Identification of sitosteryl glucoside palmitate in a chloroform-derived fraction of Phyllanthus niruri with antiplasmodial and peripheral antinociceptive properties
Ezenyi Ifeoma Chinwude, Kulkarni Roshan, Joshi Swati, Salawu Oluwakanyinsola Adeola, Emeje Martins
1Department of Pharmacology and Toxicology, National Institute for Pharmaceutical Research and Development, Idu, Abuja, Nigeria
2Organic Chemistry Division, National Chemical Laboratory, Pune 411008, India
3Centre for Nanomedicine and Biophysical Drug Delivery, Department of Pharmaceutical Technology and Raw Material Development, National Institute for Pharmaceutical Research and Development, Idu, Abuja, Nigeria
Identification of sitosteryl glucoside palmitate in a chloroform-derived fraction of Phyllanthus niruri with antiplasmodial and peripheral antinociceptive properties
Ezenyi Ifeoma Chinwude1,2*, Kulkarni Roshan2, Joshi Swati2, Salawu Oluwakanyinsola Adeola1, Emeje Martins3
1Department of Pharmacology and Toxicology, National Institute for Pharmaceutical Research and Development, Idu, Abuja, Nigeria
2Organic Chemistry Division, National Chemical Laboratory, Pune 411008, India
3Centre for Nanomedicine and Biophysical Drug Delivery, Department of Pharmaceutical Technology and Raw Material Development, National Institute for Pharmaceutical Research and Development, Idu, Abuja, Nigeria
PEER REVIEW
Peer reviewer
Prof. Dr. Mpiana Pius Tshimankinda (PhD), Department of Chemistry, Faculty of Science, P.O. Box 190 Kinshasa XI, University of Kinshasa, Democratic Republic of the Congo.
Tel: +2438 1811 6019
E-mail: ptmpiana@yahoo.fr
Comments
This is an interesting work in which the authors have isolated and elucidated the structure of an antiplasmodial phytochemical sitosteryl-6-β-D-glucoside-6’-palmitate by the mean of NMR spectroscopy experiments and mass spectrometry. Modern cell biology techniques were used to validate the bioactivity and the safety of the pant, thus justifying pharmaceutical standardization of recipes from this plant material.
Details on Page 712
Objective:To evaluate the antiplasmodial properties of fractions of chloroform portion of Phyllanthus niruri (P. niruri) methanol extract and identify a suitable chemical marker present therein.
Malaria, Medicinal plant, Plasmodium falciparum
1. Introduction
Malaria remains a serious health problem in sub-Saharan Africa, tropical and many sub-tropical regions in the world. In 2011, about 3.3 billion people globally were at risk of malaria and approximately 90% of people who die from the disease reside in the WHO African region[1].Plasmodium falciparum(P. falciparum)causes the mostlethal forms of malaria: cerebral malaria and severe malaria anaemia which account for most malaria-associated mortalities, especially in children less than five years old[2]. Chloroquine- and multidrug-resistantP. falciparumstrains now prevail in malaria-endemic areas with high transmission rates; thus, artemisinin based combination therapies (ACT) for treating malaria in these areas are used. Even so, inaccessibility and high costs of the drugs hinder the use of ACTs and evidence suggests that the parasite has developed resistance to ACTs in certain areas, which implies the possibility of a rise in recrudescent disease and mortalities[3,4]. With few alternative antimalarial regimens, the search for new options that are safe, effective and affordable remains a priority. In most cases, plants have been investigated as sources of new leads as they show enormous potential. According to the identification of artemisinin, quinine and other antimalarial pharmacophores come from different plants[5-8]. The herb,Phyllanthus niruri(P. niruri) (family: Euphorbiaceae) is widely used as medicine in nearly all tropical countries where it is found. Decoctions of aerial parts of the plant are employed as a remedy for fever, diabetes, liver and kidney ailments. Previous investigations show its useful pharmacological properties[9-12]. Different investigators have also reported the antiplasmodial actions ofP. niruriextracts[13-15]. From the boiled aqueous extract ofP. niruri, four known compounds with antiplasmodial activity were isolated[13]. However, Sholikhahet al.described the methanol extract ofP. nirurias the most active extract, compared with the aqueous and chloroform extract[14]. Similarly, our earlier investigation ofP. nirurimethanol extract also showed that the chloroform portion of the extract was more effective againstPlasmodium bergheiin early infection than its ethanol and aqueous portions in mice[15]. The present study was carried out to determine active fractions of the organic chloroform fraction and identify compounds therein by antiplasmodial activity-guided fractionation, usingin vitroantiplasmodial activity against chloroquine-sensitive and chloroquine-resistantP. falciparumas bio-activity guide.
