Comparison of plasma microRNA-1 and cardiac troponin T in early diagnosis of patients with acute myocardial infarction
Li-ming Li, Wen-bo Cai, Qin Ye, Jian-min Liu, Xin Li, Xiao-xing Liao
1Department of Emergency Medicine, Pengpai Memorial Hospital of Haifeng, Haifeng, China
2Department of Emergency Medicine, First Af fi liated Hospital, Sun Yat-Sen University, Guangzhou, China
Corresponding Author:Xiao-xing Liao, Email: liaowens@163.com
Comparison of plasma microRNA-1 and cardiac troponin T in early diagnosis of patients with acute myocardial infarction
Li-ming Li1, Wen-bo Cai1, Qin Ye1, Jian-min Liu1, Xin Li2, Xiao-xing Liao2
1Department of Emergency Medicine, Pengpai Memorial Hospital of Haifeng, Haifeng, China
2Department of Emergency Medicine, First Af fi liated Hospital, Sun Yat-Sen University, Guangzhou, China
Corresponding Author:Xiao-xing Liao, Email: liaowens@163.com
BACKGROUND:Early reperfusion can effectively treat acute myocardial infarction (AMI) and reduce the mortality signi fi cantly. This study aimed to compare the role of plasma microRNA-1 (miR-1) and cardiac troponin T (cTnT) in early diagnosis of AMI patients.
METHODS:From May 2011 to May 2012, plasma samples were collected from 56 AMI patients and 28 non-AMI controls. The expression of plasma miR-1 was measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR), and the level of plasma cTnT was measured using electrochemiluminescence-based methods on an Elecsys 2010 Immunoassay Analyzer. SPSS 16.0 was used for the statistical analysis of the results. Data were expressed as mean±standard deviation unless otherwise described. The differences about clinical characteristics between the AMI patients and controls were tested using Student's t test or Fisher's exact test. The Mann-Whitney U test was conducted to compare the expression of microRNAs between the AMI patients and controls. MicroRNAs expression between different intervals of the AMI patients was compared using Wilcoxon's signed-rank test. The receiver operating characteristic (ROC) curve was established to discriminate the AMI patients from the controls.
RESULTS:In the present study, the expression of plasma miR-1 was signi fi cantly increased in the AMI patients compared with the healthy controls (P<0.01). The plasma miR-1 in the AMI patients decreased to the normal level at 14 days (P>0.05). The expression of plasma miR-1 was not related to the clinical characteristics of the study population (P>0.05). ROC curve analyses demonstrated that miR-1 was speci fi c and sensitive for the early diagnosis of AMI, but not superior to cTnT.
CONCLUSION:Plasma miR-1 could be used in the early diagnosis of AMI, but it is similar to cTnT.
MicroRNA-1; High sensitive cardiac troponin T; Acute myocardial infarction; Biomarker; Early diagnosis; Speci fi city; Sensitivity
INTRODUCTION
Acute myocardial infarction (AMI), which has a high morbidity and mortality worldwide, is the acute necrosis of myocardial tissue due to persistent and severe ischemia. According to a survey in 2008, approximately three to four million people were estimated to suffer from AMI each year.[1]Early reperfusion can effectively treat AMI and reduce its mortality signi fi cantly.
How to make a timely diagnosis of AMI is important in clinical practice. The circulating levels of cardiac tropnins (cTns) are considered the "gold standard" for the early diagnosis of AMI because cTns are both highly specific and sensitive for cardiac injury. However, studies[2,3]have shown that cTn concentrations were alsoelevated in patients with end-stage renal disease (ESRD) and that these molecules might serve as biomarkers for the outcome and prognosis of the disease. Therefore, there are still some weaknesses of cTns in the diagnosis of AMI.
