Toxicity and antifeedant activity of essential oils from three aromatic plants grown in Colombia against Euprosterna elaeasa and Acharia fusca (Lepidoptera: Limacodidae)
Ricardo Hernández-Lambraño, Karina Caballero-Gallardo, Jesus Olivero-Verbel
Environmental and Computational Chemistry Group, Faculty of Pharmaceutical Sciences, University of Cartagena. Cartagena, Colombia
Toxicity and antifeedant activity of essential oils from three aromatic plants grown in Colombia against Euprosterna elaeasa and Acharia fusca (Lepidoptera: Limacodidae)
Ricardo Hernández-Lambraño, Karina Caballero-Gallardo, Jesus Olivero-Verbel*
Environmental and Computational Chemistry Group, Faculty of Pharmaceutical Sciences, University of Cartagena. Cartagena, Colombia
ARTICLE INFO
Article history:
Received 3 Apr 2014
Received in revised form 4 May 2014
Accepted 8 Jun 2014
Available online 24 Jun 2014
Elaeis guineensis
Objective:To determine the biological effects of essential oils (EOs) isolated from Cymbopogon nardus, Cymbopogon flexuosus and Cymbopogon martinii grown in Colombia against two Lepidoptera larvae, common pests in the oil palm.
1. Introduction
All the organs of the African oil palm (Elaeis guineensisJacquin 1763) can be attacked by insects. Although this species was originally found in West Africa, the majority of the pests of economic importance that attacks the plant are from Tropical America, which adapted to the new crop[1-5]. The leaves constitute the main source of food for a diverse number of insect pests. Most of these belong to the order Lepidoptera but also include various species of Coleoptera and some Orthoptera[2,6].
In Colombia, the Lepidoptera insects attack the majority of African oil palm crops[5,7]. All of them are phytophagous in the larval stages and are considered as the most important pests of agricultural crops, by feeding on the leaves and theparenchyma. These negatively affect the competitiveness of oil palm sector, by causing declines in yield, an increase in the use of agricultural inputs and then increasing costs[2,8].
Euprosterna elaeasaDyar (Lepidoptera: Limacodidae) (E. elaeasa) andAcharia fuscaStoll (Lepidoptera: Limacodidae) (A. fusca) highlight as insect crops that cause extensive defoliation in the palm areas[5,6]. The main damage is caused by the larvae. In fact, larva one specimen ofE. elaeasacan consume during its larval stage, 50 cm2of leaflet, leaving just the midrib, and an entire colony can cause up to 80% defoliation whereas a larva ofA. fuscacan consume 350 cm2of foliage throughout their lives[1,5]. These pests are commonly controlled using chemical insecticides, but over time, insects have acquired some physiological and behavioral resistance. This has forced many plantations to increase the doses of insecticides and application frequencies, with serious implications in terms of production costs, environmental pollution and natural agroecosystem imbalance[6].
Over the recent years, essential oils (EOs) have long been touted as attractive alternatives to synthetic chemical insecticides for pest management. This arises from the factthat these botanical mixtures reputedly pose little threat to the environment or to human health[9]. A significant number of authors have studied the antifeedant effect of EOs in Lepidoptera[10-14], as well as their toxicity on larvae[15-17], even though they have also been used as oviposition deterrents[18].
In this study, EOs from three species of the Colombian flora were tested for toxicity and antifeedant activity againstA. fuscaandE. elaeasa, two common defoliators of African oil palm plantation in Colombia.
2. Materials and methods
2.1. EOs
Cymbopogon nardus (C. nardus),Cymbopogon flexuosus(C. flexuosus)andCymbopogon martinii(C. martinii)EOs were obtained from plant material (300 g in 0.3 L of water), by microwave assisted hydrodistillation and were characterized as previously reported[19] at the Research Center of Excellence, CENIVAM, Industrial University of Santander, Bucaramanga. The oils were provided by Dr. Elena Stashenko, and stored at -4 °C until used for conducting experiments. Each extraction was repeated in triplicate. The chemical composition of the EOs were presented in the supplementary information.
