新型胆酸缩氨基硫脲衍生物的合成与抗菌活性研究
张丽娜, 姜羽佳, 陈佰灵, 赵志刚
(西南民族大学化学与环境保护工程学院, 四川 成都 610041)
新型胆酸缩氨基硫脲衍生物的合成与抗菌活性研究
张丽娜, 姜羽佳, 陈佰灵, 赵志刚
(西南民族大学化学与环境保护工程学院, 四川 成都 610041)
通过甾酮与取代氨基硫脲缩合, 合成了一系列新型的胆酸缩氨基硫脲衍生物. 其结构均经1H NMR, IR, ESI-MS及元素分析所证实. 此外, 所有目标化合物都进行了对金黄色葡萄球菌, 枯草芽孢杆菌, 绿脓杆菌, 大肠杆菌的抗菌活性测试. 化合物4b, 4g和4h对绿脓杆菌和大肠杆菌都具有良好的抑制效果.
胆酸; 缩氨基硫脲; 抗菌活性
近年来, 各种各样的致病细菌对动植物造成了严重破坏[1], 并给社会和经济带来了严重性后果. 由于细菌感染, 每年有许多人正遭受着身心的巨大痛苦, 不幸的是, 一些人甚至死于此. 随着耐药细菌多样性的出现[2], 发展新型且有效的抗菌药物来降低感染率是迫在眉睫的需求.
在生物活性的研究领域, 作为一类广谱有机化合物, 缩氨基硫脲类衍生物已受到了越来越多化学家与生物学家的广泛关注[3]. 近期的研究结果表明, 缩氨基硫脲类化合物具有良好的生物活性[4], 包括抗细菌[5-7], 抗真菌[8-9],抗肿瘤[10-11], 抗寄生虫[12], 抗疟疾[13-14], 抗病毒[15], 抗艾滋病和抗单纯疱疹病[16-17].
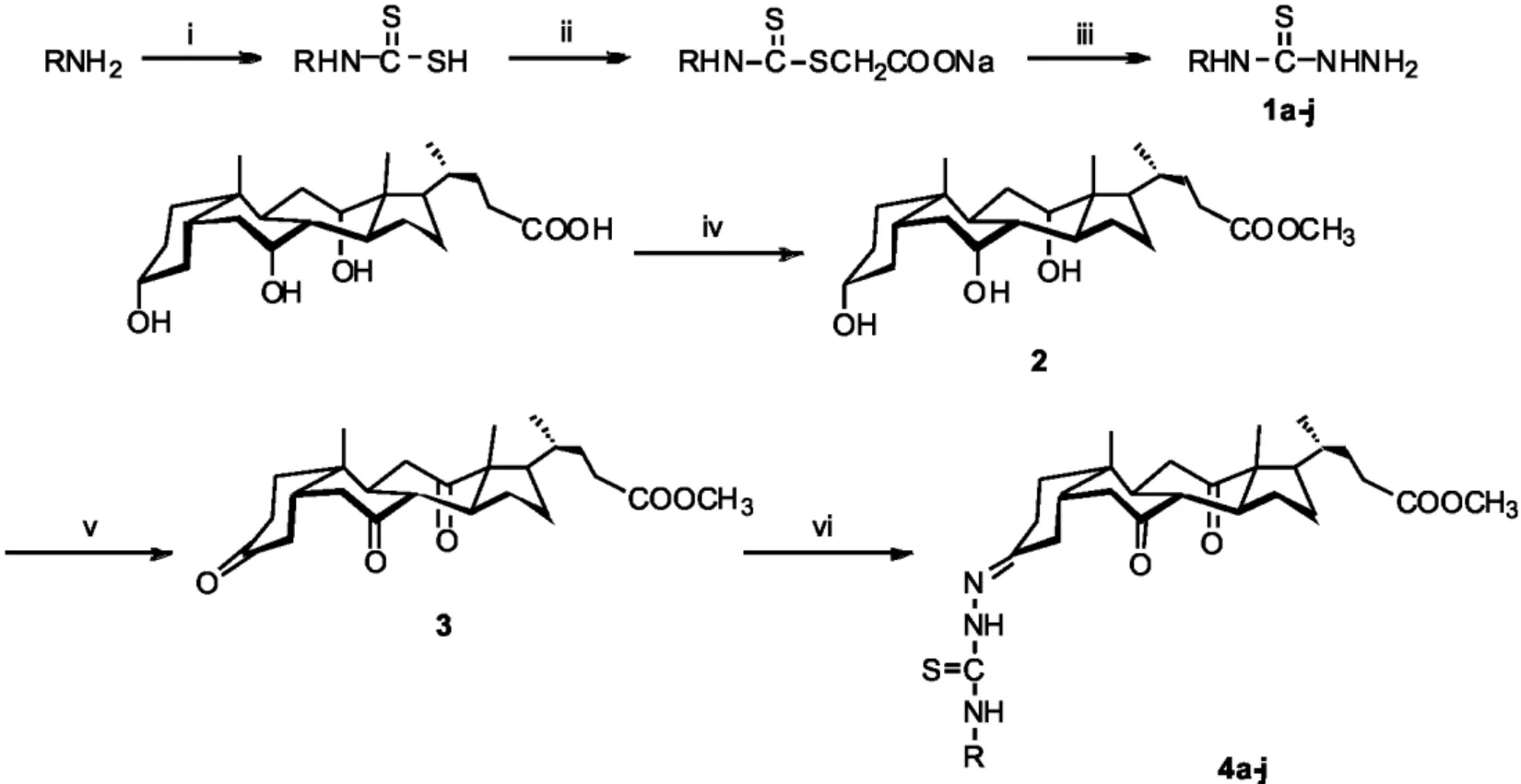
目前, 虽然已有许多基于甾体单元修饰的文章, 包括衍生化, 环化[18], 但关于胆酸席夫碱却鲜有报道. 众所周知, 胆酸在生物系统中丰富且十分重要, 同时其显示出相对低的毒性以及良好的生物相容性[19]. 根据活性因子叠加原理, 我们预期含有胆酸单元和硫脲基团的缩氨基硫脲衍生物具有高效的抗菌活性. 在此, 我们报告新型胆酸缩氨基硫脲衍生物的合成以及这些化合物对四种致病菌株(大肠杆菌、枯草芽孢杆菌、绿脓杆菌、金黄色葡萄)的抗菌活性评估. 其合成路线见Scheme 1.
1 实验部分
1.1 仪器与试剂