Recent studies[4,5]have suggested that microRNAs (miRNAs) can regulate gene expression and play critical roles in various pathophysiological processes. Each miRNA can regulate several distinct target mRNAs, and conversely, single mRNAs can be targeted by several distinct miRNAs.[6]Many miRNAs, which demonstrate tissue- or cell-specific distributions, can be detected in the peripheral blood or plasma.[7,8]In addition, the levels of circulating miRNAs are characteristically altered in individuals with various pathological conditions, indicating that circulating miRNAs may be useful diagnostic biomarkers for speci fi c diseases.[9,10]
Studies[11–17]have shown that the levels of several miRNAs in the blood and/or plasma alter during AMI, suggesting that circulating myocardial-derived miRNAs might be good biomarkers for the diagnosis of AMI.
The objectives of the study were to measure the level of circulating miR-1, the concentration of cardiac troponin T (cTnT) in AMI patients, so as to analyze the utility of miR-1 as a novel biomarker for the early diagnosis of AMI and compare its diagnostic value with that of cTnT.
METHODS
Clinical specimens
Fifty-six patients diagnosed with AMI as well as twentyeight healthy volunteers without AMI were enrolled in this study. In accordance with the guidelines, patients with increased cTnT or creatine kinase-MB (CK-MB) levels combined with chest pain lasting for >30 minutes and electrocardiogram (ECG) fi ndings such as new pathological Q waves or ST-segment elevation or depression were diagnosed with AMI.[18]The study protocol was approved by the Ethics Committees of Sun Yat-Sen University, and written informed consent was obtained from all participants.
Plasma collection and storage
Venous blood samples (5 mL) from AMI patients were collected on day 1 within 12 hours and at 24 hours of the onset of symptoms as well as on day 14. Due to the progression of disease, only 12 samples from patients with AMI were analyzed on day 14. Venous blood samples from the 28 volunteers were collected regardless of time period. All the samples were collected using K2-EDTA-coated tubes. Plasma was isolated by centrifugation at 1 000 g for 10 minutes at 4 ˚C, followed by centrifugation of the supernatant at 14 000 g for 15 minutes at 4 ˚C. The plasma was transferred into RNase/DNase-free tubes and stored at–70 ˚C until RNA extraction.
RNA isolation
Total RNA was isolated from plasma using the TRIzol LS®reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and dissolved in 10 mL of diethylpyrocarbonate (DEPC)-treated water. The concentration and quality of the RNA samples were determined using a BioPhotometer (Eppendorf).
Quantitative reverse transcriptionpolymerase chain reaction (qRT-PCR)
The Bulge-LoopTM miRNA qRT-PCR Primer Set (RiboBio Co, Guangzhou, China) was used to detect and quantify the expression of miR-1 according to the manufacturer's instructions; a Caenorhabditis elegans microRNA (cel-miR-39) was used as the internal control.[9]Total RNA (0.5 μg) from each sample was reverse transcribed using microRNA-specific primers and M-MLV reverse transcriptase (Promega, Madison, WI, USA). The reaction was carried out for 60 minutes at 42 ˚C, followed by 10 minutes at 70 ˚C, and the resulting cDNA was stored at –20 ˚C until use.
The qRT-PCR reactions were performed on the PRISM 7900HT sequence detection system (Applied Biosystems, Carlsbad, CA, USA) in 20 μL reactions containing 2 μL of reverse transcription product, 2 μL of PCR forward primer (5 μmol), 2 μL of Universal Adaptor PCR primer (5 μmol), 9 μL of Platinum SYBR Green qPCR SuperMix-UDG reagent (Invitrogen, Carlsbad, CA, USA), and 5 μL of ddH2O. The reactions were incubated at 95 ˚C for 2 minutes, followed by 40 cycles of 95 ˚C for 15 seconds, 60 ˚C for 30 seconds and 95 ˚C for 15 seconds. The samples were then heated from 60 ˚C to 95 ˚C to obtain melting curves. Reactions containing either no reverse transcriptase or no template were used as negative controls, and all assays were performed in triplicate. The relative expression values of the miR-1 were calculated using the 2–ΔΔCt method.[19]
cTnT determination
The levels of plasma cTnT were determined using electro-chemiluminescence-based methods with the Elecsys 2010 Immunoassay Analyzer (Roche Diagnostics, Switzerland) according to the manufacturer's protocol. cTnT<0.04 ng/mL was de fi ned as normal.
