Effects of aspirin on the expression of nuclear factor-κB in a rat model of acute pulmonary embolism
Ling-cong Wang, Rong-lin Jiang, Wei Zhang, Li-ling Wei, Ru-hui Yang
1Intensive Care Unit, First Af fi liated Hospital of Zhejiang University of Traditional Chinese Medicine, Hangzhou, China
2Zhejiang University of Traditional Chinese Medicine, Hangzhou, China
3Hangzhou Hebei Science & Technology Co,. Ltd, Hangzhou, China
Corresponding Author:Ling-cong Wang, Email: wlc501@139.com
Effects of aspirin on the expression of nuclear factor-κB in a rat model of acute pulmonary embolism
Ling-cong Wang1, Rong-lin Jiang1, Wei Zhang2, Li-ling Wei2, Ru-hui Yang3
1Intensive Care Unit, First Af fi liated Hospital of Zhejiang University of Traditional Chinese Medicine, Hangzhou, China
2Zhejiang University of Traditional Chinese Medicine, Hangzhou, China
3Hangzhou Hebei Science & Technology Co,. Ltd, Hangzhou, China
Corresponding Author:Ling-cong Wang, Email: wlc501@139.com
BACKGROUND:Acute pulmonary embolism (APE) is a disorder involving the pulmonary circulation resulting from a blockage of the pulmonary artery. The present study aimed to investigate the effects of aspirin on the nuclear factor-κB (NF-κB) activity in a rat model of APE.
METHODS:A total of 108 healthy male Sprague-Dawley rats were randomly assigned into six groups (n=18 rats per group): control group, sham operation group, APE model group, and low-, medium- and high-dose aspirin groups. Six, 24, and 72 hours after the induction of APE, rats in the low-, medium- and high-dose aspirin groups were given aspirin at a respective daily dose of 150, 300, and 600 mg/kg by gavage for three consecutive days. Rats in the other groups were treated with equal volumes of normal saline. Six rats in each group were anesthetized with 10% chloral hydrate solution at each time point, and then the lung tissues were collected and analyzed using immunohistochemical staining.
RESULTS:Positive immunohistochemical staining was present in the bronchial epithelial cells, alveolar cells, macrophages, and surrounding bronchial smooth muscle cells. When compared with the APE model group, the number of positive cells was signi fi cantly lower in the other groups at each time point (P<0.001). Statistically signi fi cant differences were also observed among the aspirin-treated groups at 6 hours (P<0.05, P<0.001). Compared with the APE model group, NF-κB protein expression was reduced in the other groups at each time point (P<0.05, P<0.001). Rats from the APE model group had thrombosis, damaged alveolar walls, and pulmonary hemorrhage, along with different degrees of in fl ammatory cellular in fi ltration at each time point. However, pathological changes such as pulmonary hemorrhage and in fi ltration of in fl ammatory cells were attenuated after the aspirin treatment.
CONCLUSION:Aspirin can signi fi cantly inhibit NF-κB activity in the lung of rats with APE in a dose-dependent manner, and can alleviate lung injury after APE.
Aspirin; Acute pulmonary embolism; Nuclear factor-κB
INTRODUCTION
Acute pulmonary embolism (APE) is a disorder involving the pulmonary circulation resulting from a blockage of the pulmonary artery. It is associated with high incidence and mortality rates in Western countries. Recently, the incidence of APE has been rising in Chinese individuals. It has been demonstrated that there is an extensive in fl ammatory response in the lung tissues of rats with APE, along with greatly increased levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-8.[1]In our previous studies[1,2]a high level of nuclear factor (NF)-κB was also observed in rats after the induction of APE. In addition, aspirin was capable of alleviating lung injury and reducing the levels of in fl ammatory cytokines in rats with APE. However, few studies[3,4]in the effects of aspirin on the expression of NF-κB have been published. Therefore, the present study was designed to evaluate the effects of aspirin on NF-κB activity in a rat model of APE.
METHODS
Animals and grouping
A total of 108 male speci fi c-pathogen-free Sprague-Dawley rats weighing 250±20 g were purchased from the Huishan Laboratory Animal Center, Wuxi, Jiangsu, China (license number: SCXK (Su) 2009–0005). The rats were rank-ordered according to weight and randomly assigned into six groups: control group, sham operation group, APE model group, and low-, medium- and highdose aspirin groups (n=18 rats per group).
