Effects of exogenous ABA application on post-anthesis dry matter redistribution and grain starch accumulation of winter wheat with different staygreen characteristics
Dongqing Yng,Yongli Luo,Yingli Ni,Ynping Yin,Weibing Yng,Dinling Peng,Zhengyong Cui,Zhenlin Wng,*
aState Key Laboratory of Crop Biology,Agronomy College of Shandong Agricultural University,Tai'an,Shandong 271018,China
bAgriculture Bureau of Rencheng District of Shandong Province,Jining,Shandong 272000,China
1.Introduction
In winter wheat (Triticum aestivum L.),starch is an important part of the endosperm.Generally,starch contributes 65%–80%of the final dry weight and is considered a key component of grain weight[1].The supply of assimilates to kernels originates from current assimilation transferred directly to kernels and from the remobilization of assimilates stored temporarily in vegetative plant parts [2].It is reasonable to hypothesize that increasing starch accumulation and promoting dry matter remobilization will increase grain yield.
Plant hormones play important roles in plant growth and yield formation[3].ABA,one of the phytohormones,is gaining increased attention from researchers on crop growth.ABA is suggested to be involved in plant responses to stresses such as water stress[4,5]and heavy-metal stress[6].A higher ABA level in growing kernels reduced the expression of genes responsible for metabolism of sucrose to ADP-glucose [7].ABA regulates activities of key enzymes in starch synthesis and accumulation in kernels,including SS and SPS [8].Staygreen genotypes are crop mutants that retain high photosynthetic activity and maintain assimilate drain from vegetative organs to grain during filling stage[9–11].In wheat,staygreen varieties exhibit higher yield potential than non-staygreen varieties [12].At present,our understanding of differences between staygreen and non-staygreen varieties in yield-forming mechanisms,including for example differences in starch content and in redistribution of dry matter in different organs,is very weak.
The effect of ABA on plant growth and development has been confirmed in many crops.Exogenous ABA may regulate starch accumulation and dry matter redistribution,but whether it regulates high yield in staygreen wheat is unknown.In the present study,we conducted a two-year experiment with a staygreen and a non-staygreen wheat variety sprayed with exogenous ABA.We attempted to(i)identify differences between the two genotypes in starch content,grain yield,and dry matter remobilization; (ii) elucidate the effect of exogenous ABA on starch accumulation and grain filling in staygreen wheat; and(iii) cast light on the regulating mechanism of exogenous ABA during yield formation in staygreen winter wheat.
2.Materials and methods
2.1.Plant materials and growth condition
Experiments were conducted in two growing seasons from October 2010 to June 2011 and from October 2011 to June 2012 at Shandong Agricultural University Farm,Tai'an,Shandong Province,China (36°09′ N,117°09′ E,and 128 m of elevation).Two wheat cultivars(T.aestivum L.),staygreen variety Wennong 6 and control variety Jimai 20,were grown in experimental plots.Plot size was 9 m2(3 m × 3 m) with 10 rows (0.25 m between rows).The soil contained 12.3 g kg-1organic matter,0.91 g kg-1total N,87.2 mg kg-1available N,8.6 mg kg-1Olsen-P,57.5 mg kg-1Olsen-K.Initially 108 g N,90 g P2O5,and 90 g K2O per plot were incorporated into the soil and another 108 g N per plot was applied at the jointing stage.Seeds were sown on October 10,2010 and October 10,2011 at a density of 225 plants m-2.Pests,diseases,and weeds were controlled by appropriate chemical applications during the growing period.Other cultural practices followed the precision high-yielding cultivation system of Yu[13].
2.2.Treatments and experimental design
The experiment consisted of sprays with water (control) or a 10 mg L-1solution of ABA(Sigma).Exogenous ABA was sprayed at anthesis,stage 60 of the scale of Zadoks[14]on 10 May 2011 and 7 May 2012.Starting 1 DAA,ABA was sprayed at the rate of 100 mL m-2on the whole plants for 3 days at 5:00 p.m.(concentration and volume were determined according to Yang et al.[15]and a preliminary experiment).All the solutions contained Tween-20 at final concentrations of 0.5% (v/v),respectively.Each treatment was an area of 9 m2with three replications.Treatments and cultivars were arranged as a randomized block design.