Statistical analysis
The Statistical Package for Social Sciences,version 17.0 (SPSS, Inc., Chicago, IL, USA), was used for the statistical analysis. All data are presented as the mean±standard deviation (χ–±s) unless otherwise noted. The microRNA levels measured in each AMI patient at different time points were compared using the Wilcoxon's signed-rank test. The Mann-Whitney U test was conducted to compare the expression of microRNAs between the AMI patients and controls. The significance of the differences in clinical characteristics between the AMI patients and controls was tested using Student's t test or Fisher's exact test. Receiver operating characteristic (ROC) curves were established to discriminate between the AMI patients and controls. P<0.05 was considered statistically signi fi cant.
RESULTS
Clinical characteristics of the study populationIn the present study, we enrolled 56 patients with AMI and 28 controls. The clinical characteristics of each group are listed in Table 1. The mean age of the AMI patients was 63.95±11.34 years, and the mean age of the controls was 60.50±9.10 years (P=0.166). Both groups were predominantly males (44/56 in the AMI group and 20/28 in the control group; P=0.468). The mean cTnT level at admission in the AMI patients was 1.35±1.10 ng/mL, which was significantly higher than the mean cTnT level in the control group (P<0.01). There were no signi fi cant differences in any other clinical characteristics between the AMI and control groups.
Circulating miR-1 level was elevated in the AMI patients