Aspirin pretreatment and dosing
Rats in the low-, medium- and high-dose aspirin groups were administered with a single-dose gavage of aspirin (Yung-Shin Pharmaceutical Industrial Co., Ltd., Kunshan, Jiangsu, China; batch number: W026) at 150, 300, and 600 mg/kg, respectively. Rats in the control, sham operation, and APE model groups received equal volumes of normal saline by gavage.
Establishment of APE model
After pretreatment, the model of APE was produced in all animals, except those in the control and sham operation groups, using the autologous blood clot method.[1,2]Briefly, 4 hours before the induction of APE, blood clotting suspensions were obtained from the blood taken from the orbital venous plexus of the rats. Afterward, approximately 15–20 autologous blood clots with a size of 2 mmX3 mm were inserted into the external jugular vein. The successful establishment of the rat APE model was indicated by the symptom of short breath. Meanwhile, the rats in the sham operation group were given equal volumes of normal saline, and those in the control group did not receive any treatment.
Intervention protocols
At the respective time points of 6, 24, and 72 hours post-treatment, rats in the low-, medium- and high-dose aspirin groups were given aspirin at a respective daily dose of 150, 300, and 600 mg/kg by gavage for three consecutive days. Rats in the other groups were treated with 2 mL of normal saline in the same manner. All rats were allowed free access to food and water throughout the treatment period.
Immunohistochemical analysis
After the model was established, six rats from each group were anesthetized with intramuscular 10% chloral hydrate solution at 6, 24, and 72 hours. Next, the lung tissues were quickly removed, and a small piece of the lung was routinely embedded in paraffin and used for immunohistochemical staining of NF-κB. Four lung sections from each rat were analyzed, and three highmagnification fields of vision were randomly selected from each section, and the mean was calculated.
Western blot analysis
The upper lobes of the left lung were collected for the detection of NF-κB protein expression by Western blot analysis. The optical intensity of the visualization signal was analyzed using an Image J densitometric analysis system (National Institutes of Health, Bethesda, MD, USA), and NF-κB protein expression was normalized to β-actin.
Pathological analysis
The lung tissues in each group were fi xed with 10% formaldehyde for 24 hours. Paraffin-embedded sections were cut into slices, stained with hematoxylin-eosin, and analyzed by experienced pathologists under an optical microscope.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 19.0 statistical software (IBM Corporation, Armonk, NY, USA). Data were expressed as means and standard deviations. Multi-factor analysis of variance (ANOVA) was used to compare the immunohistochemistry results between the groups, and the differences of NF-κB activity analyzed by Western blotting were tested using one-way ANOVA. Additionally, Fisher's least-significantdifference test was used to compare the means between the groups. Values of P<0.05 were considered statistically signi fi cant.
RESULTS
Treatment with aspirin signi fi cantly reduced the number of NF-κB-positive cells
Positive immunohistochemical staining (positive cells were stained brown) of NF-κB was primarily present in the bronchial epithelial cells, alveolar cells, macrophages, and surrounding bronchial smooth muscle cells. Rats in the APE model group exhibited the strongest positive staining (Figure 1).
As demonstrated in Table 1, compared with the APE model group, the number of cells with positive staining was signi fi cantly lower in the other groups at each time point (P<0.001). There were obvious differences in the number among the aspirin-treated groups at 6 hours after the induction of APE (P<0.05, P<0.001). In addition, a significantly decreased number of NF-κB-positive cellswas found in the APE model and medium-dose aspirin groups at 72 hours when compared with the number of cells at 24 hours (P<0.001).

Figure 1. Immunohistochemical staining of NF-κB in lung tissues of rats from different groups (original magni fi cationX400). A: control group; B: sham operation group; C: APE model group; D: low-dose aspirin group; E: medium-dose aspirin group; F: high-dose aspirin group. Positive immunohistochemical staining of NF-κB is primarily present in the bronchial epithelial cells, alveolar cells, macrophages, and surrounding bronchial smooth muscle cells; positive staining of NF-κB appears brown. Rats from the APE model group exhibited the strongest positive staining.