2.2. Test procedures
2.2.1. Experimental units
Third instar larva specimens ofA. fuscaandE. elaeasawere collected directly from oil palm plantations in the municipality of Maria La Baja, Bolivar-Colombia (9°58’52” N, 75°17’55” W) where used for the assays (Figure 1). Organisms were stored in glass containers covered with a plastic mesh with a diet of fresh oil palm leaflets at (26±2) °C, relative humidity of 70%-85% and photoperiod 10:14 h (light: dark) and kept under these conditioning until used for assays, within 96 h after collection.

Figure 1. Third instar larva of A. fusca (A) and E. elaeasa (B).
2.2.2. Antifeedant assay
The antifeedant activity was assessed through the binary choice method described by Wellsowet al. using leaves of oil palm impregnated with EOs[20]. The leaves were cut disc shaped of 2 cm in diameter and weighed using an analytical balance to the nearest 0.1 mg (Ohaus Pioneer). EOs were dissolved in acetone and 60 μL of respective solutions where applied on the leaves to produce final concentrations of 0.002, 0.020, 0.200, 0.400 and 0.600 μL/cm2on 2 cm discs. A commercial repellent formulation (Stay off Colombia), which contains a 150 mL/L solution [ethyl 3-(N-acetyl-N-butylamino) propionate] (IR3535), was employed as positive control. Ten larvae were individually placed in Petri dishes (9 cm×1.2 cm) with a single treated or vehicle control (60 μL acetone) leaf disc. After 12 h, the remained leaf fraction was weighted and used to calculate the feedrate using formula[21]:
FI (%)=[1-(T/C)]×100
Where T=consumption on treated dish; C=consumption of control dish. An FI=100% indicates complete feeding inhibition. Three replicates were used for each tested concentration of EO (n=30), and the assays were repeated twice.
2.2.3. Contact toxicity assays
The contact toxicity of the EOs was evaluated using a topical application test[14,17]. Dilutions of the tested EOs (0.1-30.0 mL/L) were prepared using acetone as a solvent. Each larva was individually weighed using an analytical balance (Ohaus Pioneer) and received 40 μL of solution per treatment, with acetone alone as the control. Doses used were between 0.02 μL/g and 8.00 μL/g of larva, and solutions were applied topically to the dorsal surface of the larvae using a micropipette. After 24 h exposure dead larvae were counted and data tabulated for mortality assessment. To determine whether the larva was alive or dead, the palpation method was utilized (the larva was touched with a soft painting brush; if it makes any movement, it is considered alive, otherwise it is considered dead)[17]. Five replicates were used for each tested concentration of EO (n=50), and each assay was repeated twice.
2.3. Statistical analysis
The results are presented as mean±SE. The sign obtained in the calculation of FI (%) was employed to qualify the antifeedant (positive) or phagostimulant (negative) action of the EO. FI50and median lethal dose (LD50) of EOs and their confidence intervals at 95% were calculated using Probit Analysis[22]. Normal distribution and equality between variances were checked by Kolmogorov-Smirnov’s and Bartlett’s tests, respectively. Comparisons of the FI (%) and mean mortality between evaluated EOs and positive controlwere performed using ANOVA, with Dunnett’s post-test used to compare treated with control group, Tukey’s posttest to compare between the concentrations of the EOs andt-test to compare between the concentrations of EOs for both pest insects. Statistical analysis was performed with Statgraphics Plus 5.1[23], and Graph pad Prism 5 for Windows[24].
3. Results
3.1. Antifeedant activity of EOs
The results of the antifeedant activity assays for tested EOs are presented in Table 1. Data showed that at all tested concentrations, the EOs presented antifeedant properties against both examined organisms, with a clear dosedependent activity (Tables 1 and 2). The maximum feed rate inhibitions were obtained forC. martiniiat the highest tested concentration (0.600 μL/cm2) with values of 98% and 88% forA. fuscaandE. elaeasa, respectively.
