Combined use of non-biological arti fi cial liver treatments for patients with acute liver failure complicated by multiple organ dysfunction syndrome
Department of Intensive Care, Xuzhou Central Hospital, Affiliated to School of Medicine, Southeast University, Xuzhou 221009, China
Corresponding Author:Mao-qin Li, Email: limaoqinxi@126.com
Combined use of non-biological arti fi cial liver treatments for patients with acute liver failure complicated by multiple organ dysfunction syndrome
Mao-qin Li, Jun-xiang Ti, Yun-hang Zhu, Zai-xiang Shi, Ji-yuan Xu, Bo Lu, Jia-qiong Li, Xiao-meng Wang, Yan-jun Xu
Department of Intensive Care, Xuzhou Central Hospital, Affiliated to School of Medicine, Southeast University, Xuzhou 221009, China
Corresponding Author:Mao-qin Li, Email: limaoqinxi@126.com
BACKGROUND:Acute liver failure (ALF) caused by viral and non-viral hepatitis is often accompanied with severe metabolic disorders, the accumulation of toxic substances and continuous release and accumulation of a large number of endogenous toxins and inflammatory mediators. The present study aimed to investigate the effects of various combined non-biological arti fi cial liver treatments for patients with acute liver failure (ALF) complicated by multiple organ dysfunction syndrome (MODS).
METHODS:Thirty-one patients with mid- or late-stage liver failure complicated by MODS (score 4) were randomly divided into three treatment groups: plasmapheresis (PE) combined with hemoperfusion (HP) and continuous venovenous hemodiafiltration (CVVHDF), PE+CVVHDF, and HP+CVVHDF, respectively. Heart rate (HR) before and after treatment, mean arterial pressure (MAP), respiratory index (PaO2/FiO2), hepatic function, platelet count, and blood coagulation were determined.
RESULTS:Signi fi cant improvement was observed in HR, MAP, PaO2/FiO2, total bilirubin (TBIL) and alanine aminotransferase (ALT) levels after treatment (P<0.05). TBIL and ALT decreased more signi fi cantly after treatment in the PE+CVVHDF and PE+HP+CVVHDF groups (P<0.01). Prothrombin time (PT) and albumin were signi fi cantly improved only in the PE+CVVHDF and PE+HP+CVVHDF groups (P<0.05). TBIL decreased more significantly in the PE+HP+CVVHDF group than in the HP+CVVHDF and PE+CVVHDF groups (P<0.05). The survival rate of the patients was 58.1% (18/31), viral survival rate 36.4% (4/11), and non-viral survival rate 70% (14/20).
CONCLUSION:Liver function was relatively improved after treatment, but PE+HP+CVVHDF was more efficient for the removal of toxic metabolites, especially bilirubin. The survival rate was signi fi cantly higher in the patients with non-viral liver failure than in those with viral liver failure.
Severe acute liver failure; Artificial liver; Plasma exchange; Hemoperfusion; Continuous veno-venous hemodia fi ltration
INTRODUCTION
Acute liver failure (ALF) caused by viral and non-viral hepatitis is often accompanied with severe metabolic disorders, the accumulation of toxic substances and continuous release and accumulation of a large number of endogenous toxins and inflammatory mediators. In turn, this promotes liver damage and inhibits the regeneration of liver cells, creating a vicious cycle. Despite internal conventional therapies such as protecting liver function, improving jaundice, and a variety of medical support treatments, the clinical mortality of patients with severe ALF is still as high as 70%.[1]Artificial liver support system (ALSS) replaces liver function temporarily and partially, while removingall harmful substances and supplying biological active substances to create a good environment for regeneration and functional recovery of hepatic cells of the patient. Due to the limited effect of single ALSS, various combinations of non-biological artificial liver methods have become the hot topic. The present study aimed to observe the efficacy and safety of three combined ALSS regimens for the treatment of severe ALF: plasmapheresis (PE) combined with hemoperfusion (HP) and continuous venovenous hemodiafiltration (CVVHDF), PE+CVVHDF and HP+CVVHDF.
