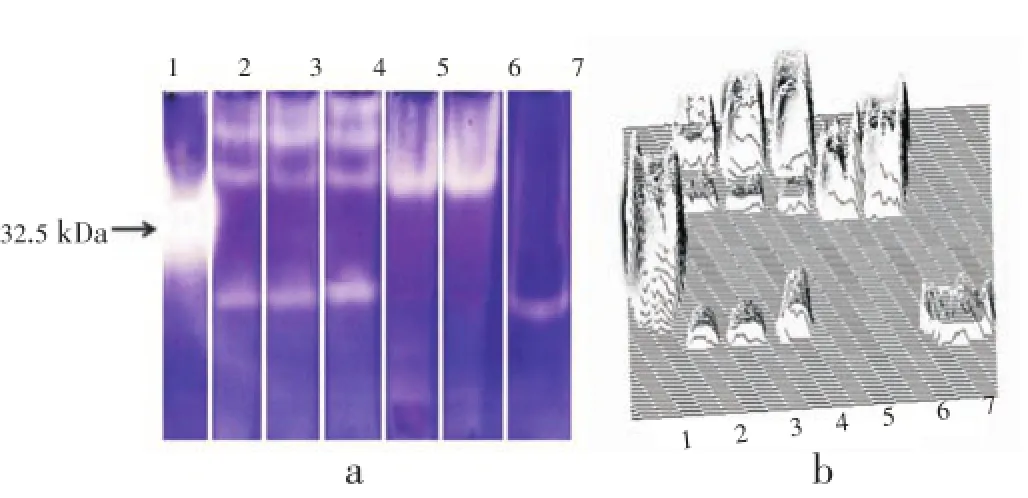
Figure 2. SOD isozyme profile from different H. contortus extracts and host tissue.a: The original gel; b: 3-D densitogram prepared using Image J. Lane 1: Purified bovine liver Cu/Zn SOD; Lane 2: Extracts of males; Lane 3: Nonovigerous adult female; Lane 4: Ovigerous female; Lane 5: Infected host tissue; Lane 6: Non-infected tissue; Lane 7: E/S products of both male and female H. contortus. Lanes were fractionated on 12.5% polyacrylamide gel electrophoresis and zones of SOD activity visualized as described in the Materials and methods section.
3.3. Characterization ofSOD
Appreciable amount of enzyme activity was inhibited by KCN which is a known inhibitor for Cu/Zn SOD, but not by H2O2and NaN3(Figure 3). Similarly, no activity band of SOD was detected following treatment of gels with 5 mmol/L KCN, whereas other inhibitors didn’t abolish the enzyme activity but only reduced the enzyme activity (data not shown). It demonstrates the noticeable effect of KCN, which reveals that KCN is the most potent inhibitor of SOD.
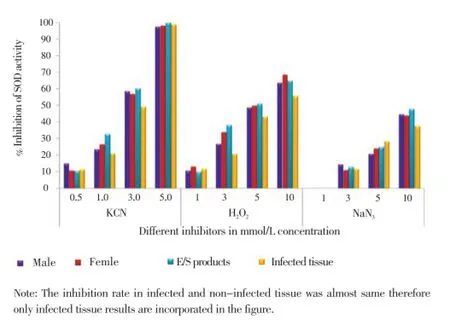
Figure 3. Effect of inhibitors on the SOD activity of male and female H. contortus, their E/S products and infected tissue.The SOD activity was assayed in the presence or absence of inhibitors and inhibition was presented as percentage of inhibition of the total SOD activity. KCN: Potassium cyanide, NaN3: Sodium azide, H2O2: Hydrogen peroxide.