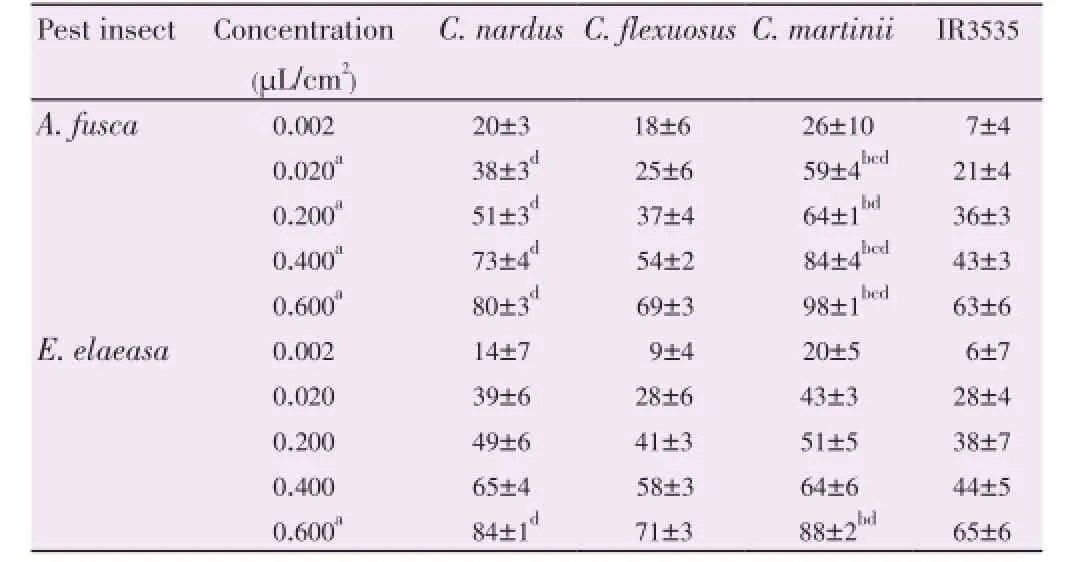
Table 1 Feed rate inhibition (%) on A. fusca and E. elaeasa exposed to EOs of three different leaves and IR3535.

Table 2 FI50at 12 h after of A. fusca and E. elaeasa exposed with three EOs and positive control (IR3535) at five concentrations.
At concentrations between 0.020 and 0.600 μL/cm2, there were statistical differences between the antifeedant properties of tested EOs againstA. fusca(0.020 μL/cm2,F=16.15;P=0.000 2; 0.200 μL/cm2,F=22.26;P<0.000 1; 0.400 μL/cm2,F=21.53;P<0.000 1; 0.600 μL/cm2,F=34.67;P<0.000 1; Table 1). However, forE. elaeasa, these differences occurred only at 0.600 μL/cm2(F=20.57;P<0.0001; Table 1). Post-test analysis revealed that for some concentrations at which there were statistical differences between EOs, forA. fusca,C. martiniishowed significant differences againstC. nardusandC. flexuosus; whereas forE. elaeasa, the only detected difference was observed betweenC. martiniiandC. flexuosusat 0.600 μL/ cm2. On the other hand, when comparingA. fuscavs.E. elaeasa, only the EO ofC. martiniipresented greater activity on the first, and this happened at 0.020, 0.400 and 0.600 μL/ cm2(T=3.54;P=0.005;T=2.97;P=0.01;T=4.29;P=0.002; Table 1). The activities of tested EOs were compared to that elicited by the commercial repellent IR3535. ForA. fusca, only the oils fromC. martiniiandC. narduswere significantly greater than the positive control at concentrations greater than 0.002 μL/cm2, whereas forE. elaeasa, such difference was registered forC. martiniiat the greatest tested concentration.
Finally, based on the FI-values (Table 2), the antifeedant properties of the EOs againstA. fuscadecreased in the orderC. martinii≈C. nardus>C. flexuosus, whereas forE. elaeasait wasC. martinii≈C. nardus>C. flexuosus. In both cases, the EO isolated fromC. flexuosuswas the least potent.
3.2. Contact toxicity of EOs
The results of the contact toxicity assays for examined EOs are shown in Figure 2. All EOs showed toxicity activity againstA. fuscaandE. elaeasa, with a clear dose-dependent toxicity (Table 3). The maximum mortality percentage obtained forA. fuscawas reached withC. martinii70%, whereas forE. elaeasait was 63%, also with the same EO at the highest applied concentration.