2.3.Sampling
Thirty plants from each treatment were sampled weekly after anthesis and divided into two parts,one stored at-40 °C for endogenous hormone measurement and the other dried for 48 h at 70 °C for starch-content measurement.The first and second kernels of each spikelet were designated as superior and the third and fourth as inferior kernels.Thirty panicles were sampled at 3-or 6-d intervals according to the experiment,dried at 70 °C to constant weight,dehulled,and weighed.These data were used to simulate the grain-filling process.At maturity,the plants in an area of 1 m2were harvested to determine yield,number of kernels per spike,and 1000-grain weight,and each measurement was performed on plants from three different pots.
The grain filling process was fitted by the Richards growth equation as described by Zhu et al.[16]:

where W is grain weight(g),A is final grain weight(g),t is time after anthesis(d),and B,k,and N are coefficients determined by regression.The active grain-filling period(D)was defined as the period during which W constituted from 5%(t1)to 95%(t2)of A.Grain filling rate (G) was calculated as the derivative of Eq.(1):
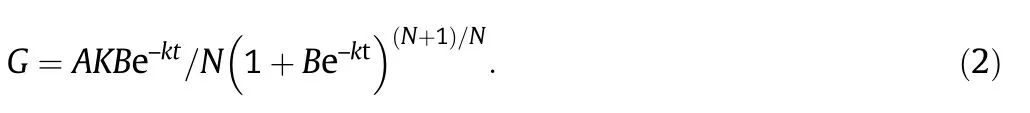
Integration of Eq.(2) gives the mean grain-filling rate:Gmean= Ak/(2N + 4),and the maximum grain-filling rate:Gmax= Ak (1 + N)-(N+1)/N.The actual filling terminus (T3) was calculated by T3=-ln {[(100/99)N-1]/B}/k.
2.4.Assay of grain starch
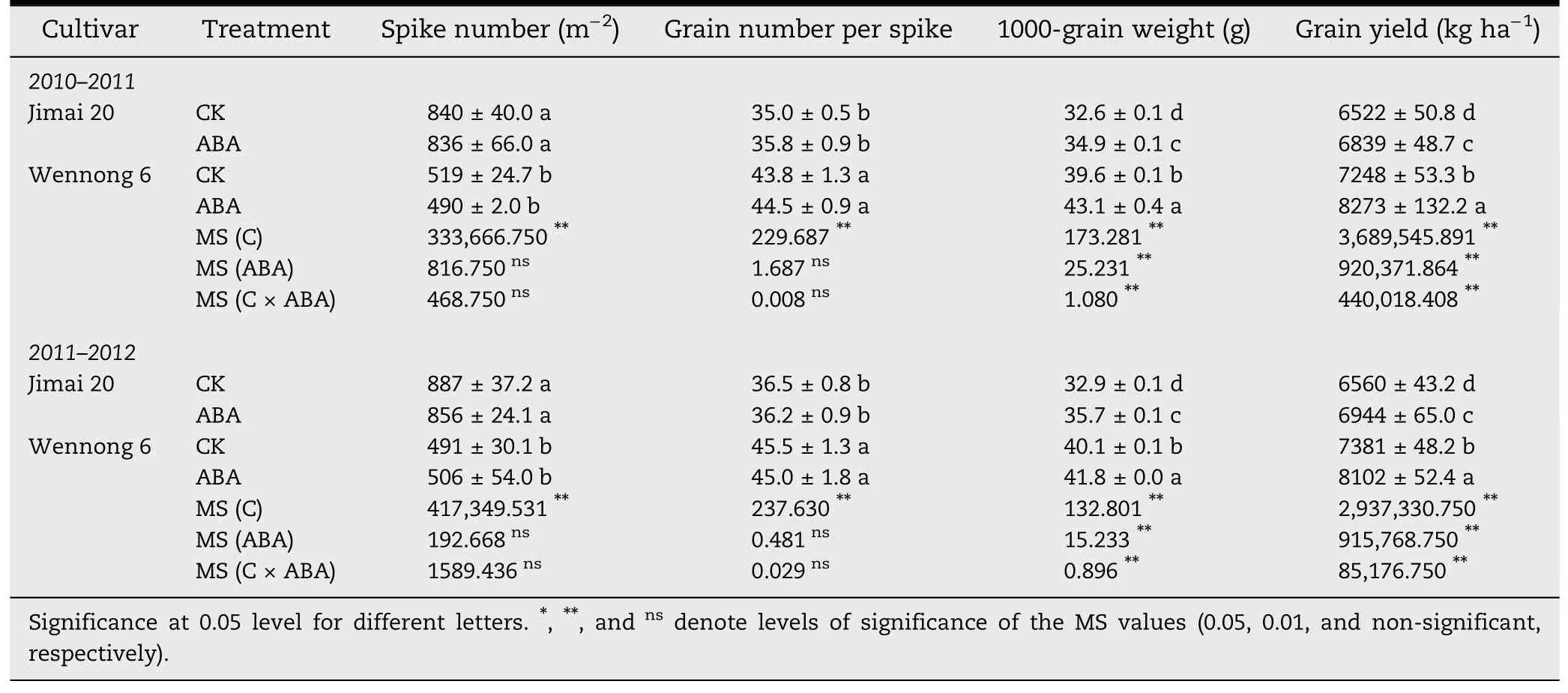
Table 1-Effect of exogenous ABA on factors of wheat yield.Mean squares (MS) for the effects of hormone(ABA),cultivars(C),and their interactions(ABA × C)are also shown.
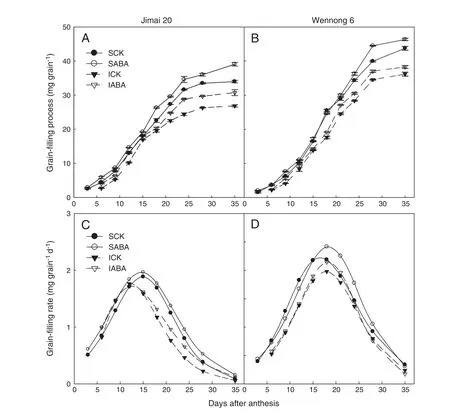
Fig.1-Grain weights(A and B)and grain-filling rates(C and D)of superior and inferior kernels of wheat.SCK and SABA represent superior kernels sprayed with water (containing Tween-20)and ABA(10 mg L-1),respectively.ICK and IABA represent inferior kernels sprayed with water (containing Tween-20)and ABA(10 mg L-1),respectively.
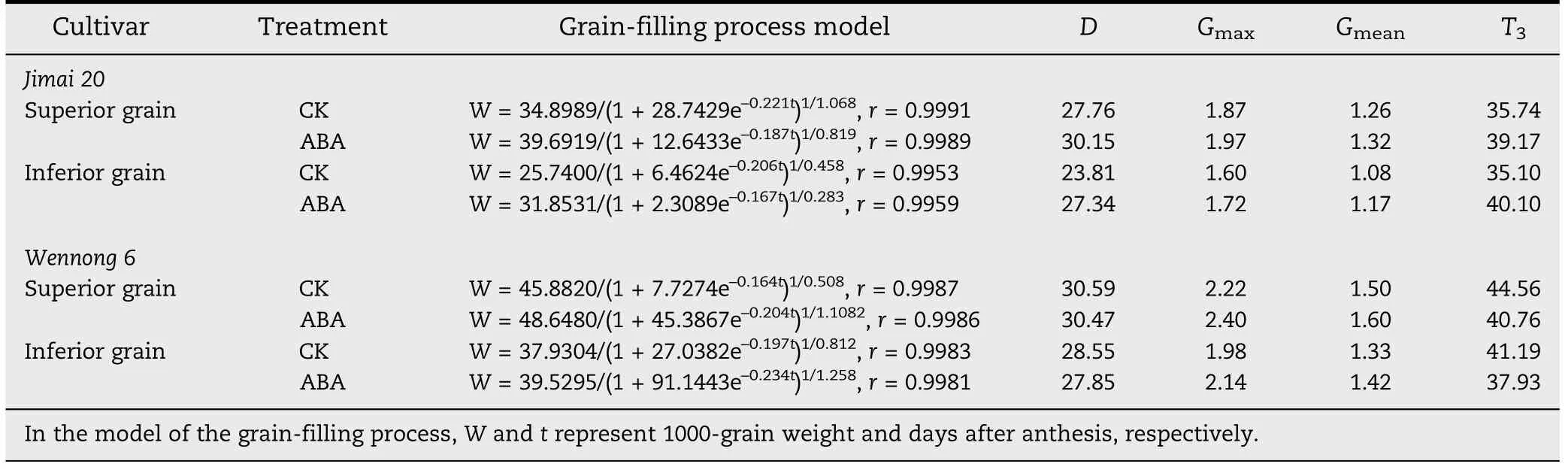
Table 2-Grain-filling parameters and grain-filling process model.