WRS-1B型数字显示显微熔点仪(温度计未经校正); Wzz-2B型自动旋光仪(CH2Cl2为溶剂); Agilent-400MHz型核磁共振仪(TMS作内标, CDCl3作溶剂); PERKIN-ELMER1700型红外光谱仪(KBr压片); FINNIGAN-LCQ型质谱仪; VarioMICRO自动元素分析仪; 三申-YX280A型手提式高压蒸汽灭菌锅; 东联- DL-CJ-1N型垂直层流洁净工作台; 友联-HY-2A型数显调速多用振荡器; 锐品-ECA-9272型电热恒温培养箱, 所用试剂均为分析纯.
1.2 取代氨基硫脲1a-j的合成
在装有0.01 mol取代胺溶液、15 mL无水乙醇的三颈瓶中, 加入2 mL浓氨水, 不断搅拌下缓慢滴加0.8 mL二硫化碳, 并控制此混合溶液在15-20 ℃反应1-2 h, 可见反应逐渐析出固体. 浑浊体系中加入1.2 g(0.01 mol)氯乙酸钠, 搅拌后加入1.2 mL 85%的水合肼, 于60 ℃下冷凝回流反应4 h, TLC监测反应. 反应完全后, 冷却, 减压抽滤. 将固体粗产物用乙醇重结晶, 得到纯品取代氨基硫脲1a-j.[20]
1.3 中间体2的合成
在烧瓶中加入0.41 g(1.0 mmol)胆酸, 15 mL无水甲醇, 0.3 mL浓盐酸, 室温反应4 h, TLC监测反应. 反应完全后, 减压蒸馏除去过量甲醇, 固体用20 mL乙酸乙酯溶解后, 依次用饱和NaHCO3溶液(3×20 mL)洗涤, 饱和食盐水(3×20 mL)洗涤, 再加无水Na2SO4干燥过夜. 减压抽滤后, 粗产物经柱层析分离纯化, 得白色晶体.[21]
1.4 中间体3的合成
0.42g(1.0 mmol) 中间体2溶于20 mL干燥的CH2Cl2, 加入0.69 g(3.2 mmol)PCC, 室温搅拌9 h, TLC监测反应. 反应完全后, 旋干CH2Cl2, 加入20 mL乙酸乙酯溶解, 粗产物经柱层析分离纯化, 得白色晶体.[21]
1.5 胆酸缩氨基硫脲4a-j的合成通法
0.42g(1.01 mmol)中间体3和1.0 mmol取代氨基硫脲溶于20 mL无水乙醇中, 加入两滴浓盐酸, 室温反应4-6 h, 并用TLC监测反应进程. 反应完全后, 减压抽滤, 固体粗产物用乙醇重结晶得纯品目标物.