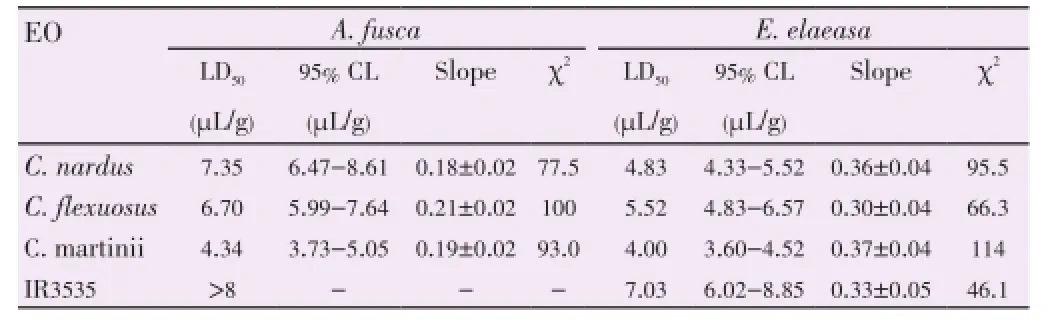
Table 3 Lethal doses (LD50) at 24 h after of A. fusca and E. elaeasa were exposed with three EOs and positive control (IR3535) at five concentrations.

Figure 2. Mortality of A. fusca (A) and E. elaeasa (B) after 24 h exposure to EOs from C. nardus, C. flexuosus, C. martinii and the positive control (IR3535). a: Significant difference between activities of EOs and the positive control (P<0.05). Error bars represent the SE.
Based on LD50values (Table 3), the dermal toxicity of EOs againstA. fuscadecreased in the orderC. martinii>C. flexuosus≈C. nardus. However, forE. elaeasa, although the LD50was lower forC. martinii, the variability of the data was greater within EOs, with clear overlapping between confidence intervals. Interestingly, the positive control, IR3535, was not only less potent but also presented lower efficacy than the examined EOs.
After 24 h exposure to EOs,A. fuscaandE. elaeasalarvae depicted characteristic behavioral changes, consisting of extreme agitation, random walking and wandering, dieresis and convulsions, finally leading to paralysis and death. However, onlyA. fuscalarvae exhibited discoloration, changing their body color to a dark brown (Figure 3). Larvae treated with vehicle-control did not show any change.
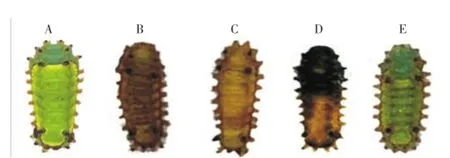
Figure 3. Representative specimens of A. fusca larva exposed for 24 h to vehicle-control and EOs from C. nardus, C. flexuosus, C. martinii and IR3535.A: Vehicle-control; B: C. nardus; C: C. flexuosus; D: C. martinii; E: IR3535.
4. Discussion
Plants with insecticidal properties have been traditionally used for crop protection, but only recently, the potential for the development of products utilized in pest management applications has been recognized[25]. In general, EOs are mostly considered nontoxic to vertebrates[26]. On the other hand, they act as broad spectrum pesticides due to their diverse mode of action, including repellency and antifeedant activity, disruption of molting and cuticle, as well as retardation of growth and fecundity[27,28]. Recent reports indicated a strong antifeedant effect of plant derivatives and recommended their widespread use as they showed great environmental safety[29-31]. However, it should be kept in mind that some EOs may posse neurotoxic effects, evident from their rapid action against some pest insects[26].
The present study demonstrated that the EOs isolated fromC. nardus,C. flexuosusandC. martinii, exhibited strong toxicity and antifeedant activity towardA. fuscaandE. elaeasalarvae. The EO fromC. martiniiwas the most active against both species, whether it was evaluated as a larvicidal or as a feeding deterrent. In terms of acute toxicity and antifeedant properties, tested EOs were better than the synthetic repellent IR3535 on both insects.