The anthrone colorimetric method[17,18]was used to measure the starch content in kernels.A dried grain sample of 0.1 g was weighed in a 10 mL centrifuge tube and 5 mL water was added.The sample was heated in a 100 °C water bath for 30 min,cooled,and centrifuged at 4000 ×g for 5 min.The supernatant was collected,and the extraction was repeated twice.The residue was used for starch content measurement and transferred to a 50 mL volumetric flask with 20 mL distilled water.The solution was heated in boiling water for 15 min,2 mL of cold 9.2 mol L-1perchloric acid was added,and the mixture was gelatinized in boiling water for 15 min,cooled,and centrifuged at 2500 ×g for 10 min.The supernatant was collected and the extraction was repeated twice.Distilled water was added to a final volume of 50 mL.Anthrone reagent (6 mL) was added to 2 mL of extract and the mixture was boiled for 5 min.After cooling,the absorption of the solution was recorded at 620 nm with a spectrophotometer.Starch content(%)was calculated as 100 × (0.9 × C × V/a) / (W × 106),where 0.9 represents the starch coefficient from glucose conversion,C the glucose value (μg)obtained from the standard curve,V the total volume of the extracted solution (mL),a the volume of sample solution for color development(mL),and W the sample weight(g).
Starch accumulation was calculated as the product of starch content and grain weight.The starch accumulation rate was calculated as (Cn-Cn-7) / 7,where Cnrepresents starch content at n DAA.
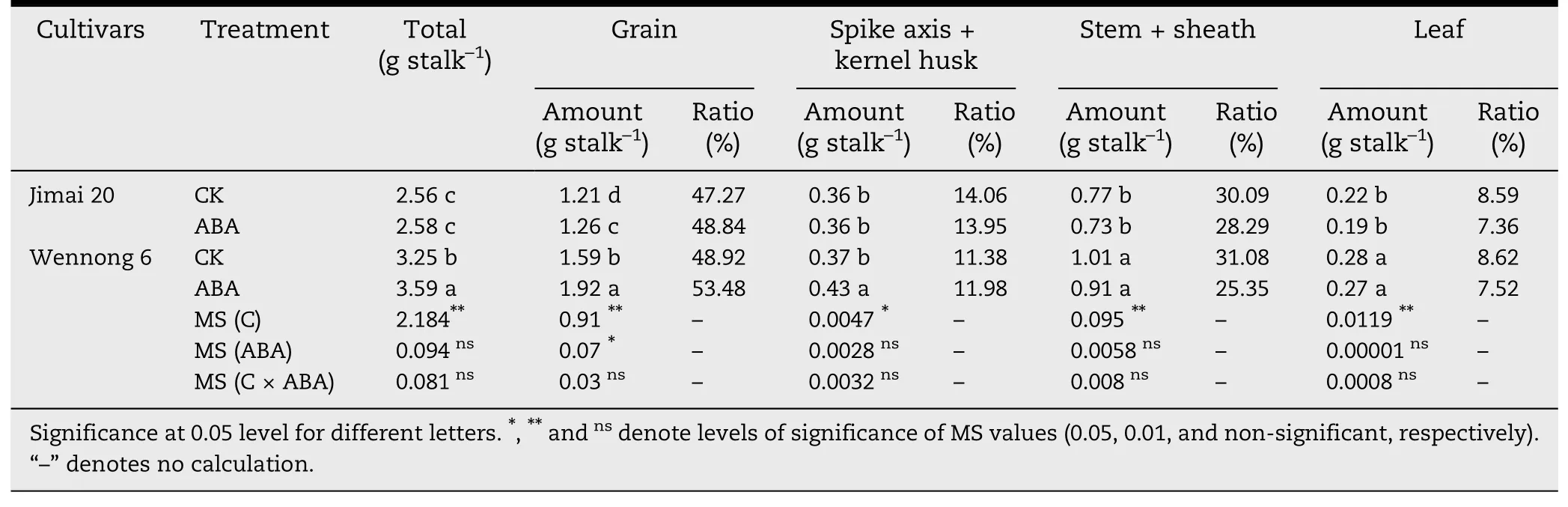
Table 3-Effects of exogenous ABA on dry matter distribution in different organs at maturity in wheat.