4. Discussion
The presence of appreciable amount of SOD in adultH. contortusand its E/S products indicate that an active antioxidant system for evasion of host generated free radical attack is evident, which would ensure survival of parasite. Comparatively, higher SOD activity was found in female than maleH. contortusin our study. Contrarily, Ouet al.[14] and Sharma and Rathaur[23] reported higher SOD activity in males than females ofB. malayiandS. cervirespectively. FemaleH. contortusexpressed higher SOD levels than males and therefore may survive for prolonged period, as the survival of parasite in the host has been shown to be dependent on the level of free radical scavenging enzymes[8,10]. Furthermore, we recovered higher numbers of female than male parasite from infected abomasums which also provided support for prolonged survival of females. The high level of SOD in females as compared to males could be attributed to the greater need for protection of eggs and offspring from free radicals generated within the parasite. It has been pointed out that due to higher metabolic rate during reproductive phase of female, there is greater consumption of oxygen accompanied by liberation of superoxide anion radicals which could be lethal to the eggs if they are not neutralized by appropriate level of SOD[24]. Liddell and Knox[30], reported high SOD activity in the uterine region of adultH. contortusfemales by indirect immuno fluorescent staining and suggested that SOD may be required at some stage of egg production. The femaleH. contortusmay be more exposed to oxyhaemoglobin derived oxygen radicals because of sucking more blood than males for egg production[31], therefore they increase the induction of SOD to neutralize free radicals as suggested by Kotze[32], for higher catalase activity in the female parasites. Thus, females as compared to males may detoxify free radicals more effectively by increasing the production of SOD and may, therefore, offer more resistance to killing by free radicals, as also suggested forHeligmosomoides polygyrusby Ben Smithet al[8]. Therefore, differential level of SOD in male and femaleH. contortusin the present study could also be correlated with the susceptibility and resistance to highly reactive free radicals.
High level of SOD activity was detected in the culture medium as compared to somatic extract ofH. contortus, indicating that the enzyme is actively secreted by the parasite. The SOD activity in E/S products of male and femaleH. contortuswas about 6 times higher than their respective somatic extracts. Ouet al.[14] found 10 and 13 times more SOD activity in the E/S products of male and femaleB. malayi, respectively, than their somatic extracts. Several fold higher SOD activity in E/S products of adultFasciola hepaticaandParagonimus westermanihas also been reported as compared to their respective somatic extracts[33,34]. The E/S SOD may provide protection against free radicals generated at host-parasite interface and play an offensive role by causing damage to the inflammatory cells[11]. Protection of new born larvae ofT. spiralisfrom killing byin vitrogenerated oxidant have been demonstrated by co culturing the larvae with adultT. spiralis[35]. Thus the importance of antioxidant enzymes in E/S is quite significant for the successful establishment of parasites since they may provide a protective barrier against the host immune responses. Therefore, SOD is rightly called as the immune defense protein.
The decline of SOD activity in infected abomasums was found to be dependent on worm burden similar to those reported earlier for infected sheep red blood cell withTheilariasp. andBabesia ovis[36,37]. Contrary to this, Łuszczaket al.[38] and Assadyet al.[39] reported an increase in SOD level in infected bovine muscles and ovine liver withTaenia saginataandFasciolaspp. respectively. Significantly lower SOD activities in the infected abomasums in the present study indicate a decline in the antioxidant defence and enhance oxidative damage to the animals. Similar phenomenon has also been reported to occur in the ovine liver infected withFasciolasp. andDicrocoelum dentriticumand also in ovine skin infested withPsorptes ovis[40,41]. The fall of SOD activity in the infected host tissue could be explained by the superoxide anion dismutation to hydrogen peroxide caused by the overproduction of the superoxide anion linked to oxidative stress[42]. The superoxide anion also causes inactivation of Cu/Zn SOD[43]. The decrease in host SOD activity may also be due to the leakage of the cell content into the gut due to constant piercing activity byHaemonchusduring its haematophagous mode of feeding. Hypoproteinemia is an important consequence of haemonchosis, which is responsible for protein loosing enteropathy. Infected animals loose large quantities of serum protein into the gut and it was reported that mean daily faecal clearance of plasma fromHaemonchusinfected animal was 210-340 mL/day[3].
The fractionation of SOD isozymes by polyacrylamide gel electrophoresis revealed in the present study 4 and 3 SOD activity band in adult female and male worms respectively. The extra band in females may be an adaptive response to host generated free radicals attack and could be exploited for further studies. The infected and non-infected host tissue showed one activity band at the same level to that of the parasite SOD. The common activity band between parasite and the host tissue homogenates indicate that one SOD activity band of parasite may be of host origin, sinceactive exchange of materials between host and the parasite has been reported[44]. Similarly by IEF analysis, Honget al.[44] reported that SOD activity band of adult Schistosomes and hamster red blood cell had the same pI value and suggested that the schistosomes may acquire host SOD during the intravascular life cycle. The sensitivity of SODs to KCN, suggests the presence of Cu/Zn form of SOD in the parasites, their E/S products and host tissue. Similarly, many workers have suggested the presence of Cu/Zn SOD in different helminth parasites includingH. contortuson the basis of inhibition of SOD activity with KCN[19,44-46].