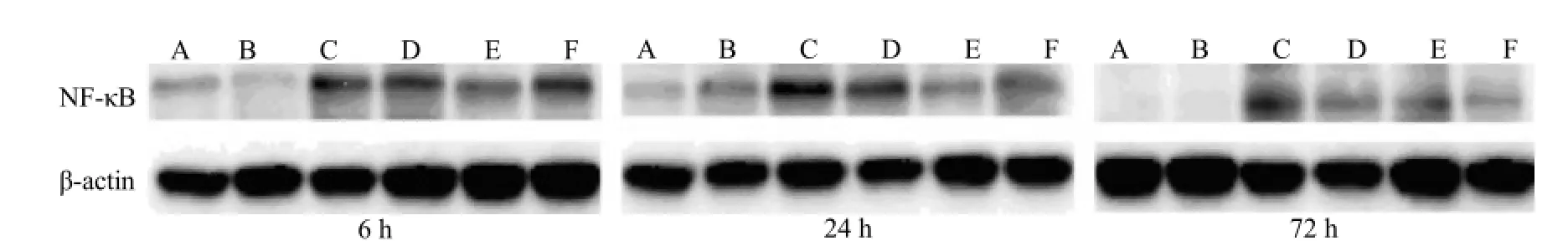
Figure 2. Western blot analysis of NF-κB protein expression in the lung tissues of rats from different groups. A: control group; B: sham operation group; C: APE model group; D: low-dose aspirin group; E: medium-dose aspirin group; F: high-dose aspirin group. Rats from the APE model group exhibited the highest NF-κB protein expression. The NF-κB protein expression in rats receiving aspirin gradually declined in a dose-dependent manner.
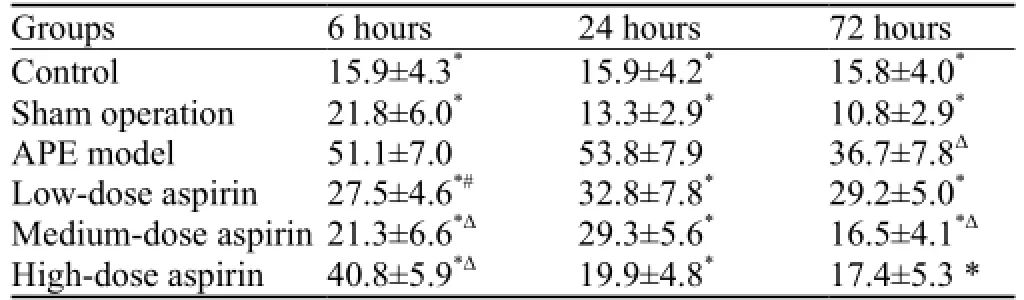
Table 1. Comparison of the number of NF-κB-positive cells in different groups of rats (mean ±SD)
Treatment with aspirin signi fi cantly reduced NF-κB protein expression
NF-κB protein expression determined by Westernblotting is shown in Table 2. When compared with the APE model group, a significant reduction in NF-κB protein expression was observed in the other groups at each time point (P<0.05, P<0.001). As shown in Figure 2, the highest NF-κB protein expression was seen in the APE model group. In contrast, NF-κB protein expressionin rats receiving aspirin gradually declined in a dosedependent manner.