The EOs extracted from the genusCymbopogonhave been evaluated by numerous authors as repellents and insecticides for protecting crop as well as for preventing from mosquito bites[32-36], making this genus a great source of natural repellents of worldwide popularity[37-39]. The composition of these oils has been previously published[19], and some of their components, such as citronellal and citronellol have been reported for their ability as contact insecticides, repellents and antifeedant chemicals[12,14,17,37,40-44].
It should be pointed that synergistic effects of complex mixtures such as EOs are thought to be important in plant defense against herbivore predators. Plants usually present defenses as a set of compounds, thus, complex EOs may be more efficient than individual pure compounds[45,46].
Although several extracts and EOs isolated from this genus have been evaluated against other insect species. This is the first report showing the use of EOs to controlA. fuscaandE. elaeasa. These promising results should encourage the development of field tests to validate these results, with the aim of to be included together with other effective control options, in the management of defoliator insect in crop of African oil palm.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to thank the Scholarship Program for Young Researchers and Innovators “Virginia Gutiérrez de Pineda” from Colciencias, the Vice-Presidency for Research of the University of Cartagena for the financial aid through the Program to Support Research Groups (2012-2013). K. Caballero is sponsored by the National Program for Doctoral Formation (Colciencias, 567-2012).
[1] Genty PH, Desmier de Chenon R, Morin JP. [Oil palm pests in Latin America]. Oleagineux 1978; 33: 325-419. Spanish.
[2] Howard FW, Moore D, Giblin-Davis RM, Abad RG. Insects on palms. Biol Plantarum 2002; 45(2): 196.
[3] Mexzón RG, Chinchilla C, Rodríguez R. [The bag worm Oiketicus kirbyi lands guilding (Lepidoptera: Psychidae), oil palm pest]. ASD Oil Palm Papers 2003; 25: 17-28. Spanish.
[4] Mexzón RG, Chinchilla C. [The bag worms, Stenoma cecropia Meyrick in oil palm on Central America]. ASD Oil Palm Papers 2004; 27: 27-31. Spanish.
[5] Aldana RC, Aldana JA, Calvache H, Franco PN. [Manual pest of oil palm in Colombia]. Bogotá: Cenipalma Press; 2010, p. 198. Spanish.
[6] Martinez LC, Hurtado REC, Araque LT, Rincon VL. [Progress in the regional campaign for the use of information on defoliator insects in the central zone]. Palmas 2009; 30: 11-21. Spanish.
[7] Martínez LC, Plata-Rueda A. Lepidoptera vectors of Pestalotiopsis fungal disease: first record in oil palm plantations from Colombia. Int Trop Insect Sci 2013; 33(4): 239-246.
[8] Araque LT, Forero DC. [Analysis of the information capture and processing systems for decision-making in the management of leaf-eating insects on palm oil plantations in Colombia]. Palmas 2009; 30: 53-67. Spanish.
[9] Benzi V, Stefanazzi N, Ferrero AA. Biological activity of essential oils from leaves and fruits of pepper tree (Schinus molle L.) to control rice weevil (Sitophilus oryzae L.). Chil J Agric Res 2009; 69(2): 154-159.
[10] Bhathal SS, Singh D, Singh S, Dhillon RS. Effect of crude root oils of Inula racemosa and Saussurea lappa on feeding, survival and development of Spodoptera litura (Lepidoptera: Noctuidae) larvae. Eur J Entomol 1993; 90: 239-240.
[11] Larocque N, Vincent C, Bélanger A, Bourassa JP. Effects of tansy essential oil from Tanacetum vulgare on biology of oblique-banded leafroller, Choristoneura rosaceana. J Chem Ecol 1999; 25(6): 1319-1330.
[12] Isman MB. Plant essential oils for pest and disease management. Crop Prot 2000; 19(8-10): 603-608.
[13] González-Coloma A, Martín-Benito D, Mohamed N, García-Vallejo MC, Soria AC. Antifeedant effects and chemical composition of essential oils from different populations of Lavandula luisieri L. Biochem Syst Ecol 2006; 34(8): 609-616.
[14] Koul O, Singh R, Kaur B, Kanda D. Comparative study on the behavioral response and acute toxicity of some essential oil compounds and their binary mixtures to larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Ind Crop Prod 2013; 49: 428-436.