It is now clear from above discussion that SOD is a fundamental enzyme needed for the establishment and persistence ofH. contortuswithin the host. The possibility of interfering or blocking this enzyme could be the target of further investigations to weaken parasitic strategies which is the need of the hour as mass drug administration for parasite control has resulted in anthelmintic resistance across the globe and threatens the viability of sheep and goat industry in many regions of the world. Drug induced depression in the level of antioxidant enzymes of the parasite has been held responsible for elimination ofN. brasiliensis[47]. SOD is, therefore, been recommend as a valuable vaccine candidate.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are grateful to the Chairman of the Department of Zoology, Aligarh Muslim University, Aligarh for providing the laboratory facilities and to Professor Athar Ali Khan, Department of Statistics and Operations Research for his guidance in statistical analysis. This work was funded by University Grants Commission, New Delhi (F.40-3(M/ S)/2009(SA-III/MANF).
[1] Alberti EG, Zanzani SA, Ferrari N, Bruni G, Manfredi MT. Effects of gastrointestinal nematodes on milk productivity in three dairy goat breeds. Small Rumin Res 2012; 106(S): S12-S17.
[2] Al-Khayat DA, Al-Jebory ZE. The effect of Haemoncus contortus infection on productive performance of Awassi sheep and black Iraqi goats. J Adv Biomed Pathobiol Res 2012; 2: 25-29.
[3] Bordoloi G, Jas R, Ghosh JD. Changes in the haematobiochemical pattern due to experimentally induced haemonchosis in Sahabadi sheep. J Parasit Dis 2012; 36(1): 101-105.
[4] Yin F, Gasser RB, Li F, Bao M, Huang W, Zou F, et al. Genetic variability within and among Haemonchus contortus isolates from goats and sheep in China. Parasit Vectors 2013; 6: 279-287.
[5] Callahan HL, Crouch RK, James ER. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today 1988; 4(8): 218-225.
[6] Chiumiento L, Bruschi F. Enzymatic antioxidant systems in helminth parasites. Parasitol Res 2009; 105: 593-603.
[7] Batra S, Srivastava JK, Gupta S, Katiyar JV, Srivastava VM. Role of reactive oxygen species in expulsion of Nippostrongylus brasiliensis from rat. Parasitology 1993; 106: 185-192.
[8] Ben-Smith A, Lammas DA, Behnke JM. Effect of oxygen radicals and differential expression of catalase and superoxide dismutase in adult Heligmosomoides polygyrus during primary infections in mice with differing response phenotypes. Parasite Immunol 2002; 24: 119-129.
[9] Smith NC, Bryant C. Free radicals generation during primary infections with Nippostrongylus brasiliensis. Parasite Immunol 1989; 11: 147-160.
[10] Smith NC, Bryant C. The role of host generated free radicals in helminth infections: Nippostrongylus brasiliensis and Nematospiroides dubius compared. Int J Parasitol 1986; 16: 617-622.
[11] Henkle - Dührsen K, Kampkötter A. Antioxidant enzyme families in parasitic nematodes. Mol Biochem Parasitol 2001; 114: 129-142.
[12] Batra S, Chatterjee RK, Srivastava VML. Antioxidant enzymes in Acanthocheilonema vitea and effect of antifilarial agents. Biochem Pharmacol 1990; 40(10): 2363-2369.
[13] Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995; 64: 97-112.
[14] Ou X, Tang LA, Mccrossan M, Henkleduhrsen K, Selkirk ME. Brugia malayi: localisation and differential expression of extracellular and cytoplasmatic CuZn superoxide dismutases in adults and microfilariae. Exp Parasitol 1995; 80: 515-529.
[15] Tang L, Ou X, Henkle-Duhrsen K, Selkirk ME. Extracellular and cytoplasmic CuZn superoxide dismutases from brugia lymphatic filarial nematode parasites. Infect Immun 1994; 62(3): 961-967.
[16] Geller BL, Winge DR. A method for distinguishing Cu, Zn, and Mn-containing superoxide dismutase. Anal Biochem 1983; 128: 86-92.
[17] Hadas E, Rodriguez-Caabeiro F, Jimenez Gonzales J. Superoxide dismutase of Trichinella spiralis and Trichinella pseudospiralis larvae. Trop Med Parasitol 1993; 44: 195-196.