4a: 白色固体, 产率90%, m.p. 188-189℃;= -136.12 (c 0.57, CH2Cl2); IR (KBr): 3328, 2973, 2884, 1735, 1705, 1600, 1539, 1476, 1441, 1383, 1348, 1264, 1180, 1101, 1048, 756 cm-1;1H NMR (400 MHz, CDCl3) δ: 9.23 (s, 1H, NH), 8.62 (d, 1H, J = 11.2 Hz, NH), 7.63 (d, 2H, J =7.6 Hz, ArH), 7.38 (m, 2H, ArH), 7.22 (m, 1H, ArH), 3.67 (s, 3H, COOCH3) , 1.37 (s, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.85 (d, 3H, J = 6.4 Hz, 21-CH3); ESI-MS m/z (%): 588 ([M+23]+, 100). Ana1. Calcd for C32H43N3O4S: C, 67.93; H, 7.66; N, 7.43. Found: C, 67.82; H, 7.64; N, 7.45.
4b: 白色固体, 产率85%, m.p. 211-212℃;= -109.73 (c 0.60, CH2Cl2); IR (KBr): 3298, 2968, 2882, 1741, 1707, 1584, 1532, 1481, 1391, 1269, 1186, 1067, 1006, 821 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.19 (s, 1H, NH), 8.64 (d, 1H, J = 6.4 Hz, NH), 7.56 (d, 2H, J = 8.8 Hz, ArH), 7.48 (d, 2H, J = 8.8 Hz, ArH), 3.67 (s, 3H, COOCH3), 1.38(s, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.85 (d, 3H, J = 6.4 Hz, 21-CH3); ESI-MS m/z (%): 668 ([M+23]+, 100). Ana1. Calcd for C32H42BrN3O4S: C, 59.62; H, 6.57; N, 6.52. Found: C, 59.71; H, 6.59; N, 6.54.
4c: 白色固体, 产率87%, m.p. 148-149℃;= -76.58 (c 0.56, CH2Cl2); IR (KBr): 3315, 2968, 2881, 1736, 1708, 1600, 1539, 1460, 1381, 1236, 1189, 1108, 1029, 747 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.75 (s, 1H, NH), 8.67 (t, 1H, J = 6.8 Hz, ArH), 8.57 (d, 1H, J = 11.2 Hz, NH), 7.13 (t, 1H, J = 8.0 Hz, ArH), 6.99 (t, 1H, J = 8.0 Hz , ArH), 6.91 (d, 1H, J = 8.0 Hz, ArH), 3.89 (s, 3H, Ar-OCH3), 3.67(s, 3H, COOCH3), 1.38 (s, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.85 (d, 3H, J = 6.8 Hz, 21-CH3); ESI-MS m/z (%): 618 ([M+23]+, 100). Ana1. Calcd for C33H45N3O5S: C, 66.53; H, 7.61; N, 7.05. Found: C, 66.62; H, 7.63; N, 7.03.