2. Materials and methods
2.1. Parasite strain and THP-1 cell line
Chloroquine-sensitivePlasmodium berghei(NK 65 strain) was sourced from the National Institute for Medical Research, Lagos, Nigeria. The parasite was maintained in the Department of Pharmacology and Toxicology, National Institute for Pharmaceutical Research and Development (NIPRD) by continuous reinfection by intraperitoneal passage in mice.
Chloroquine-sensitive (HB3) and chloroquine-resistant (FcM29)P. falciparumstrains were obtained from Centre National d’Application des Recherches Pharmaceutiques, Madagascar. THP-1 cell line was obtained from the National Centre for Cell Science, Pune, India.
2.2. Animals
Adult albino Wistar rats of either sex weighing 100-150 g were used. Prior to the study, the rats were acclimatized to laboratory conditions in the animal facility centre in NIPRD. They were housed in stainless steel cages, fed with standard rodent diet and allowed free access to potable drinking water. All animal experiments were carried out in accordance with NIPRD standard operating procedures and the updated National Institutes of Health guideline for the care and use of laboratory animals[16].
2.3. Drugs and chemicals
Chloroquine, artemisinin, acetylsalicylic acid (ASA), acetic acid, chloroform and ethylacetate purchased from Sigma Aldrich (St Louis MO, USA) and quinine dihydrate (Acros organics NJ, USA) were used. Other reagents used were of analytical grade.
2.4. Plant material, extraction and fractionation
Fresh aerial parts ofP. niruriwere collected from Orba, Enugu State, Nigeria on 25 July 2011. A voucher specimen was prepared and deposited in NIPRD herbarium (NIPRD/ H/6565). The roots of the plant were cut off and the aerial parts dried at room temperature for two weeks then pulverized to coarse powder using an electric hammer mill.
The powdered plant material (2.03 kg) was extracted with 10 L methanol (98% v/v) for 48 h with occasional mechanical shaking (4 h/day) and afterwards, the mixture was filtered using ashless filter paper. The filtrate was concentrated under reduced pressure using a rotary evaporator to give 100 g of the methanol extract. The extract was portioned between water and chloroform (4×600 mL) using a separatory funnel. The combined lower chloroformlayer was concentrated to dryness under vacuum by rotary evaporation at 40 °C and the slurry obtained was designated F1 (35 g).
F1 was further separated by column chromatography (CC) over silica gel (100-200 mesh size) and eluted withn-hexane first, then successively in a gradient manner withn-hexane: ethylacetate, ethylacetate: methanol and finally with methanol. Eluates that showed similar thin layer chromatography (TLC) profiles were pooled together to afford 15 fractions labelled 1-15. The fractions were concentrated by rotary evaporation under vacuum. Solvent-free fractions were stored in screw-cap glass bottles in a refrigerator until required in the experiments.
2.5. Antiplasmodial assay
2.5.1. In vitro parasite culture
Chloroquine-sensitive (HB3) and chloroquine-resistant (FcM29)P. falciparumstrains were maintained in culture according to the modified method of Trager and Jensen[17]. The parasites were cultured in human erythrocytes (blood group AB) in complete media, comprising RPMI 1640 medium supplemented with 10% heat inactivated human blood type O+ serum, 25 mmol/L HEPES, 25 mmol/L gentamycin and NaHCO3. Prior to assay, infected red blood cells were washed thrice in incomplete media (without serum). Parasitaemia was determined by light microscopy from thin smears that were fixed in absolute methanol, blotted dry and stained with commercial Romanowski stain (Diff-Quik). Only cultures with 7%-10% parasitaemia were adjusted accordingly and used for the assay.
2.5.2. Estimation of parasite growth inhibition
This assay was performed as described previously[18]. Briefly, stock solutions of fractions 1-15 were made by dissolving known quantities in dimethylsulfoxide (DMSO). Stock solutions was serially double diluted using RPMI 1640 (incomplete media) as diluent in 96 well round bottom microplates, test concentrations ranged from 0.39-50 µg/ mL. Chloroquine, artemisinin and quinine were used as reference drugs, assay was done in duplicate for each concentration used. From each dilution, 50 µL volumes was transferred to wells in a flat bottom plate each containing 50 µL incomplete media before the addition of 100 µL of infected red blood cells (adjusted to 1% parasitaemia and 2% haematocrit in complete media) to give a final volume of 200 µL per well. The plates were incubated in 95% CO2at 37oC for 72 h for parasite growth. After 72 h, parasite growth was determined using a SYBR Green I-based assay.
The concentration of the test fraction/drug that produced 50% inhibition of parasite growth (IC50) was determined graphically, from a plot of the relative growth versus the concentrations of the fraction/drug; where
Relative growth= (parasitaemia (%) in treated well/maximum parasitaemia (%) in untreated wells)×100%.