To determine the circulating miR-1 level in the AMI patients, we investigated the difference of the plasma level of miR-1 between the AMI patients and controls. As shown in Figure 1, the plasma concentration of miR-1 was markedly elevated in the AMI patients relative to the controls (P<0.01).
We also measured the level of miR-1 in plasma fromthe AMI patients on day 14, fi nding that the miR-1 level had decreased to the baseline level, which was not signi fi cantly different from the level in the controls (P>0.05) (Figure 2).
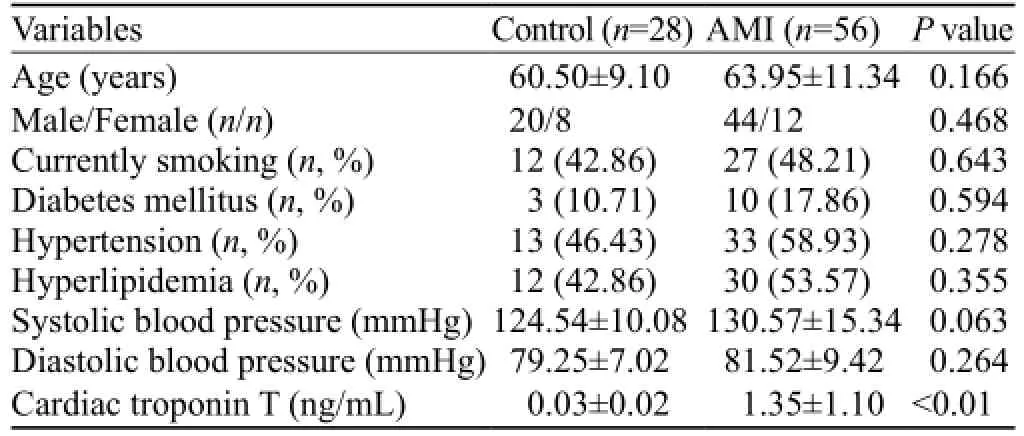
Table 1. Clinical characteristics of the study population
Circulating miR-1 as potential predictor of AMI
由于现实中的合作社鱼龙混杂,存在不少虚假合作社,加上很多合作社组织机构不健全且大多虚置,权责关系混乱,登记注册股份成员大多与实际不相符,使得合作社社会形象的认可度较低,制约了合作社外部商业资源的获取。此外,合作社也难以获取需要全部社员签名确认的政府扶持资源和合作机会。其原因在于:一是农户大多具有根深蒂固的小农意识,对签名行为十分谨慎,加上普通社员获得的盈余分配激励往往较低甚或没有,使得社员配合签名的意愿较低;二是社员数量众多,可能存在部分兼业化社员外出打工、转业社员未注销退出等现象,使得合作社很难及时获取这些社员农户的签名。
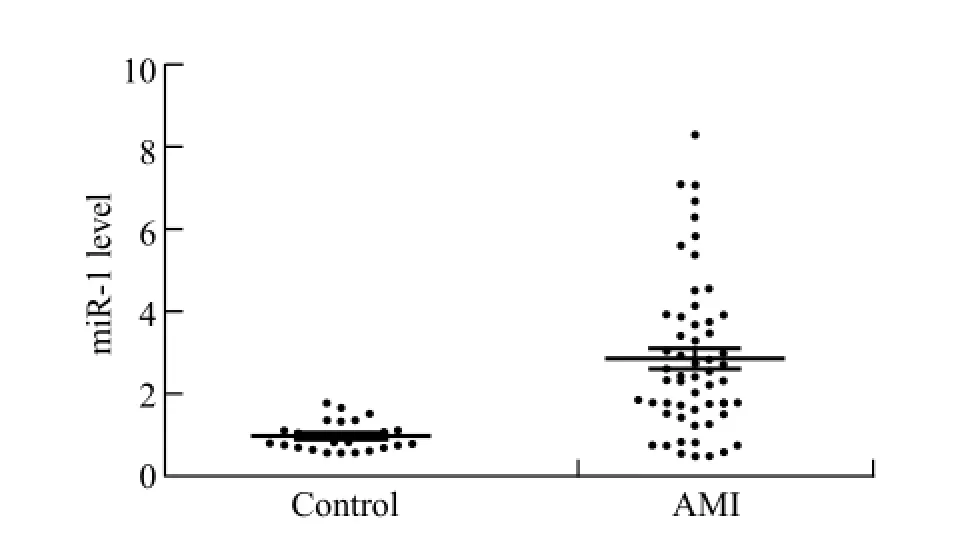
Figure 1. Level of circulating miR-1 in the AMI patients at time of admission in hospital. The plasma levels of miR-1 were signi fi cantly increased in the AMI patients (n=56) compared with the controls (n=28) (P<0.01).
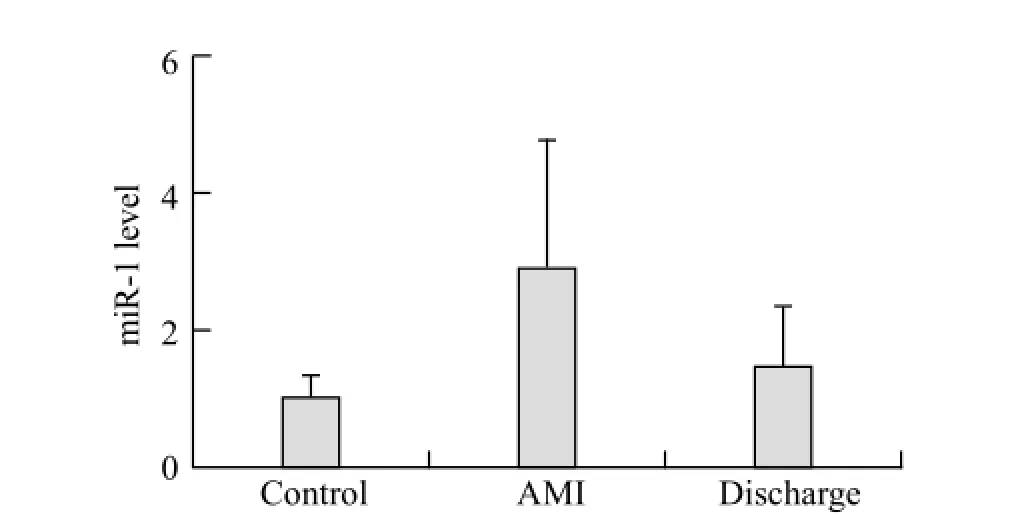
Figure 2. Alteration of plasma miR-1 levels in the AMI patients. MiR-1 was markedly increased in plasma samples gathered within 12 hours after the onset of AMI (day 1). At hospital discharge (day 14), miR-1 had returned to baseline levels similar to those in the controls (n=28 controls, n=56 AMI patients at day 1, n=12 AMI patients at day 14). The values represent the fold changes of miR-1 in the AMI patients relative to the controls. The results are presented as the means ± standard deviation.*P<0.01 vs. the controls.