4d: 白色固体, 产率87%, m.p. 186-187℃;= -85.31 (c 0.60, CH2Cl2); IR (KBr): 3285, 2966, 2884, 1745, 1707, 1598, 1537, 1463, 1380, 1297, 1166, 1044, 781, cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.24 (s, 1H, NH), 8.59 (d, 1H, J = 10.0 Hz, NH), 7.45 (s, 1H, ArH), 7.26 (t, 1H, J = 8.0 Hz, ArH), 7.12 (d, 1H, J = 8.0 Hz, ArH), 6.77 (d, 1H, J = 8.4 Hz, ArH), 3.82 (s, 3H, Ar-OCH3), 3.67 (s, 3H, COOCH3), 1.38 (s, 3H, , 19-CH3), 1.07 (s, 3H, 18-CH3), 0.85 (d, 3H, J = 6.8 Hz, 21-CH3); ESI-MS m/z (%): 618 ([M+23]+, 100). Ana1. Calcd for C33H45N3O5S: C, 66.53; H, 7.61; N, 7.05. Found: C,66.45; H, 7.59; N, 7.07.
4e: 白色固体, 产率85%, m.p. 197-198℃;= -97.27 (c 0.60, CH2Cl2); IR (KBr): 3316, 2955, 2887, 1747, 1705, 1583, 1530, 1488, 1448, 1382, 1273, 1210, 1174, 1100, 741 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 8.98 (s, 1H, NH), 8.67 (d, 1H, J = 12.0 Hz, NH), 7.64 (t, 1H, J = 6.8 Hz, ArH), 7.25-7.18 (m, 3H, ArH), 3.67 (s, 3H, COOCH3), 2.30 (s, 3H, Ar-CH3), 1.38 (s, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.85 (d, 3H, J = 6.8 Hz, 21-CH3); ESI-MS m/z (%): 1197 ([2M+39]+, 100). Ana1. Calcd for C33H45N3O4S: C, 68.36; H, 7.82; N, 7.25. Found: C,68.47; H, 7.84; N, 7.23.
4f: 白色固体, 产率90%, m.p. 196-197℃;= -100.56 (c 0.57, CH2Cl2); IR (KBr): 3307, 2967, 2885, 1744, 1707, 1599, 1538, 1483, 1440, 1379, 1276, 1168, 1045, 781 cm-1;1H NMR (400 MHz, CDCl3) δ∶1H NMR (400 MHz, CDCl3) δ∶ 9.28 (s, 1H, NH), 9.63 (d, 1H, J = 8.0 Hz, NH), 7.66 (d, 1H, J = 10.4 Hz, ArH), 7.37-7.29 (m, 2H, ArH), 6.91 (t, 1H, J = 7.6 Hz, ArH), 3.67 (s, 3H, COOCH3), 1.38 (d, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.84 (d, 3H, J = 6.8 Hz, 21-CH3); ESI-MS m/z (%): 606 ([M+23]+, 100). Ana1. Calcd for C32H42FN3O4S: C, 65.84; H, 7.25; N, 7.20. Found: C,65.95; H, 7.22; N, 7.18.
4g: 白色固体, 产率88%, m.p. 191-192℃;=110.92 (c 0.55, CH2Cl2); IR (KBr): 3318, 2974, 2879, 1735, 1705, 1597, 1535, 1473, 1439, 1383, 1345, 1265, 1180, 834 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.13 (s, 1H, NH), 8.66 (d, 1H, J = 7.6 Hz, NH), 7.57-7.53 (m, 2H, ArH), 7.07 (t, 2H, J = 8.4 Hz, ArH), 3.67 (s, 3H, COOCH3), 1.38 (s, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.81 (d, 3H, J = 6.8 Hz, 21-CH3); ESI-MS m/z (%): 606 ([M+23]+, 100). Ana1. Calcd for C32H42FN3O4S: C, 65.84; H, 7.25; N, 7.20. Found: C,65.75; H, 7.27; N, 7.22.
4h: 白色固体, 产率86%, m.p. 168-169℃;= -52.84 (c 0.56, CH2Cl2); IR (KBr): 3307, 2950, 2886, 1736, 1707, 1586, 1529, 1486, 1437, 1375, 1267, 1186, 1101, 752 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.71 (s, 1H, NH), 8.69 (d, 1H, J = 12.0 Hz, NH), 8.57 (t, 1H, J=7.2 Hz, ArH), 7.42 (d, 1H, J = 8.0 Hz, ArH), 7.31 (t, 1H, J = 7.2 Hz , ArH), 7.14 (t, 1H, J = 7.6 Hz, ArH), 3.67 (s, 3H, COOCH3), 1.37 (d, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.84 (d, 3H, J = 6.8 Hz, 21-CH3); ESI-MS m/z (%): 622 ([M+23]+, 100). Ana1. Calcd for C32H42ClN3O4S: C, 64.03; H, 7.05; N, 7.00. Found: C,64.15; H, 7.07; N, 6.98.