[18] Hadaś E, Stankiewicz M. Superoxide dismutase and total antioxidant status of larvae and adults of Trichostrongylus colubiformis, Haemonchus contortus and Ostertagia circumcincta. Parasitol Res 1998; 84: 646-650.
[19] Knox DP, Jones DJ. A comparison of superoxide dismutase (SOD,EC: 1.15.1.1) distribution in gastro-intestinal nematodes. Int J Parasitol 1992; 22(2): 209-214.
[20] Callahan HL, Hazen-Martin D, Crouch RK, James ER. Immunolocalization of superoxide dismutase in Dirofilaria immitis adult worms. Infect Immun 1993; 61: 1157-1163.
[21] Batra S, Chatterjee RK, Srivastastava VM. Antioxidant system of Litomosoides carinii and Setaria cervi: effect of a microfilaricidal agent. Vet Parasitol 1992; 43: 93-103.
[22] Britton C, Knox DP, Kennedy MW. Superoxide dismutase (SOD) activity of Dictyocaulus viviparus and its inhibition by antibody from infected and vaccinated bovine hosts. Parasitology 1994; 109: 257-263.
[23] Sharma M, Rathaur S. In vitro released filarial superoxide dismutase: its use in the diagnosis of lymphatic filariasis. J Parasit Dis 1998; 22: 82-88.
[24] Sanchez-Moreno M, Monteoliva M, Fatou A, García-Ruiz MA. Superoxide dismutase from Ascaris suum. Parasitology 1998; 97: 345-353.
[25] Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47: 469-474.
[26] Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem 1978; 86: 142-146.
[27] Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680-685.
[28] Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gel. Anal Biochem 1971; 44: 276-287.
[29] R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011.
[30] Liddell S, Knox DP. Extracellular and cytoplasmic Cu/Zn superoxide dismutases from Haemonchus contortus. Parasitology 1998; 116: 383-394.
[31] Musongong GA, Ndofor-Foleng HN, Abdurashid M. Prevalence and intensity of Haemonchus contortus infection in two breeds of small ruminants in Maiduguri, and arid zone of Northern Nigeria. Dairy Cattle Forum; 2011. [Online] Available from: http:// en.engormix.com/MA-dairy-cattle/health/forums/prevalenceintensity-haemonchus-contortus-t5167/165-p0.htm [Accessed on 23rd September, 2013]
[32] Kotze AC. Catalase induction protects Haemonchus contortus against hydrogen peroxide in vitro. Int J Parasitol 2003; 33: 393-400.
[33] Chung YB, Kong Y, Cho SY, Kang SY, Choi BC, Lee HS. Characterization of a peroxidase in excretory-secretory product of adult Paragonimus westermani. Korean J Parasitol 1993; 31(3): 259-267.
[34] Piacenza L, Radi R, Carmona C. CuZn superoxide dismutase activities from Fasciola hepatica. Parasitology 1998; 117: 555-562.
[35] Kazura JW, Meshnick SR. Scavenger enzymes and resistance to oxygen mediated damage in Trichinella spiralis. Mol Biochem Parasitol 1984; 10: 1-10.
[36] Nazifi S, Razvi SM, Kianiamin P, Rakhshandehroo E. Evaluation of erythrocyte antioxidant mechanisms: antioxidant enzyme, lipid peroxidation, and serum trace elements associated with progressive anemia in ovine maligant theileriosis. Parasitol Res 2011; 109: 275-281.
[37] Esmaeilnejad B, Tavassoli M, Asri-Rezaei S, Dalir-Naghadeh B. Evaluation of antioxidant status and oxidative stress in sheep naturally infected with Babesia ovis. Vet Parasitol 2012; 185: 124-130.
[38] Łuszczak J, Ziaja-Sołtys M, Rzymowska J. Anti-oxidant activity of superoxide dismutase and glutathione peroxidase enzymes in skeletal muscles from slaughter cattle infected with Taenia saginata. Exp Parasitol 2011; 128: 163-165.
[39] Assady M, Farahnak A, Golestani A, Esharghian MR. Enzyme activity assay in Fasciola spp. parasites and liver tissue extract. Iran J Parasitol 2011; 6: 17-22.
[40] Değer Y, Ertekin A, Değer S, Mert H. Lipid peroxidation and antioxidant potential of sheep liver infected naturally with distomatosis. Turkiye Parazitol Derg 2008; 32: 23-26.