Figure 3. Comparisons of the sensitivity and speci fi city of the diagnosis by plasma miRNA-1 and cTnT in the AMI patients. ROC curves were constructed to evaluate the diagnostic values of miR-1 for the AMI patients in comparison with cTnT. AUC: area under the ROC curve.
To evaluate the predictive power of circulating miR-1 for AMI, we performed ROC analysis for 56 patients with AMI. As shown in Figure 3, the areas under the ROCcurve (AUC) for miR-1 was 0.8540 (95% confidence interval, 0.8116–0.8964) and the AUC measured for cTnT was 0.9592. This result demonstrated that miR-1 had marked sensitivity and specificity for AMI. However, it was not superior to cTnT for the diagnosis of AMI.
DISCUSSION
In our study, we found that the level of miRNA-1 was significantly increased in patients after AMI compared with the level in healthy volunteers, and our data indicated that the elevated expression of miR-1 was coincident with the progression of AMI. As we know, in the early stages of AMI, pathological changes such as myocardial ischemia, hypoxia, edema, and necrosis occur rapidly, followed by the release of necrotic products, such as cardiac troponins (cTns), creatine kinase (CK) and brain natriuretic peptide (BNP), into the blood-stream. These established biomarkers can be useful for the early diagnosis of AMI. The results of the present work suggest that miRNA-1 might leak out of the necrotic myocardium and into the circulation during the early stages of AMI, and the level of miR-1 would thus become elevated as the AMI progresses; therefore, it might be a useful biomarker for the early diagnosis of AMI.
AMI is a leading cause of death in both developed and developing countries, and the mortality rate of this disease can be reduced by early diagnosis. According to the recent redefinition of myocardial infarction, troponins are more sensitive and specific measures of myocyte necrosis with respect to AMI diagnosis than CK-MB, BNP, and CRP.[20–22]Nevertheless, cTns, as is said previously, have several weaknesses. Therefore, it is important to continue the search for new biomarkers with higher sensitivity and specificity for the early diagnosis of AMI.
Several recent studies have reported that the levels of circulating, cardiac-specific miRNAs were altered in conjunction with the progression of cardiovascular diseases; therefore, these miRNAs might be promising novel biomarkers for the diagnosis of AMI.
The findings from the present study are consistent with those reported in recent articles, indicating that level of the circulating miR-1 was increased during AMI and were significantly higher than those in the healthy volunteers. The level of miR-1 decreased to normal on day 14. We also analyzed the concentration of cTnT during AMI. We performed ROC curve analyses to determine the diagnostic value of miR-1 and to compare it with cTnT. The results of these analyses indicated that circulating miR-1 is specific and sensitive for the early diagnosis of AMI and that it is a promising novel biomarker for AMI. We found that although there were positive relationships between miR-1 and cTnT during the progression of AMI, circulating miR-1 was not superior to cTnT for the early diagnosis of AMI.