[15] Marimuthu S, Gurusubramanian G, Krishna SS. Effect of exposure of eggs to vapours from essential oils on egg mortality, development and adult emergence in Earias vittella (F.) (Lepidoptera: Noctuidae). Biol Agric Hortic 1997; 14(4): 303-307.
[16] Jeyasankar A. Antifeedant, insecticidal and growth inhibitory activities of selected plant oils on black cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae). Asian Pac J Trop Dis 2012; 2(Suppl 1): S347-S351.
[17] Kostić I, Petrović O, Milanović S, Popović Z, Stanković S, Todorović G, et al. Biological activity of essential oils of Athamanta haynaldii and Myristica fragrans to gypsy moth larvae. Ind Crops Prod 2013; 41: 17-20.
[18] Jeyasankar A, Elumalai K, Raja N, Ignacimuthu S. Effect of plant chemicals on oviposition deterrent and ovicidal activities against female moth, Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Int J Agric Sci Res 2013; 2(6): 206-213.
[19] Rodríguez RQ, Ruiz CN, Arias GM, Castro HS, Martínez J, Stashenko E. [Comparative study of the essential oil compositions of four Cymbopogon (Poaceae) species grown in Colombia]. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2012; 11: 77-85. Spanish.
[20] Wellsow J, Grayer RJ, Veitch NC, Kokubun T, Lelli R, Kite GC, et al. Insect-antifeedant and antibacterial activity of diterpenoids from species of Plectranthus. Phytochemistry 2006; 67(16): 1818-1825.
[21] Valencia E, Valenzuela E, Barros E, Hernandez M, Lazo C,Gutierrez C, et al. [Phytochemical study antifeedant and activity of Senna stipulaceae]. Bol Soc Chil Quím 2000; 45: 297-301. Spanish.
[22] Finney DJ. Probit analysis. Cambridge: Cambridge University Press; 1971, p. 188.
[23] Corporation SG. Statgraphics plus for Windows 5.1[computer program]. Informer Technologies, Inc.; 1994-2001.
[24] Motulsky HJ. Analyzing data with GraphPad Prism. San Diego: GraphPad Software Inc.; 1999. [Online] Available from: http:// graphpad.com/manuals/analyzingdata.pdf [Accessed on 27 June, 2014]
[25] Isman MB, Miresmailli S, Machial C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev 2011; 10(2): 197-204.
[26] Isman MB, Machial CM. Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M, Carpinella MC, editors. Naturally occurring bioactive compounds.Amsterdam: Elsevier; 2006, p. 29-44.
[27] Cosimi S, Rossi E, Cioni PL, Canale A. Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J Stored Prod Res 2009; 45(2): 125-132.
[28] Sertkaya E, Kaya K, Soylu S. Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite (Tetranychus cinnabarinus Boisd.) (Acarina: Tetranychidae). Ind Crops Prod 2010; 31(1): 107-112.
[29] Xu D, Huang Z, Cen YJ, Chen Y, Freed S, Hu XG. Antifeedant activities of secondary metabolites from Ajuga nipponensis against adult of striped flea beetles, Phyllotreta striolata. J Pest Sci 2009; 82(2): 195-202.
[30] Sandoval-Mojica AF, Capinera JL. Antifeedant effect of commercial chemicals and plant extracts against Schistocerca americana (Orthoptera: Acrididae) and Diaprepes abbreviatus (Coleoptera: Curculionidae). Pest Manag Sci 2011; 67(7): 860-868.
[31] Akhtar Y, Pages E, Stevens A, Bradbury R, da Camara CAG, Isman MB. Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol Entomol 2012; 37(1): 81-91.
[32] Kumar R, Srivastava M, Dubey NK. Evaluation of Cymbopogon martinii oil extract for control of postharvest insect deterioration in cereals and legumes. J Food Prot 2007; 70(1): 172-178.
[33] Olivero-Verbel J, Caballero-Gallardo K, Jaramillo-Colorado B, Stashenko EE. [Repellent activity of the essential oils from Lippia origanoides, Citrus sinensis and Cymbopogon nardus cultivated in Colombia against Tribolium castaneum, Herbst]. Rev Univ Ind Santander Salud 2009; 41: 244-250. Spanish.