METHODS
General information
Thirty-one patients with severe ALF were admitted to intensive care unit of Xuzhou Central Hospital from January 2007 to March 2013. The patients met the following criteria: liver score IV (total bilirubin>240 µmol/L) according to multiple organ dysfunction syndrome (MODS) score; and severe ALF complicated with MODS. In this series, 22 patients were male, and 9 were female, with a mean age of 38.1±14.8 years (range 20–82 years). Their chronic health evaluation II score (APACHE II) was 28.7±7.8, and sequential organ failure assessment score (SOFA score) was 13.67±3.31. Primary diseases in these patients included acute viral hepatitis (1 patient), ALF and chronic toxicity (10 patients), non-viral liver injury (cardiac surgery in 1 patient, poisoning in 6, pregnancy in 5, poisonous mushroom in 1, severe infection in 5, and others in 2). Liver failure staging[6]showed that all patients were in the stage of advanced liver failure with prothrombin activity (PTA)≤30%, including 23 patients with renal dysfunction, 15 patients with hepatic encephalopathy, 21 patients with gastrointestinal bleeding, 31 patients with blood clotting abnormalities, and 15 patients with circulatory dysfunction.
The trial protocol was approved by the Ethics Committee of Xuezhou Central Hospital. All patients or their legal surrogates provided written informed consent for participation.
Treatment
In addition to conventional medicine and supportive treatment, the 31 patients were randomly treated with the following three regimens: PE+HP+CVVHDF, PE+CVVHDF or HP+CVVHDF.
In the PE+HP +CVVHDF group, after establishing temporary access of the femoral vein or jugular vein catheterization, patients received plasmapheresis at bedside using a Swiss Campbell PF2000N plasma separator (permutation of fresh plasma 1 500–2 500 mL, plasma exchange fl ow rate 80–120 mL/min, plasma separation speed 25–30 mL/min, and replacement time 2–3 hours). After a single plasma exchange was completed, patients received HP, using neutral macroporous resin ( a HA330-II type hemoperfusion device produced by Zhuhai Franc). The hemoperfusion device was removed while its perfusion adsorption capacity for saturation and blood perfusion lasted 2–3 hours. Then, the patients were subjected to CVVHDF for (32.4±24.4) hours (range 10–49 hours).
In the PE+CVVHDF group, patients underwent CVVHDF therapy after plasmapheresis. In the HP +CVVHDF group, patients underwent CVVHDF after HP.
In the study, the 31 patients received a total of 81 treatments, including 23 treatments with PE+HP+CVVHDF, 26 treatments with PE+CVVHDF, and 32 treatments with HP+CVVHDF.
Indicators
The following indicators were observed: consciousness, heart rate (HR), mean arterial pressure (MAP), arterial blood gas (pH, PaO2, PaCO2), hepatic and renal function, blood count, electrolytes, and coagulation of each patient before and after treatment in the three groups.
Statistical analysis
Data of the three groups were expressed as the mean±SD. All analyses were performed using the SPSS 12.0 statistical package (SPSS Inc., Chicago, IL). Twoway ANOVA was used to compare the data between the three groups. All tests were two-tailed, and P values less than 0.05 were considered statistically signi fi cant.
RESULTS
General data
There were no significant differences in age, SOFA score and treatment time between the three groups (P> 0.05) (Table 1).

Table 1. Patients' age, SOFA score, and treatment time (mean ±SD)

Table 2. HR, MAP, PaO2/FiO2and PLT before and after treatment (mean±SD)
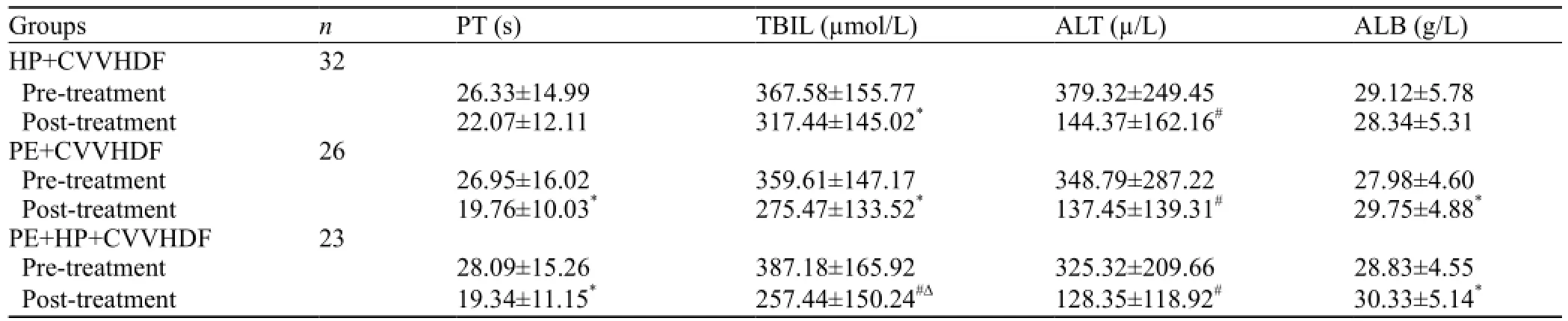
Table 3. Coagulation and liver function (mean ±SD)
HR, MAP, PaO2/FiO2and platelets before and after treatment
There were no significant differences in platelet changes before and after treatment in the three groups (P>0.05). HR, MAP and PaO2/FiO2increased more significantly after treatment than before treatment in the three groups (P<0.05). HR and PaO2/FiO2showed a significant change between the PE+HP+CVVHDF and HP+CVVHDF groups (P<0.05) (Table 2).