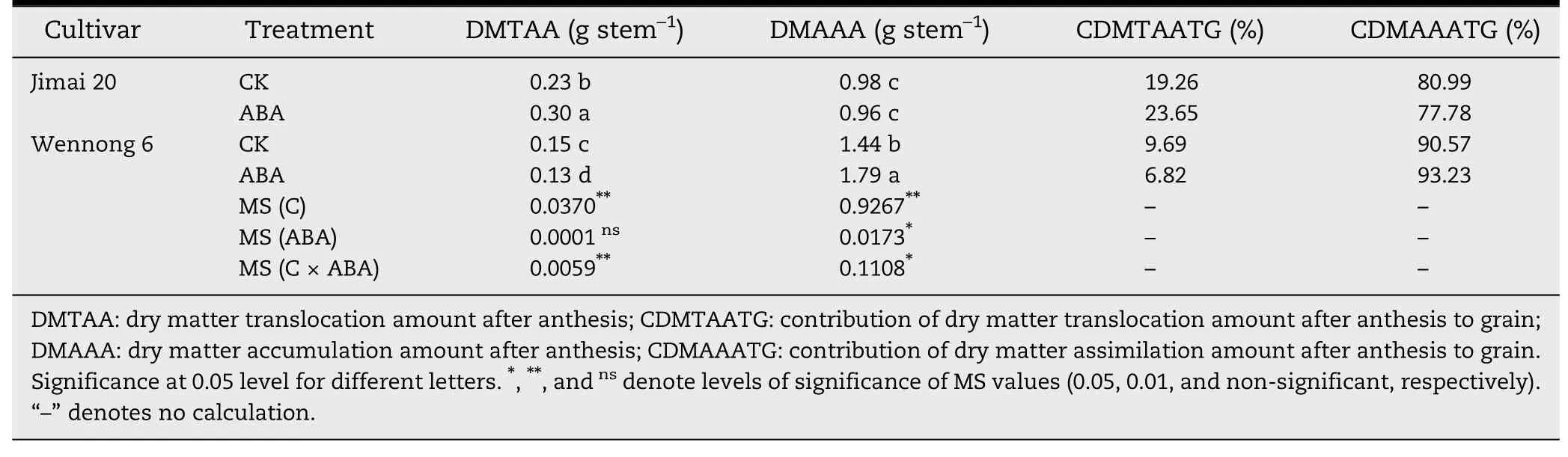
Table 4-Effects of exogenous ABA on dry matter translocation amount from vegetative organ to kernels after anthesis.
2.5.Dry matter translocation and remobilization
At anthesis and maturity,20 wheat plants were harvested and the samples were separated into leaves,stems and sheath,spike axis and kernel husk,and kernels.Samples were dried at 70 °C to constant weight for dry matter determination.The following parameters were calculated according to Despo and Gagianas[19]:
Dry matter translocation amount after anthesis(DMTAA) =dry matter of vegetative organs at anthesis-dry matter of vegetative organs at maturity.