According to these results, cTnT seems to be more sensitive and speci fi c than circulating miR-1 in patients with AMI. This study verified the diagnostic value of miR-1 in the early stage of AMI and compared it with the established biomarker cTnT. We found that miR-1 might serve as a novel biomarker for the diagnosis of AMI; however, the diagnostic value of circulating miR-1 was not superior to that of cTnT. We propose that because of its high sensitivity and specificity miR-1 could be used to distinguish AMI in AMI patients with ESRD or other diseases so as to reduce the mortality of AMI. However, our work has some limitations, including the small sample size. Therefore, additional studies with larger cohorts of healthy volunteers and patients are needed to demonstrate the diagnostic value of miR-1 as a practical biomarker.
Funding:This study was supported by grants from the National Natural Science Foundation of China (81071030), the Science and Technology Foundation of Guangdong Province (2011B080701006).Ethical approval:The study protocol was approved by the Ethics Committees of Sun Yat-Sen University, Guangzhou, China.
Con fl icts of interest:We have no con fl icts of interest relevant to the study.
Contributors:Li LM proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
2 Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, et al. It's time for a change to a troponin standard. Circulation 2000; 102: 1216–1220.
3 Collinson PO, Hadcocks L, Foo Y, Rosalki SB, Stubbs PJ, Morgan SH, et al. Cardiac troponins in patients with renal dysfunction. Ann Clin Biochem 1998; 35: 380–386.
4 Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation 2005; 112: 3088–3096.
5 Pheasant M, Mattick JS. Raising the estimate of functional human sequences. Genome Res 2007; 17: 1245–1253.
6 Soifer HS, Rossi JJ, Saetrom P. MicroRNAs in disease and potential therapeutic applications. Mol Ther 2007; 15: 2070–2079.
7 van Rooij E, Liu N, Olson EN. MicroRNAs fl ex their muscles. Trends Genet 2008; 24: 159–166.
8 Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol 2002; 12: 735–739.
9 Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 2006; 11: 441–450.
10 Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518.
11 Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470–1476.
12 Zhou H, He XY, Zhuang SW, Wang J, Lai Y, Qi WG, et al. Clinical and procedural predictors of no-re fl ow in patients with acute myocardial infarction after primary percutaneous coronary intervention. World J Emerg Med 2014; 5: 96–102.
13 Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun 2010; 391: 73–77.
14 Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 2010; 115: 163–169.
15 Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem 2009; 55: 1944–1949.
16 Liu M, Wang HR, Liu JF, Li HJ, Chen SX, Shen S, et al. Therapeutic effect of recombinant tissue plasminogen activator on acute cerebral infarction at different times. World J Emerg Med 2013; 4: 205–209.
17 Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res 2010; 106: 1035–1039.
18 Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010; 3: 499–506.
19 Thygesen K, Alpert JS, White HD, Joint ESCAAHAWHFTF-ftRoMI. Universal de fi nition of myocardial infarction. Eur Heart J 2007; 28: 2525–2538.
20 Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001; 25: 402–408.
21 Myocardial infarction redefined-a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000; 21: 1502–1513.
22 Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined-a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36: 959–969.
Received March 3, 2014
Accepted after revision June 19, 2014
World J Emerg Med 2014;5(3):182–186
10.5847/ wjem.j.issn.1920–8642.2014.03.004
 World journal of emergency medicine2014年3期
World journal of emergency medicine2014年3期
- World journal of emergency medicine的其它文章
- Cardiac arrest: a case-based review
- Life-threatening complications of ascariasis in trauma patients: a review of the literature
- Instructions for Authors
- Abdominal cocoon in a young man
- Effects of aspirin on the expression of nuclear factor-κB in a rat model of acute pulmonary embolism
- Attitudes towards child restrains and seat belts usage in the learned population of Karachi, Pakistan