Coagulation and liver function before and after treatment
Total bilirubin (TBIL) and alanine aminotransferase (ALT) levels decreased more significantly after treatment than before treatment (P<0.05) in the PE+HP+ CVVHDF group and PE+CVVHDF group (P<0.01). However, TBIL decreased more significantly in the PE+HP+CVVHDF group than in the HP+CVVHDF and PE+CVVHDF groups. The changes of prothrombin time (PT) and albumin (ALB) level were statistically significant between the PE+HP+CVVHDF and PE+ CVVHDF groups (P<0.05) (Table 3).
Clinical outcomes
Of the 31 patients, 18 (58.1%) survived after treatment with the ALSS. In these patients, 4 were from the 11 patients with virus-caused liver failure, and 14 were from the 20 patients with non-virus-caused liver failure.
DISCUSSION
ALF is characterized by rapid onset, progression, and poor prognosis.[2]Actually, it is a kind of MODS. The treatment effect of simple conventional supportive care is not satisfactory, and blood purification technology can replace liver metabolic functions, thus supporting multiple organ function effectively.[3]The combined use of ALSS treatments plays a crucial role in decreasing the level of serum bilirubin, removing or reducing toxic substances, and improving the internal environment of ALF in liver failure patients. It has been reported that the survival rate of patients was 80%–90 % in the early course of ALF. Their interim survival rate was 60%–70%, while the survival rate of patients with advanced ALF was less than 20%.[4]Ye et al[5]reported that the survival rate was 48.3% for chronic severe hepatitis B patients treated with hemo fi ltration and plasmapheresis. He et al[6]reported that the survival rate was 45.5% for MODS patients with ALF in the ICU after treatment with pairing plasma separation adsorption and hemofiltration. The survival rate was 42.5% for MODS patients with ALF after treatment with plasma exchange and CVVHDF.[7]In our study, the liver function score was≥IV according to MODS score, and PTA was less than 30%. All patients were in the stage of advanced liver failure according to liver failure treatment guidelines.[8]We used three combined non-biological arti fi cial liver therapies, and 18 (58.1%) of the 31 patients survived. A significant improvement was observed in thesurvival rate compared to another study.[7]
In our study, the survival rate of patients with nonvirus-caused liver failure was 70%, but the survival rate of patients with virus-caused liver failure was 36.4%. The finding suggested that the combined use of nonbiological artificial liver technology plays a significant role in improving the clinical outcomes. Different combinations of non-bioarti fi cial therapies were effective in improving severe metabolic disorders and removing accumulated toxic substances and inflammatory mediators caused by serious damage of liver cells. Thus, combined non-bioartificial therapies play a significant role in improving clinical outcomes. For patients with non-virus-caused severe ALF, combined ALSS plays a signi fi cant role in reducing the mortality.
Three combined therapies with the non-biological arti fi cial liver revealed that there are signi fi cant differences in heart rate, mean arterial pressure, respiratory index after treatment compared with before treatment (P<0.05). TBIL and ALT in the PE+HP+CVVHDF group and PE+CVVHDF group decreased more significantly (P<0.01). PT and ALB changed significantly before and after treatment in the PE+HP+CVVHDF group and PE+CVVHDF group (P<0.05). The decrease of TBIL was more significant in the PE+HP+CVVHDF group than in the HP+CVVHDF and PE+CVVHDF groups. The results suggested that the combination of PE+HP+CVVHDF is more conducive to remove metabolites and scavenge poisons in addition to maintain homeostasis. PE can widely remove endogenous toxins (such as endotoxin, bilirubin, and bile acids) and macromolecules binding with plasma protein and circulating immune complexes.