2.6. THP-1 cytotoxicity assay
This was done according to the method described by Ezenyiet al[18]. Briefly, cells were grown in Eagle’s minimum essential medium (MEM; GIBCO, NY, USA) supplemented with 10% fetal bovine serum in 25-mL cellculture flasks incubated at 37oC in 5% CO2. About 100 µL of cell culture (cell density of about 107) was seeded at an approximate concentration 104cells/mL in minimum essential medium and 10% fetal bovine serum containing 100 µg/mL of streptomycin and ampicillin respectively in a sterile 96-well plate. Stock sample solutions of fractions 12, 13 and 14 (10 mg/mL each) were prepared in DMSO (Sigma Chemical Co., St. Louis, MO) and 1 µL (100 µg/mL) was added in triplicate to test wells. Blank wells contained culture media in place of cell culture while control wells contained DMSO instead of the sample solution. The plates were incubated at 37oC for 72 h and on the 4th day, 10 µL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide dye solution (5 mg/mL) was added to each well and further incubated for 1 h. After 4 h, 200 µL isopropanol was added to the culture and absorbance was read at 490 nm.
2.7. Effect of active fractions on acetic acid-induced abdominal writhing test in rats
Fractions 12, 13, 14 that exhibited high antiplasmodial activity were further screened for antinociceptive activity. The method of Tajiket al.was adopted with minor modification[19]. Sixty rats were divided into twelve groups of five rats each. Groups 1 received 5 mL/kg body weight distilled water. Groups 2-4, 5-7 and 8-10 were treated with 7.5-30 mg/kg body weight doses of fractions 12, 13 and 14 respectively. Group 11 was administered with 100 mg/kg body weight ASA. All treatment was done orally via an oral cannula. Forty five minutes after treatment in each group, 1 mL of 2% acetic acid was injected intraperitoneally per rat. Immediately after the injection of acetic acid, each rat was transferred into a plastic observation chamber and after a latency time of 10 min, the number of full writhes (a wave of abdominal muscle contraction followed by hind limb extension) was counted during a 15 min observation time.
The percentage reduction in writhing in the treated groups was calculated as:

2.8. TLC of active fractions
Fingerprints of fractions 12, 13 and 14 were obtained by TLC. Samples were spotted on pre-coated silica gel plates (2 cm×5 cm, Merck, Darmstadt, Germany). Mobile phase systems used were methanol: chloroform (7:93) for fraction 12; and methanol: chloroform (20:80) for fractions 13 and 14 while sitosteryl glycoside was used as reference standard. The resolved spots were air dried, then derivatized with 10% H2SO4in methanol with heating at 110oC for 1 min.
2.9. High performance liquid chromatography (HPLC) fingerprinting
HPLC fingerprint was obtained for fraction 12 and a separate fingerprint obtained for combined fractions 13 and 14 based on TLC and activity profile. A Shimadzu HPLC chromatography system consisting of Ultra-Fast LC-20AB prominence equipped with SIL-20AC auto-sampler; DGU-20A3 degasser; SPD-M20A UV-diode array detector; column oven CTO-20AC, system controller CBM-20Alite and Windows LCsolution software (Shimadzu Corporation, Kyoto Japan); column VP-ODS 5 µm and dimensions of 150 mm×4.6 mm. The chromatographic conditions included mobile phase: solvent A: 0.2% v/v formic acid; solvent B: acetonitrile and methanol (50:50); mode: linear gradient; flow rate 0.6 mL/ min; injection volume of 20 µL solution of compounds and fractions in methanol; detection ultraviolet 254 nm. The HPLC operating conditions were programmed to give the following: at 0.01 min, solvent B: 30%; at 15 min, solvent B: 60%; at 20 min, solvent B: 90%; at 25 min, solvent B: 30%. Column oven temperature was 40 °C. The total run time was 25 min.
2.10. Column separation and spectroscopy
About 0.945 g quantity of fraction 12 was separated by CC over silica gel (2.5 cm×60 cm, 200-400 mesh size) by gradient elution starting with methanol: chloroform 2:98 (200 mL), 4:96 (200 mL), 8:92 (300 mL), 10:90 (200 mL), 25:75 (200 mL) and 100% methanol (200 mL) to collect 9 fractions based on TLC profiles. Sub fraction 3 was subjected to preparative TLC with methanol: chloroform (8:92) followed by further purification by CC separation (1 cm×40 cm) with isocratic elution using acetone: petroleum ether (20:80). This afforded 6.6 mg of a colourless amorphous material, which was subjected to spectral analyses.