[34] Kumar P, Mishra S, Malik A, Satya S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med Vet Entomol 2011; 25(3): 302-310.
[35] Caballero-Gallardo K, Olivero-Verbel J, Stashenko EE. Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. J Stored Prod Res 2012; 50: 62-65.
[36] Olivero-Verbel J, Tirado-Ballestas I, Caballero-Gallardo K, Stashenko EE. Essential oils applied to the food act as repellents toward Tribolium castaneum. J Stored Prod Res 2013; 55: 145-147.
[37] Peterson C, Coats J. Insect repellents-past, present and future. Pestic Outlook 2001; 12(4): 154-158.
[38] Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol 2010; 101(1): 372-378.
[39] Akhila A. Essential oil-bearing grasses: the genus Cymbopogom: medicinal and aromatic plants-industrial profiles. Boca Raton: CRC Press; 2010, p. 262.
[40] Tyagi BK, Shahi AK, Kaul BL. Evaluation of repellent activities of Cymbopogon essential oils against mosquito vectors of Malaria, filariasis and dengue fever in India. Phytomedicine 1998; 5(4): 324-329.
[41] Papachristos DP, Karamanoli KI, Stamopoulos DC, Menkissoglu-Spiroudi U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say). Pest Manag Sci 2004; 60(5): 514-520.
[42] Kordali S, Kesdek M, Cakir A. Toxicity of monoterpenes against larvae and adults of Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Ind Crops Prod 2007; 26: 278-297.
[43] Caballero-Gallardo K, Olivero-Verbel J, Stashenko EE. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum herbst. J Agric Food Chem 2011; 59(5): 1690-1696.
[44] Kumar P, Mishra S, Malik A, Satya S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole). Parasitol Res 2013; 112(1): 69-76.
[45] Don-Pedro KN. Investigation of single and joint fumigant insecticidal action of citruspeel oil components. Pestic Sci 1996; 46(1): 79-84.
[46] Hori M. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J Chem Ecol 1998; 24(9): 1425-1432.
10.12980/APJTB.4.2014APJTB-2014-0178
*Corresponding author: Jesús Olivero Verbel, PhD, Environmental and Computational Chemistry Group Campus of Zaragocilla, Faculty of Pharmaceutical Sciences, University of Cartagena, Cartagena, Colombia.
Tel: +57-5-6698180.
Fax: +57-5-6699771.
E-mail: jesusolivero@yahoo.com
Foundation Project: Supported by the Vice-Presidency for Research of the University of Cartagena for the financial aid through the Program to Support Research Groups (2012-2013) and K. Caballero is sponsored by the National Program for Doctoral Formation (Colciencias, 567-2012).
Pest
Lepidoptera
Defoliators
Methods:Specimens were captured in the field and the antifeedant activity and dermal contact lethality of EOs were measured against Acharia fusca and Euprosterna elaeasa (Lepidoptera: Limacodidae) at various concentrations 0.002-0.600 μL/cm2and 0.002-8 μL/g, respectively.Results:All EOs exhibited strong antifeedant and toxicity activity toward Acharia fusca and Euprosterna elaeasa larvae. Cymbopogon martinii oil was the most active against both pest insect species, although all tested EOs were better than the synthetic repellent IR3535 on both insects.
Conclusions:Colombian EOs have potential for integrated pest management programs in the oil palm industry.
 Asian Pacific Journal of Tropical Biomedicine2014年9期
Asian Pacific Journal of Tropical Biomedicine2014年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Hematological and serum biochemical aspects associated with a camel (Camelus dromedarius) naturally infected by Trypanosoma evansi with severe parasitemia in Semnan, Iran
- Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study
- Rate of carcass and offal condemnation in animals slaughtered at Yazd Slaughterhouse, central Iran
- Phytochemical screening and antioxidant activity of ethanol extract of Tithonia diversifolia (Hemsl) A. Gray dry flowers
- Acute brucellosis as unusual cause of immune thrombocytopenia: a case report and review of the literature
- A case report of cutaneous larva migrans in a Mexican population of high marginalization