4i: 白色固体, 产率88%, m.p. 206-207℃;= -65.15 (c 0.57, CH2Cl2); IR (KBr): 3301, 2967, 2883, 1741, 1706, 1586, 1531, 1483, 1381, 1269, 1186, 1092, 826 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.20 (s, 1H, NH), 8.66 (d, 1H, J = 6.4 Hz, NH), 7.60 (d, 2H, J = 8.8 Hz, ArH), 7.34 (d, 2H, J = 8.4 Hz, ArH), 3.67 (s, 3H, COOCH3), 1.37 (s, 3H, J=4.8 Hz, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.84 (d, 3H, J = 6.4 Hz, 21-CH3); ESI-MS m/z (%): 622 ([M+23]+, 100). Ana1. Calcd for C32H42ClN3O4S: C, 64.03; H, 7.05; N, 7.00. Found: C,64.17; H, 7.03; N, 7.02.
4j: 白色固体, 产率82%, m.p. 182-183℃;= -80.72 (c 0.56, CH2Cl2); IR (KBr): 3305, 2965, 2884, 1746, 1706, 1594, 1533, 1479, 1379, 1243, 1182, 823 cm-1;1H NMR (400 MHz, CDCl3) δ∶ 9.06 (s, 1H, NH), 8.62 (d, 1H, J = 14.4 Hz, NH), 7.45 (d, 2H, J = 8.8 Hz, ArH), 6.91 (d, 2H, J = 8.8 Hz, ArH), 3.82 (s, 3H, Ar-OCH3), 3.67 (s, 3H, COOCH3), 1.38 (s, 3H, 19-CH3), 1.07 (s, 3H, 18-CH3), 0.84 (d, 3H, J = 6.4 Hz, 21-CH3); ESI-MS m/z (%): 618 ([M+23]+, 100). Ana1. Calcd for C33H45N3O5S: C, 66.53; H, 7.61; N, 7.05. Found: C,66.40; H, 7.64; N, 7.03.
2 结果与讨论
2.1 光谱分析
化合物4a-j的其结构均经1H NMR, IR, ESI-MS及元素分析所确认. 质谱均显示出预期的高强度分子峰. 红外光谱中, 3328-3285 cm-1的强吸收峰为N-H伸缩震动的特征峰, 1600-1583 cm-1与1297-1236 cm-1范围内的强吸收峰分别归属于C=N与C=S基团. 1707-1705 cm-1范围内的强吸收峰为甾酮中C=O基团的特征峰, 1747-1735 cm-1则为COOCH3中C=O的吸收峰. 核磁氢谱中, δ 8.98-9.75 ppm间的单峰为NH的质子峰, δ 8.56-8.71 ppm间的双峰则为另一个NH的质子峰. 此外, 位于δ 1.37-1.38 ppm和δ 1.07 ppm的单峰与δ 0.84-0.86 ppm的双峰为甾体上甲基质子的特征峰, δ 3.67 ppm处的单峰为COOCH3的质子峰.
2.2 体外抗菌活性测试
化合物4a-j的体外抗菌活性测试使用两种革兰氏阳性细菌(金黄色葡萄球菌、枯草芽孢杆菌)和两种革兰氏阴性细菌(绿脓杆菌、大肠杆菌), 阿莫西林(25 μg)与环丙沙星(25 μg)作为标准对照药物. 采用二倍稀释法来计算试管中含菌体数为105CFU mL−1的标准培养液的MIC. 测试前, 先将化合物溶解于1 mL DMSO中, 依次稀释至最终浓度分别为256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 μg mL−1, 每支试管中加入1 mL 0.5马克法兰氏浊度标准的菌液. MIC直观地决定于在37 ℃孵化16 h后明显增长的抑制. 此外, IC50值则由含菌体数为105CFU mL−1的标准培养液的抑菌圈测试来计算. 测试前, 先将待测物溶解于2 mL DMSO中, 依次稀释至最终浓度分别为640, 320, 160, 80, 40, 20, 10, 5 μg mL−1, 0.5马克法兰氏浊度标准的菌液在肉汁培养基中培养后, 用待测物稀释位于表面增长的细菌使滤纸(直径6 mm)饱和. 37 ℃孵化16 h后得到抑菌圈直径, 由此计算出IC50值. MIC与IC50值见表1.