1H and13C nuclear magnetic resonance (NMR) spectra were recorded using 400 MHz and 100 MHz Bruker FT-NMR Ultra Shield spectrometers. Chemical shifts were reported in parts per million (δ). Residual solvent peaks in respective deuterated solvents were used as internal reference; central peaks were 7.22 and 150.30 for Pyridine-d5. Electrospray ionization mass spectroscopy was carried out on surveyor MSQ (ThermoFinnigan).
2.11. Statistical analysis
Data were analysed using Graph Pad Prism 5.0. Results were expressed as mean±standard error of mean and subjected one way analysis of variance followed by Dunnet’s test for multiple comparisons. Differences between groups were accepted as significant atP<0.05.
3. Results
In the antiplasmodial assay of the gradient fractions, fractions 11, 12, 13 and 14 showed moderate to high potency against two strains ofP. falciparumused, with IC50<20 µg/ mL (Table 1). These fractions had lower IC50against the chloroquine resistant parasite relative to the chloroquine sensitive strain. Fractions 1-5, 10 and 15 did not show any activity at the concentrations used. Fractions 6, 7 and 8 were moderately active only against HB3 strain, with IC50of 49.82, 51.5 and 52.12 µg/mL respectively, but were not active or had higher IC50values against FcM29 strain, similar to chloroquine. The highest activity was recorded for fraction 13 against FcM29, as no parasite growth was detected at the lowest concentration used (0.39 µg/mL). Fraction 13 also exhibited maximum antiplasmodial activity against chloroquine sensitive HB3 strain (IC50=0.97 µg/mL), compared with other fractions although it was less effective than chloroquine (IC50=0.032 µg/mL).
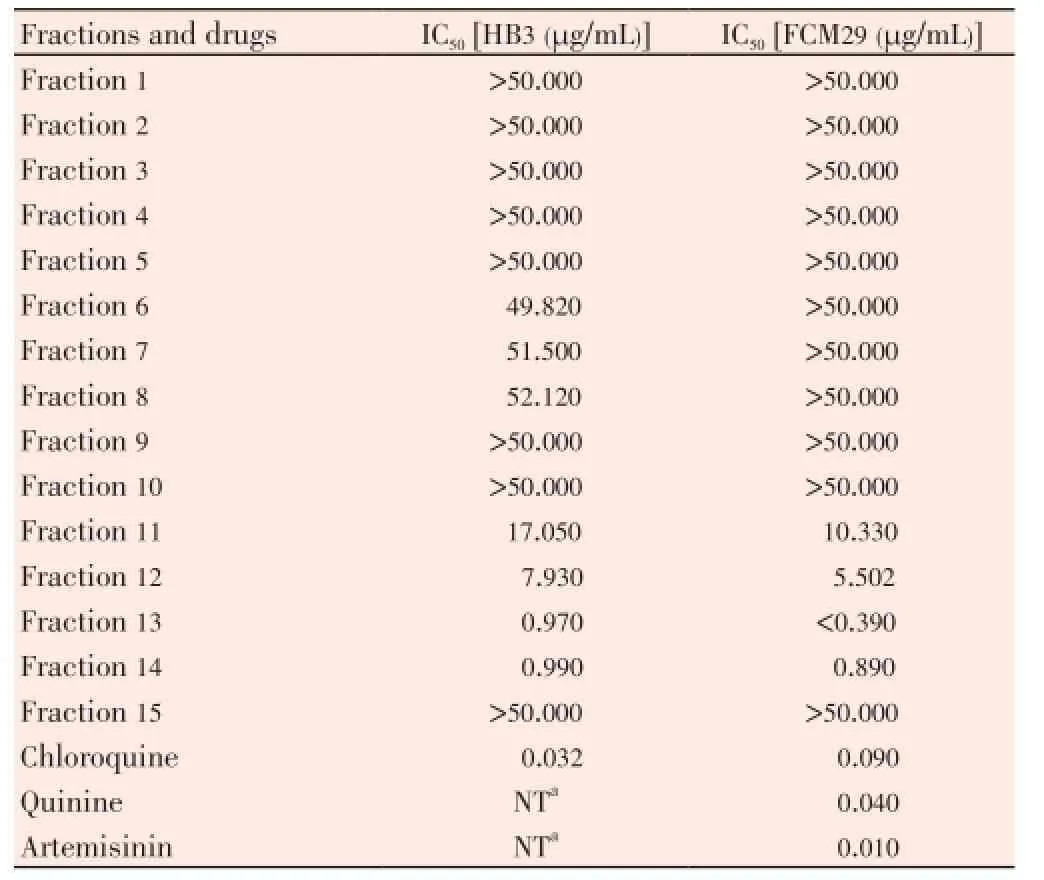
Table 1 In vitro anti-plasmodial activity of fractions against P. falciparum.