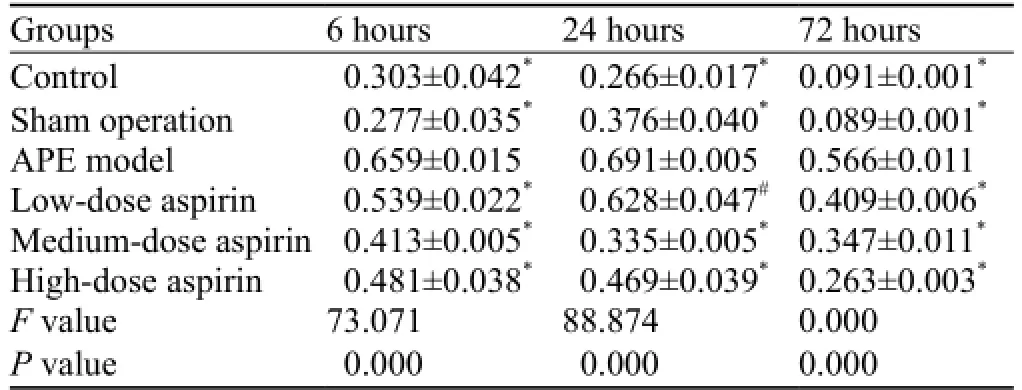
Table 2. Comparison of NF-κB protein expression in different groups of rats (mean ±SD)
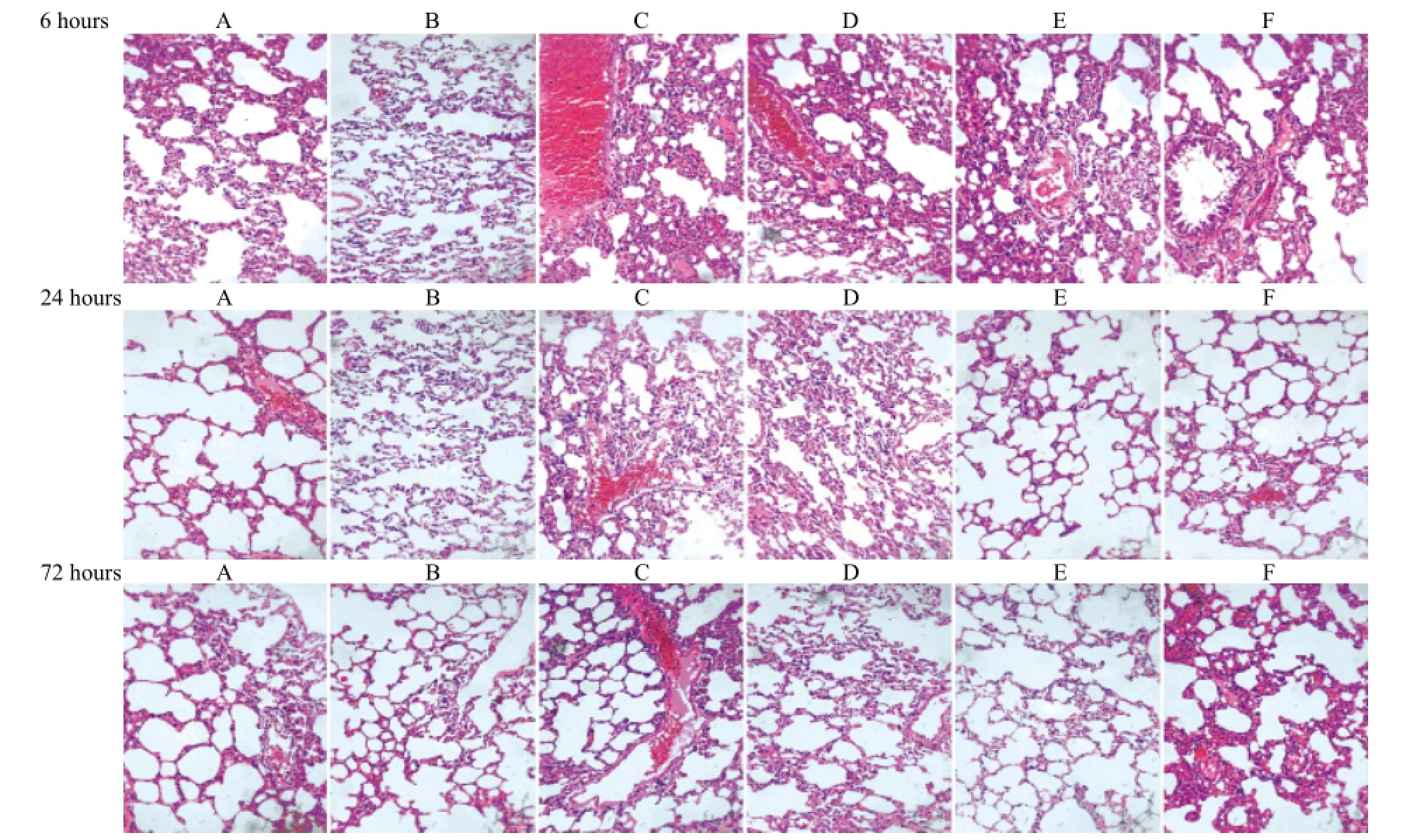
Figure 3. Morphological lung changes in different groups of rats by optical microscopy (HE staining, original magni fi cationX200). A: control group; B: sham operation group; C: APE model group; D: low-dose aspirin group; E: medium-dose aspirin group; F: high-dose aspirin group. Rats from the APE model group exhibited damaged alveolar walls, pulmonary hemorrhage, and different degrees of in fi ltration of in fl ammatory cells at each time point. However, the referred changes such as pulmonary hemorrhage and in fl ammatory cellular in fi ltration were attenuated in the aspirin-treated groups.