表1 胆酸缩氨基硫脲衍生物4a-j与两种阳性对照(环丙沙星与阿莫西林)的抗菌活性Table 1 Antibacterial activity of cholic acid thiosemicarbazone derivatives 4a-j and two positive controls (Ciprofloxacin and Amoxicillin)
-, 无抑菌活性
如表1所示, 化合物4b, 4g和4h对绿脓杆菌与大肠杆菌显示出良好的抗菌活性, 其中4g对大肠杆菌的抗菌活性与阿莫西林相当, 无化合物对金黄色葡萄球菌与枯草芽孢杆菌有抗菌活性. 初步实验结果表明, 苯环上不同的取代基团, 对化合物的生物活性有不同影响. 苯环上有吸电子基团如氟、溴的化合物显示出良好的抗菌活性,然而引入给电子基团如甲基或甲氧基则会对其活性产生不利影响. 进一步的抗菌活性还在研究中.
[1] HERNANDES M Z, RABELLO M M, LEITE A C L, et al. Studies toward the structural optimization of novel thiazolylhydrazonebased potent antitrypanosomal agents[J]. Bioorg Med Chem, 2010, 18: 7826-7835.
[2] PARRILHA G L, SILVA J G D, GOUVEIA L F, et al. Pyridine-derived thiosemicarbazones and their tin(IV) complexes with antifungal activity against Candida spp[J]. Eur J Med Chem, 2011, 46: 1473-1482.
[3] PAVAN F R, MAIA P I S, LEITE S R A, et al. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones:Anti-mycobacterium tuberculosis activity and cytotoxicity [J]. Eur J Med Chem, 2010, 45: 1898-1905.
[4] KANG I J, WANG L W, HSU T A, et al. Isatin-β-thiosemicarbazones as potent herpes simplex virus inhibitors [J]. Bioorg Med Chem Lett, 2011, 21: 1948-1952.
[5] KHAN S A, KUMAR P, JOSHI R, et al. Synthesis and in vitro antibacterial activity of new steroidal thiosemicarbazone derivatives [J]. Eur J Med Chem, 2008, 43: 2029-2034.
[6] ZHU Y J, SONG K K, LI Z C, et al. Antityrosinase and antimicrobial activities of trans-cinnamaldehyde thiosemicarbazone [J]. J Agric Food Chem, 2009, 57: 5518-5523.
[7] KHANDANI M, SEDAGHAT T, ERFANI N, et al. Synthesis, spectroscopic characterization, structural studies and antibacterial and antitumor activities of diorganotin complexes with 3-methoxysalicylaldehyde thiosemicarbazone [J]. J Mol Struct, 2013, 1037: 136-143.
[8] OPLETALOVA V, KALINOWSKI D S, VEJSOVA M, et al. Identification and characterization of thiosemicarbazones with antifungal and antitumor effects: cellulariron chelation mediating cytotoxic activity [J]. Chem Res Toxicol, 2008, 21: 1878-1889.
[9] ALOMAR K, LANDREAU A, ALLAIN M, et al. Synthesis, structure and antifungal activity of thiophene-2,3-dicarboxaldehyde bis(thiosemicarbazone) and nickel(II), copper(II) and cadmium(II) complexes: Unsymmetrical coordination mode of nickel complex [J]. J Inorg Biochem, 2013, 126: 76-83.
[10] JANSSON P J, SHARPE P C, BERNHARDT P V, et al. Novel thiosemicarbazones of the ApT and DpT Series and their copper complexes: identification of pronounced redox activity and characterization of their antitumor activity [J]. J Med Chem, 2010, 53: 5759-5769.