Table 2 showed that the fractions did not display high cytotoxicity against THP-1 cell line at the concentration tested and their IC50values were estimated to be >100 µg/mL. Results in Table 3 illustrated the effect of these fractions on acetic acid-induced abdominal writhing. Fraction 12 showed significant (P<0.05) activity and reduced the mean number of writhes by 66.67% compared to the untreated control group. This effect was higher than that produced by 100 mg/kg aspirin which inhibited writhing by 65.22%. Similarly, fraction 14 had significant (P<0.05) activity at 15 and 30 mg/kg doses, although this effect was not observed to be dose-dependent. At the doses of fraction 13 used (7.5-30 mg/kg), inhibition of writhing by 43.48%-58.70% was statistically insignificant (P>0.05).

Table 2 Effect of fractions (100 µg/mL) on THP-1 cell line.
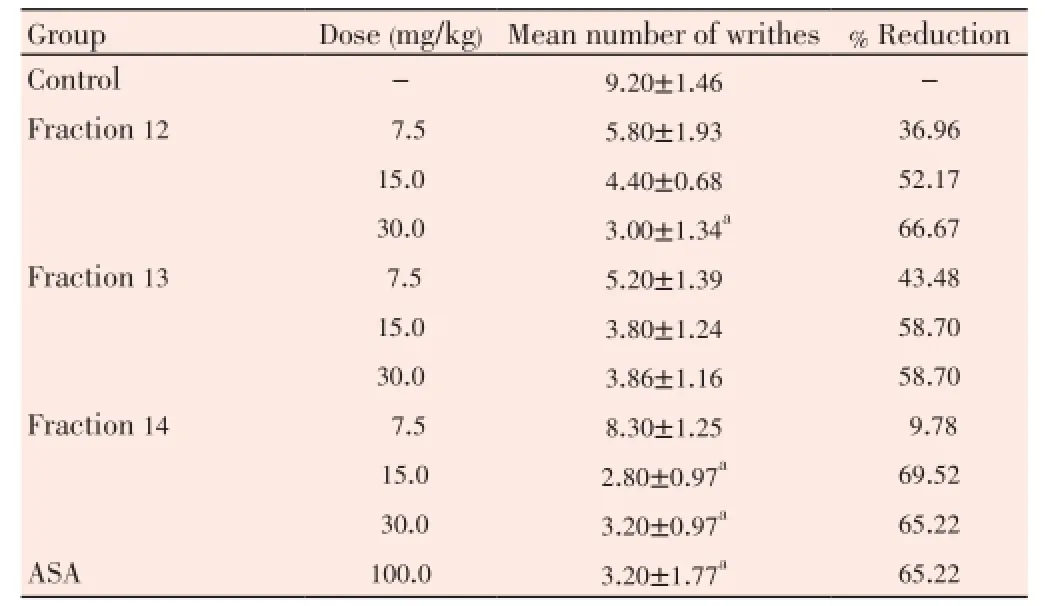
Table 3 Peripheral anti-nociceptive activity of antiplasmodial-active fractions in acetic acid-induced abdominal writhing in rats.
Further separation of the highly active antiplasmodial fractions led to the isolation and the identification of sterol glycosides and an esterified sterol glycoside. TLC of the fractions showed the presence of three major compounds in combined fractions 13 and 14, one of which had the same retention factor (Rf) value of 0.73 with standard sitosteryl glycoside marker. This was, however, not detected in fraction 12. Figure 1 and 2 showed the HPLC profiles of the fractions, which indicate their respective component peaks. Spectroscopic analysis of the compound isolated from fraction 12 revealed the presence of one sugar moiety, signals indicative of a steroid and fatty linkage in the13C NMR and DEPT spectra. Methylene at δ 63.44 in sugar region of13C NMR spectrum was indicative of glucose esterification at C-6’. Presence of one olefinic proton at δ 5.36 and six methyl resonances at δ 0.70 (3H, s, H-18), 0.83 (3H, d,J=6.9 Hz, H-26), 0.85 (3H, d,J=6.81 Hz, H-27), 0.90 (3H, t,J=7.4 Hz, H-29), 0.95 (3H, d,J=6.14 Hz, H-21) and 1.02 (3H, s, H-19) indicated steroid to be sitosterol. Thus the compound was suspected to be sitosterol glucoside with fatty acid esterified at C-6’. Mass spectroscopy gave [M+Na]+atm/z837.66 corresponding to palmitic ester. Thus, the compound was identified as sitosteryl-6-β-D-glucoside-6’-palmitate after comparison of its spectra with Sigma Aldrich reference spectra for palmitic acid and from its identification in a prior report[20,21].

Figure 1. HPLC profile of fraction 12.