Pathological assessment of the lungs
The pathological changes of lung tissues among all groups are shown in Figure 3. Rats from the APE model group exhibited damaged alveolar walls, pulmonary hemorrhage, and different degrees of infiltration of inflammatory cells at each time point. However, changes such as pulmonary hemorrhage and in fl ammatory cellular in fi ltration were attenuated in the aspirin-treated groups.
DISCUSSION
A variety of research has demonstrated the extensive degree of inflammatory responses in lung tissues of rats with APE. Moreover, the interactions between the products from blood coagulation and inflammatory cells have been found to further aggravate the pathological injury in the lung.[1–2,6]It is well established that the production of various in fl ammatory cytokines such as thromboxane A2 (TXA2), platelet activating factor, IL-1, IL-6, and IL-8 is involved in the process of APE.[7]NF-κB is a common cytokine which can be activated by many bacteria and viruses.[8]NF-κB has been shown to induce the excessive release of inflammatory cytokines including TNF-α, IL-1β, and IL-6, and then promote significant accumulation of neutrophils in inflammatory regions, thereby causing inflammatory responses. NF-κB-induced activation of TNF-α, IL-1β, and IL-6 can also up-regulate NF-κB. This positive feedback loop can further aggravate the in fl ammatory responses. Furthermore, a close correlation was found between the increase in NF-κB activity and the severity of pulmonary in fl ammation in animal models of APE; thus, this rise in the NF-κB activity was thought to have a pathogenic role in the lung injury.[3–5]Aspirin is an irreversible inhibitor for cyclooxygenase, and it can inhibit cyclooxygenase activity, thereby reducing the production of TXA2 in platelets. Additionally, aspirin can exert its anti-in fl ammatory properties by blocking NF-κB activation. However, due to the non-specificity of nonsteroidal anti-inflammatory drugs, the inhibition of NF-κB activity is more significant at high concentrations of aspirin.[9,10]So, the high-dose of aspirin was administered to rats in the present study. To date, thrombolytic therapy and oral anticoagulation with warfarin have been the main treatment regimens for APE, while aspirin is considered second-line therapy for APE.[11]Recent studies[12–14]haveindicated that aspirin is effective in the prevention and treatment of APE. Based on these fi ndings, it is indicated that the administration of a high-dose of aspirin can alleviate the in fl ammatory responses following APE, and aspirin could be considered an effective approach for the treatment of APE in clinical practice. Nevertheless, there are aspirin-related adverse events such as bleeding, aspirin should be used with extreme caution in patients with APE.
APE is mainly characterized by pathological alterations involving destruction of alveolar walls and pulmonary hyperemia. In the present study, a rat model of APE was successfully established, as indicated by the symptom of shortness of breath. Rats in the APE model group presented with apparent pulmonary hyperemia and infiltration of inflammatory cells at 6, 24, and 72 hours after model establishment. However, these pathological changes were alleviated in rats treated with aspirin. These findings suggest that aspirin can attenuate the pathological injury in the lung following APE. The results of immunohistochemical staining of NF-κB showed that the number of NF-κB-positive cells was notably lower in the aspirin-treated groups at each time point in comparison with that in the APE model group. Compared with the control and sham operation groups, a signi fi cant increase in the number of positive cells was found in the APE model group at each time point. This suggested increased NF-κB activity in the rats with APE, which was consistent with the results of the Western blot analysis. Nevertheless, the number of cells with positive staining in the low-dose aspirin group peaked at 24 and 72 hours, and the number of cells in the medium-dose aspirin group reached the maximum at 24 hours. By contrast, the number of positive cells peaked at 6 hours in the high-dose aspirin group. These results revealed a dose-dependent effect of aspirin on the number of NF-κB-positive cells. Therefore, it is thought that aspirin can reduce the levels of TNF-α and IL-1β through the suppression of NF-κB expression, and then inhibit the in fl ammatory responses after APE.