PE is not adequately effective because the small molecular weight toxins can easily pass through the blood vessel wall, and is widely distributed in tissues. HP can absorb aromatic amino acid, phenol, indole, shortchain fatty acids and others. Hemsoperfuion using HA resin perfusion, a neutral macroporous resin adsorption 500–5 000 Da major molecular weight substance, can absorb a variety of proteins binding toxins and cytotoxic substances, which inhibit regeneration of liver.[10]CVVH can continually eliminate the molecular substances, ammonia and other toxic substances such as false neurotransmitters, free fatty acids, amino acids, aromatic thiols in patients with acute liver failure, increase the content of CAMP in cerebrospinal fluid, improve energy metabolism in the brain, alleviate and ameliorate hepatic encephalopathy. CVVH can accurately control capacity, continuously and slowly remove the solute and liquid, regulate water, electrolyte and acid-base balance, and reduce the occurrence of brain edema in patients with acute liver failure.
In conclusion, liver function was improved after three artificial liver treatments in the present study. PE+HP+CVVHDF was more effective to remove the metabolites and toxins, especially bilirubin. After treatment, the survival rate of patients with non-viruscaused liver failure was signi fi cantly higher than that of those with virus-caused liver failure.
Funding:The study was supported by a grant from Xuzhou Municipal, China.
Ethical approval:This study was approved by the ethical Committees of Xuzhou Central Hospital, Jiangsu Province, China.
Con fl icts of interest:We have no con fl icts of interest to report.
Contributors:Li MQ proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
1 Zheng Z, Li X, Li Z, Ma X. Artificial and bioartificial liver support systems for acute and acute-on-chronic hepatic failure: A meta-analysis and meta-regression. Exp Ther Med 2013; 6: 929–936. Epub 2013 Jul 31.
2 Majumdar M, Ratho R, Chawla Y, Singh MP. High levels of circulating HMGB1 as a biomarker of acute liver failure in patients with viral hepatitis E. Liver Int 2013; 33: 1341–1348.
3 Song G, Li Y, Li M, Xuan R. Acute renal and liver failure due to acute fatty liver of pregnancy-complicated pre-eclampsia. J Obstet Gynaecol 2012; 32: 702–703.
4 Takikawa Y, Suzuki K. Clinical epidemiology of fulminant hepatitis in Japan. Hepatol Res 2008; 38 Suppl 1: S14–18.
5 Wu XL, Long D, Yu L, Yang JH, Zhang YC, Geng F. Urokinasetype plasminogen activator receptor as a predictor of poor outcome in patients with systemic infl ammatory response syndrome. World J Emerg Med 2013; 4: 190–195.
6 Fang K, Wang XL. Treatment of multiple organ dysfunction syndrome by Xuebijing Injection: a clinical research. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013; 33: 205–207.
7 Li LJ, Zhang YM, Liu XL, Du WB, Huang JR, Yang Q, et al. Arti fi cial liver support system in China: a review over the last 30 years. Ther Apher Dial 2006; 10: 160–167.
8 Chinese Society of Hepatology. Guidelines for liver failure. Chin J Hepatology 2006; 14: 543–646.
9 Li MQ, Li JQ, Shi ZX, Xu JY, Zhang Z, Lu F, et al. Efficacy of various combined blood purification techniques for treating patients with non-viral acute liver failure. Cell Biochem Biophys 2014; 68: 571–575.
10 Huang Z, Wang SR, Su W, Liu JY. Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial 2010; 14: 596–602.
Received January 11, 2014
Accepted after revision July 3, 2014
World J Emerg Med 2014;5(3):214–217
10.5847/ wjem.j.issn.1920–8642.2014.03.010
 World journal of emergency medicine2014年3期
World journal of emergency medicine2014年3期
- World journal of emergency medicine的其它文章
- Domestic versus imported drug-eluting stents for the treatment of patients with acute coronary syndrome
- Comparison of plasma microRNA-1 and cardiac troponin T in early diagnosis of patients with acute myocardial infarction
- International normalized ratio as a predictor of mortality in trauma patients in India
- Can venous blood gas analysis be used for predicting seizure recurrence in emergency department?
- Knowledge and skills of neonatal resuscitation of health professionals at a university teaching hospital of Northwest Ethiopia
- Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensiveintracerebral hemorrhage