[11] ALI A A, NIMIR H, AKTAS C, et al. Organoplatinum(II) complexes with 2-acetylthiophene thiosemicarbazone: synthesis, characterization, crystal structures, and in vitro antitumor activity [J]. Organometallics, 2012, 31: 2256-2262.
[12] CHELLAN P, LAND K M, SHOKAR A, et al. Exploring the versatility of cycloplatinated thiosemicarbazones as antitumor andantiparasitic agents [J]. Organometallics, 2012, 31: 5791-5799.
[13] CHELLAN P, NASSER S, VIVAS L, et al. Cyclopalladated complexes containing tridentate thiosemicarbazone ligands of biological significance: Synthesis, structure and antimalarial activity [J]. J Organomet Chem, 2010, 695: 2225-2232.
[14] KHANYE S D, WAN B, FRANZBLAU S G, et al. Synthesis and in vitro antimalarial and antitubercular activity of gold(III) complexes containing thiosemicarbazone ligands [J]. J Organomet Chem, 2011, 696: 3392-3396.
[15] KESEL A J, Broad-spectrum antiviral activity including human immunodeficiency and hepatitis C viruses mediated by a novel retinoid thiosemicarbazone derivative [J]. Eur J Med Chem, 2011, 46: 1656-1664.
[16] BAL T R, ANAND B, YOGEESWARI P, et al. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives [J]. Bioorg Med Chem Lett, 2005, 15: 4451-4455.
[17] ALTUN A, KUMRU M, DIMOGLO A. Study of electronic and structural features of thiosemicarbazone and thiosemicarbazide derivatives demonstrating anti-HSV-1 activity[J]. J Mole Struct (Theochem), 2001, 535: 235-246.
[18] CHHIKARA B S, CHANDRA R, TANDON V. IBX in an ionic liquid: eco-friendly oxidation of 17α-methylandrostan-3β,17β-diol, an intermediate in the synthesis of anabolic oxandrolone [J]. Tetrahedron Lett, 2004, 45: 7585-7588.
[19] DUTTA S, KAR T, MANDAL D, et al. Structure and Properties of Cholesterol-based Hydrogelators with Varying Hydrophilic Terminals: Biocompatibility and Development of Antibacterial Soft Nanocomposites[J]. Langmuir, 2013, 29: 316-327.
[20] ZHAO Z G, LIU X L, LIU L L, et al. Microwave-assisted synthesis of new steroidal thiosemicarbazones derived from methyl 3-oxocholanate under solvent-free conditions [J]. J Chem Res, 2010, 34: 455-458.
[21] 崔建国, 黄立粱, 黄燕敏, 等. 一种具有抗肿瘤活性胆酸衍生物的合成新方法[J]. 有机化学, 2009, 29: 971-974.
Synthesis and evaluation of antimicrobial activities of novel cholic acid thiosemicarbazone derivatives
ZHANG Li-na, JIANG Yu-jia, CHEN Bai-ling, ZHAO Zhi-gang
( College of Chemistry and Environmental Protection Engineering, Southwest University for Nationalities, Chengdu 610041, P. R. C. )
A series of novel cholic acid thiosemicarbazone derivatives via the condensation of steroidal ketones and substituted thiosemicarbazides were synthesized. Their structures were elucidated by1H NMR, IR, ESI-MS spectra and elemental analyses. Moreover, all target compounds were tested for antibacterial activity againstStaphylococcus aureus, Bacillus subtilis, Pseudomanas aeroginosa and Escherichia coli.Compounds 4b, 4g and 4h possess good inhibitory effects againstP. aeruginosaandE. Coli.
cholic acid; thiosemicarbazone; antimicrobial activity
O621.3,R914.5
A
1003-4271(2014)02-0219-05
10.3969/j.issn.1003-4271.2014.02.09
2014-01-17
赵志刚(1963-), 男, 教授, 博士, 硕士生导师, 研究方向: 生物有机化学, 超分子化学与分子自组装, 药物合成, 微波化学等方面的研究. E-mail: zzg63129@163.com
四川省应用基础研究项目(No.2011JY0035); 四川省科技支撑计划项目(No. 2012SZ0160); 西南民族大学2014年研究生创新型科研项目(CX2014SZ43)