In summary, the current study demonstrated that aspirin can inhibit the NF-κB activity in the lung of rats with APE in a dose-dependent manner, and then alleviate the lung injury following APE.
Funding:The study was supported by grants from the Natural Science Foundation of Zhejiang Province (Y207052, LY12H29005) and the Construction of Key Disciplines in Traditional Chinese Medicine of Zhejiang Province (2012-XK-A12).
Ethical approval:The present study was approved by the Animal Care and Use Committee of the First Af fi liated Hospital of Zhejiang University of Traditional Chinese Medicine, Hangzhou, China.
Con fl icts of interest:The authors declare that there is no con fl ict of interest.
Contributors:Wang LC proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
1 Sun C, Wang LC, Jiang HF, Yang RH. Effects of aspirin on CX3CL1 and CX3CR1 in acute pulmonary embolism rats. Zhonghua Yi Xue Za Zhi 2013; 93: 69–72.
2 Wang L, Wu J, Zhang W, Zhi Y, Wu Y, Jiang R, et al. Effects of aspirin on the ERK and PI3K/Akt signaling pathways in rats with acute pulmonary embolism. Mol Med Rep 2013; 8: 1465–1471.
3 Lu WJ, Lee JJ, Chou DS, Jayakumar T, Fong TH, Hsiao G, et al. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation: the pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. Mol Med (Berl) 2011; 89: 1261–1273.
4 Peng CK, Huang KL, Wu CP, Li MH, Lin HI, Hsu CW, et al. The role of mild hypothermia in air embolism-induced acute lung injury. Anesth Analg 2010; 110: 1336–1342.
5 Wu XL, Long D, Yu L, Yang JH, Zhang YC, Geng F. Urokinasetype plasminogen activator receptor as a predictor of poor outcome in patients with systemic infl ammatory response syndrome. World J Emerg Med 2013; 4: 190–195.
6 Smulders YM. Pathophysiology and treatments of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 2000; 48: 23.
7 Schmeck J, Koch T, Patt B, Heller A, Neuhof H, van Ackern K. The role of endothelin-1 as a mediator of the pressure response after air embolism in blood perfused lungs. Intensive Care Med 1998; 24: 605–611.
8 Chen X, Yu G, Fan S, Bian M, Ma H, Lu J, et al. Sargassum fusiforme polysaccharide activates nuclear factor kappa-B (NF-κB) and induces cytokine production via Toll-like receptors. Carbohydr Polym; 2014: 113–120.
9 Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF- kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 2001; 107: 135–142.
10 Aradhya S, Nelson DL. NF-kappaB signaling and human disease. Curr Opin Genet Dev 2001; 11: 300–306.
11 Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008; 29: 2276–2315.
12 Sobieszczyk P, Fishbein MC, Goldhaber SZ. Acute pulmonary embolism: don't ignore the platelet. Circulation 2002; 106: 1748–1749.
13 Kher A, Samama M, Haïat R. Heparin in the treatment and secondary prevention of myocardial infarction. A critical review of the main trials. Arch Mal Coeur Vaiss 1991; 84: 1581–1586.
14 Miller F, Young DC, Wang GJ. The incidence of thromboembolic disease. Clin Orthop Relat Res 1983; 176: 210–216.
Received February 11, 2014
Accepted after revision July 20, 2014
World J Emerg Med 2014;5(3):229–233
10.5847/ wjem.j.issn.1920–8642.2014.03.013
 World journal of emergency medicine2014年3期
World journal of emergency medicine2014年3期
- World journal of emergency medicine的其它文章
- Domestic versus imported drug-eluting stents for the treatment of patients with acute coronary syndrome
- Comparison of plasma microRNA-1 and cardiac troponin T in early diagnosis of patients with acute myocardial infarction
- International normalized ratio as a predictor of mortality in trauma patients in India
- Can venous blood gas analysis be used for predicting seizure recurrence in emergency department?
- Knowledge and skills of neonatal resuscitation of health professionals at a university teaching hospital of Northwest Ethiopia
- Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensiveintracerebral hemorrhage