[41] Dimri U, Sharma MC, Yamdagni A, Ranjan R, Zama MM. Psoroptic mange infestation increases oxidative stress and decreases antioxidant status in sheep. Vet Parasitol 2010; 168: 318-322.
[42] Santiard D, Ribiere C, Nordmann R, Houee-Levin C. Inactivation of Cu, Zn-superoxide dismutase by free radicals derived from ethanol metabolism: a gamma radiolysis study. Free Radic Biol Med 1995; 19: 121-127.
[43] Li AH, Kong Y, Cho SH, Lee HW, Na BK, Pak JK, et al. Molecular cloning and characterization of the copper/zinc and manganese superoxide dismutase genes from the human parasite Clonorchis sinensis. Parasitology 2005; 130: 687-697.
[44] Hong Z, Kosman DJ, Thakur A, Rekosh D, Lo Verde PT. Identification and purification of a second form of Cu/Zn superoxide dismutase from Schistosoma mansoni. Infect Immun 1992; 60(9): 3641-3651.
[45] Chung YB, Song CY, Lee HS, Kong Y, Cho SY. Purification and characterization of a Cu/Zn-superoxide dismutase from adult Paragonimus westermani. Kisaengchunghak Chapchi 1991; 29(3): 259-266.
[46] Kim TS, Jung Y, Na BK, Kim KS, Chung PR. Molecular cloning and experssion of Cu/Zn- containing superoxide dismutase from Fasciola hepatica. Infect Immun 2000; 68(7): 3941-3948.
[47] Srivastava JK, Batra S, Gupta S, Katiyar JC, Srivastava VM. Effect of anthelmintics on the antioxidant system of Nippostrongylus brasiliensis from rats. Biochem Pharmacol 1992; 43: 289-293.
10.12980/APJTB.4.2014APJTB-2014-0099
*Corresponding author: Malik Irshadullah, Section of Parasitology, Department of Zoology, Aligarh Muslim University, Aligarh-202002, U.P, India.
Tel: +966552709824
E-mail: malikirshadullah@yahoo.co.in
Foundation Project: Supported by University Grants Commission, New Delhi (F.40-3(M/S)/2009(SA-III/MANF).
Methods:The SOD activity was analysed by using the pyrogallol autoxidation assay and non-denaturing polyacrylamide gel electrophoresis followed by specific enzyme staining by riboflavin-nitroblue tetrazolium method.
Results:The adult females were found to have higher enzyme activity than the male worms. Appreciable amount of SOD activity was also detected in the worm culture medium and female worms secreted more SOD in comparison to the male parasites. The SOD activity was negatively correlated to the worm burden. Statistically significant decrease in SOD activity (P<0.05) was observed in the heavily infected host tissue in comparison to the control non-infected host tissue. SOD profile of the crude extracts of both the sexes revealed polymorphism and a fast migrating activity band being characteristic of E/S products. The SOD activities were found highly sensitive to potassium cyanide indicating the Cu/Zn form of SOD.
Conclusions:Haemonchus contortus is a key model parasite for drug and vaccine discovery. The presences of SOD activity in appreciable amount in the parasite as well as its E/S products indicate that it has a well-developed active antioxidant system to protect itself from the host immune attack. SOD could be the target for vaccine development which is the need of the hour as mass drug administration for parasite control has resulted in anthelmintic resistance across the globe and threatens the viability of sheep and goat industry in many regions of the world. The infection with Haemonchus causes a drastic reduction in SOD activity of the host tissue thus effecting its protective potential. One characteristic SOD band was found in the females which was not present in any other preparations and thus could be exploited for further studies on diagnostic/control measures.
 Asian Pacific Journal of Tropical Biomedicine2014年9期
Asian Pacific Journal of Tropical Biomedicine2014年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Hematological and serum biochemical aspects associated with a camel (Camelus dromedarius) naturally infected by Trypanosoma evansi with severe parasitemia in Semnan, Iran
- Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study
- Rate of carcass and offal condemnation in animals slaughtered at Yazd Slaughterhouse, central Iran
- Phytochemical screening and antioxidant activity of ethanol extract of Tithonia diversifolia (Hemsl) A. Gray dry flowers
- Acute brucellosis as unusual cause of immune thrombocytopenia: a case report and review of the literature
- A case report of cutaneous larva migrans in a Mexican population of high marginalization