Figure 2. HPLC profile of fraction 13 and 14.
4. Discussion
In this study, we identified an esterified sterol glycoside from an antiplasmodial active fraction ofP. niruri. Our results indicate that IC50values of fractions 11-14 were lower for the chloroquine resistant parasite and higher for the chloroquine sensitive strain, while the reverse was obtained for chloroquine with IC50value that was three times higher for the chloroquine resistant strain compared to the chloroquine sensitive strain. This implies that the antiplasmodial activity of these fractions may have been elicited through a different mechanism, unlike the antiplasmodial mechanism of action of chloroquine. Chloroquine acts against the parasite by preventing the detoxification of heme in the food vacuole of the parasite and the accumulation of this toxic metabolic product subsequently leading to the death of the parasite[22].Chloroquine-resistant parasites have been shown to circumvent the lethality of chloroquine by the rapid efflux of the drug from the parasite. Thus, the fractions may have elicited their actions via a non-heme accumulative action as shown by their higher activity against the chloroquine resistant strain. This finding is consistent with earlier report of Sholikhahet al.that showed a lower IC50value ofP. nirurimethanol extract against the chloroquine resistant FCR-3 strain, compared with its IC50against chloroquine sensitive D-10 strain[14]. These findings support and favour the ethnomedicinal application ofP. niruripreparations against malaria in tropical endemic areas where chloroquineresistant parasites are abundant. These fractions were also non-toxic and the report of Ramasamyet al.supports this observation[23]. The authors reported low cytotoxic effect (IC50>30 µg/mL) ofP. niruriextracts against four human cell linesin vitro. This implies that the fractions are specific in their antiplasmodial actions and have a high selectivity index, which is a ratio of lethal to effective concentrations of a given test substance.
The inhibition of abdominal writhing by the fractions in response to painful stimuli may be related to the inhibition of release and/or action of these mediators. This finding illustrates the anti-inflammatory activity of the fractions and supports the findings of an earlier report[10]. intraperitoneal administration of dilute acetic acid incites local tissue injury which prompts the release of chemical mediators (K+, H+, ATP and bradykinin) and inflammatory mediators (PGE2, pro-inflammatory cytokines) from inflammatory cells. These in turn trigger the release of mediators such as prostaglandins, serotonin and histamine though reaction cascades that cause sensitization of afferent nerve terminals and ultimately result in heightened response to the painful stimulus[24]. Inflammation is a pathophysiological reaction in response to injury, cellular stress or infection. The observed anti-inflammatory action of the fractions will be useful in the alleviation of malariaassociated inflammation induced by pathogen-initiated release of activated immune effector and regulatory cells which infiltrate the vascular beds of diverse target organs, including bone marrow, spleen, brain, placenta and lungs.
Our previous investigation onP. niruriand the report of Sholikhahet al.revealed thatP. nirurimethanol extract possessed higher activity than aqueous extract; thus, the chloroform fraction of the methanol extract was selected for antiplasmodial activity-guided separation in this study[14,15]. Previously, Subekiet al.isolated and reported the antiplasmodial activity of the compounds 1-O-galloyl-6-O-luteoyl-α-D-glucose (IC50=1.4 µg/mL) and β-glucogallin (4.6 µg/mL) from an aqueous extract ofP. niruriplant against chloroquine sensitive FCR-3P. falciparum[13]. The esterified sterol glucoside identified presently may be employed as a marker compound of biological activity. Sterol glycosides have been found to have wide therapeutic applications as cardiotonic, contraceptive, anti-inflammatory and anticancer agents[25], and this may also account for the observed activity of the fraction.
Our study reveals the presence of potent antiplasmodial and analgesic fractions in chloroform portion ofP. nirurimethanol extract, and the identification of sitosteryl glucoside palmitate as a potential chemical marker of activity.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The study was supported by funds from L’Oreal-UNESCO regional fellowship for women in science, 2011; and by the Research Training Fellowship for Developing Country Scientists awarded by Centre for International Cooperation in Science (CICS), India, 2012. Ezenyi I. is grateful to Dr Dhiman Sarkar and Dr Sourav Pal of the National Chemistry Laboratory, Pune for accepting to host in India.
Comments
Background
Malaria is the main cause of death in endemic regions and shows the high prevalence of resistant parasite strains to standard antimalarials. The search for new and no toxic antimalarial drugs is urgently needed.
Research frontiers
The authors have screenedP. nirurifor its antiplasmodial and cytotoxic activities. Chromatographic fractionation of the chloroform portion ofP. nirurimethanolic extract exhibited bioactivity againstP. falciparum(HB3 and FcM29) and low toxicity against THP-1 cell lines. Bio-guided assay has been conducted for the isolation and structure characterization of sitosteryl-6-β-D-glucoside-6’-palmitate by1H,13C NMR and mass spectrometry techniques.
Related reports
Many scientific reports have indicated thatP. niruripossessed antimalarial activity bothin vitroandin vivoas well as analgesic activity. There is controversial data concerning the therapeutic index. The authors’ present work confirmed thatP. niruriis potentially safe because of its great cytotoxic index value.
Innovations and breakthroughs
The authors have demonstrated thatP. niruripossess good antimalarial activity while lacking cytotoxicity.
Applications
The present study has indicated the possibility of formulating and standardizingP. nirurias phytomedicine for the management of malaria in endemic areas.
Peer review
This is an interesting work in which the authors have isolated and elucidated the structure of an antiplasmodial phytochemical sitosteryl-6-β-D-glucoside-6’-palmitate by the mean of NMR spectroscopy experiments and mass spectrometry. Modern cell biology techniques were used to validate the bioactivity and the safety of the pant, thus justifying pharmaceutical standardization of recipes from this plant material.
[1] World Health Organization. World malaria report. Geneva: World Health Organization; 2012. [Online] Available from: http:// www.who.int/malaria/publications/world_malaria_report_2012/ report/en/ [Accessed on 20 December, 2012]
[2] Olliaro P. Mortality associated with severe Plasmodium falciparum malaria increases with age. Clin Infect Dis 2008; 47: 158-160.
[3] Krishna S, Kremsner PG. Antidogmatic approaches to artemisinin resistance: reappraisal as treatment failure with artemisinin combination therapy. Trends Parasitol 2013; 29: 313-317.
[4] Malmberg M, Ngasala B, Ferreira PE, Larsson E, Jovel I, Hjalmarsson A, et al. Temporal trends of molecular markers associated with artemether-lumefantrine tolerance/resistance in Bagamoyo district, Tanzania. Malar J 2013; 12: 103.
[5] Batista R, de Jesus Silva Júnior A, de Oliveira AB. Plantderived antimalarial agents: new leads and efficient phytomedicines. part II. Non-alkaloidal natural products. Molecules 2009; 14: 3037-3072.
[6] Kaur K, Jain M, Kaur T, Jain R. Antimalarials from nature. Bioorg Med Chem 2009; 17: 3229-3256.
[7] Cocquyt K, Cos P, Herdewijn P, Maes L, Van den Steen PE, Laekeman G. Ajuga remota Benth.: from ethnopharmacology to phytomedical perspective in the treatment of malaria. Phytomedicine 2011; 18: 1229-1237.
[8] Ndjonka D, Bergmann B, Agyare C, Zimbres FM, Luersen K, Hensel A, et al. In vitro activity of extracts and isolated polyphenols from West African medicinal plants against Plasmodium falciparum. Parasitol Res 2012; 111: 827-834.
[9] Boim MA, Heilberg IP, Schor N. Phyllanthus niruri as a promising alternative treatment for nephrolithiasis. Int Braz J Urol 2010; 36: 657-664.
[10] Obidike IC, Salawu OA, Ndukuba M, Okoli CO, Osunkwo UA. The anti-inflammatory and antinociceptive properties of the chloroform fraction from Phyllanthus niruri plant is mediated via the peripheral nervous system. J Diet Suppl 2010; 7: 341-350.
[11] Okoli CO, Obidike IC, Ezike AC, Akah PA, Salawu OA. Studies on the possible mechanisms of antidiabetic activity of extract of aerial parts of Phyllanthus niruri. Pharm Biol 2011; 49: 248-255.
[12] Wei W, Li X, Wang K, Zheng Z, Zhou M. Lignans with antihepatitis B virus activities from Phyllanthus niruri L. Phytother Res 2012; 26: 964-968.
[13] Subeki S, Matsuura H, Takahashi K, Yamasaki M, Yamato O, Maede Y, et al. Anti-babesial and anti-plasmodial compounds from Phyllanthus niruri. J Nat Prod 2005; 68: 537-539.
[14] Mustofa, Sholikhah EN, Wahyuono S. In vitro and in vivo antiplasmodial activity and cytotoxicity of extracts of Phyllanthus niruri L. herbs traditionally used to treat malaria in Indonesia. Southeast Asian J Trop Med Public Health 2007; 38: 609-615.
[15] Ifeoma O, Samuel O, Itohan AM, Adeola SO. Isolation, fractionation and evaluation of the antiplasmodial properties of Phyllanthus niruri resident in its chloroform fraction. Asian Pac J Trop Med 2013; 6: 169-175.
[16] National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington DC: The National Academies Press; 2011.
[17] Chenniappan K, Johns SH. Erythrocyte invasions and receptor heterogeneity in field isolates of Nanay river basin Iquitos. Asian Pac J Trop Med 2012; 5: 589-593.
[18] Ezenyi IC, Ranarivelo L, Oluwakanyinsola SA, Emeje M. Analgesic, anti-inflammatory, and heme biomineralization inhibitory properties of Entada africana ethanol leaf extract with antiplasmodial activity against Plasmodium falciparum. J Basic Clin Physiol Pharmacol 2014; 25: 217-223.
[19] Tajik H, Tamaddonfard E, Hamzeh-Gooshchi N. The effect of curcumin (active substance of turmeric) on the acetic acidinduced visceral nociception in rats. Pak J Biol Sci 2008; 11: 312-314.
[20] Joshi H, Joshi AB, Sati H, Gururaja MP, Shetty PR, Subrahmanyam EV, et al. Fatty acids from Memecylon umbellatum (Burm.). Asian J Res Chem 2009; 2: 178-180.
[21] Yoon NY, Min BS, Lee HK, Park JC, Choi JS. A potent anticomplementary acylated sterol glucoside from Orostachys japonicus. Arch Pharm Res 2005; 28: 892-896.
[22] Nakatani K, Ishikawa H, Aono S, Mizutani Y. Heme-binding properties of heme detoxification protein from Plasmodium falciparum. Biochem Biophys Res Commun 2013; 439(4): 477-480.
[23] Ramasamy S, Wahab NA, Abidin NZ, Manickam S. Cytotoxicity evaluation of five selected Malaysian Phyllanthaceae species on various human cancer cell lines. J Med Plants Res 2011; 5: 2267-2273.
[24] De S, Maroo N, Saha P, Hazra S, Chatterjee M. Ethanolic extract of Piper betle Linn. leaves reduces nociception via modulation of arachidonic acid pathway. Indian J Pharmacol 2013; 45: 479-482.
[25] Lin AS, Engel S, Smith BA, Fairchild CR, Aalbersberg W, Hay ME, et al. Struture and biological evaluation of novel cytotoxic sterol glycosides from the marine red alga Peyssonnelia sp. Bioorg Med Chem 2010; 18: 8264-8269.
10.12980/APJTB.4.2014APJTB-2013-0025
*Corresponding author: Ezenyi Ifeoma Chinwude, Department of Pharmacology and Toxicology, National Institute for Pharmaceutical Research and Development, PMB 21, Idu, Abuja.
Tel: +234-8036225293
E-mail: iphie_odike@yahoo.com
Foundation Project: Supported by funds from L’Oreal-UNESCO regional fellowship for women in science, 2011 and the Research Training Fellowship for Developing Country Scientists awarded by Centre for International Cooperation in Science (CICS), India, 2012.
Article history:
Received 17 Dec 2013
Received in revised form 22 Feb, 2nd revised form 29 Mar, 3rd revised form 7 Apr 2014
Accepted 23 May 2014
Available online 4 Sep 2014
Methods:Chloroform portion of P. niruri methanol extract was separated from silica gel using gradient systems of hexane, ethylacetate and methanol. The fractions were screened for antiplasmodial activity against Plasmodium falciparum HB3 and FcM29. Fractions with IC50<10 µg/mL against parasites were further screened for peripheral analgesic activity, while cytotoxicity was evaluated using THP-1 cells.
Results:Fractions 12-14 were very active (IC50<10 µg/mL) against Plasmodium falciparum and showed no significant cytotoxicity. Fractions 12 and 13 exhibited significant (P<0.01) reduction in acetic acid-induced writhing in mice, decreasing the number of writhes by 66.67% and 65.22% respectively and comparable with 100 mg/kg aspirin (65.22%). From fraction 12, a compound was isolated and identified as sitosteryl-6-β-D-glucoside-6’-palmitate by1H,13C nuclear magnetic resonance and mass spectroscopies.
Conclusions:Our findings illustrate antiplasmodial column fractions of P. niruri with analgesic activity and identify sitosteryl glucoside palmitate as a chemical marker of activity.
 Asian Pacific Journal of Tropical Biomedicine2014年9期
Asian Pacific Journal of Tropical Biomedicine2014年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Hematological and serum biochemical aspects associated with a camel (Camelus dromedarius) naturally infected by Trypanosoma evansi with severe parasitemia in Semnan, Iran
- Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study
- Rate of carcass and offal condemnation in animals slaughtered at Yazd Slaughterhouse, central Iran
- Phytochemical screening and antioxidant activity of ethanol extract of Tithonia diversifolia (Hemsl) A. Gray dry flowers
- Acute brucellosis as unusual cause of immune thrombocytopenia: a case report and review of the literature
- A case report of cutaneous larva migrans in a Mexican population of high marginalization