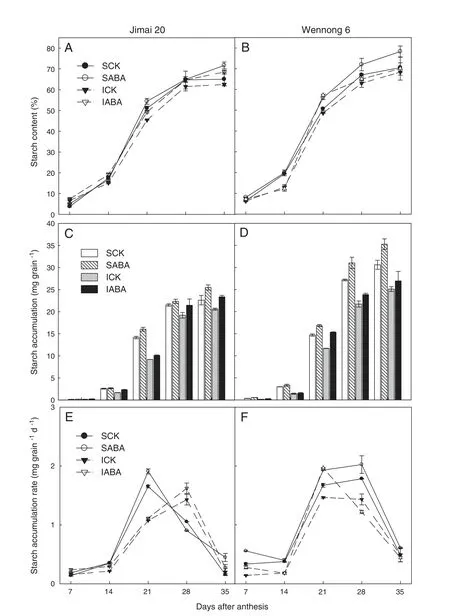
Fig.2-Effects of exogenous ABA on starch content(A and B),accumulation(C and D),and accumulation rate(E and F).SCK and SABA represent spraying of superior kernels with water(containing Tween-20)or ABA(10 mg L-1),respectively.ICK and IABA represent spraying of inferior kernels with water(containing Tween-20) or ABA(10 mg L-1),respectively.
Dry matter accumulation amount after anthesis(DMAAA) =DM of kernels at maturity-DMTAA.
Contribution of dry matter translocation amount after anthesis to grain(CDMTAATG) = (DMTAA / dry matter of vegetative organs at anthesis) × 100.
Contribution of dry matter assimilation amount after anthesis to grain (CDMAAATG) = (DMTAA / grain yield at maturity) × 100.
In these calculations,losses of dry matter due to plant respiration,pest feeding,microbial decomposition of dead issues,etc.,were not considered.It was assumed that all dry matter lost from vegetative plant parts was remobilized to developing grain sinks.For this reason,the calculated values represent apparent and not actual translocation amounts and proportions.
2.6.Determination of endogenous hormones
The method for extraction and purification of zeatin riboside(ZR),gibberellin (GA3),auxin (IAA),and abscisic acid (ABA)were modified from those described by Yang [15].1 g of kernels was ground into powder in liquid nitrogen and 4 mL acetonitrile extraction medium containing 30 mg sodium diethyldithiocarbamatre as an antioxidant was added.The extract was incubated at 4 °C for 12 h and centrifuged at 5000 ×g for 15 min.The residue was further extracted twice with the same solvent.The supernatant was concentrated to residue under low pressure at 37 °C by rotary evaporation and redissolved in 8 mL 0.4 mol L-1Na-phosphate buffer (pH 8.0),followed by addition of 6 mL chloroform and oscillation to remove pigment.To the aqueous phase was added 0.15 g insoluble polyvinylpyrrolidone and the mixture was centrifuged at 10,000 ×g for 10 min,followed by removal of 5 mL supernatant,which was adjusted to pH 3.0 with pure formic acid.The aqueous phase was extracted twice with 3 mL ethyl acetate.The ethyl acetate phase was concentrated by rotary evaporation under low pressure and redissolved in 1 mL mobile phase(acetonitrile:methanol:0.6%acetic acid = 5:50:45,v:v:v).Finally,the hormone extract was filtered through 0.2 μm hydrophobic membranes,and 10 μL samples were injected into a Waters Symmetry C18 column (4.6 mm × 150.0 mm,5 μm) using mobile phase.The flow rate was held at 0.6 mL min-1and peaks were detected with a photodiode array detector (Waters 2998 Separation Module,USA)absorbance at 254 nm.
2.7.Statistical analysis and processing
Statistical analysis was carried out using the Data Processing System software 7.05.Means were compared by Duncan's test and differences were considered significant at P <0.05.
3.Results
3.1.Yield,yield components,and grain-filling process
Compared with the control treatment,both 1000-grain weight and yield in the two cultivars were significantly (P <0.05)increased by exogenous ABA application.In the first growing season (2010–2011),1000-grain weight and grain yield in Wennong 6 were significantly(P <0.05)increased by 8.84%and 14.14%,respectively,compared with the controls.In contrast,these parameter values in Jimai 20 were increased by 7.06%and 4.86%.However,application of ABA at the full-bloom stage had no significant influence on the spike number and grain number per spike.Although the spike number of Jimai 20 was significantly higher than that of Wennong 6,1000-grain weight and grain yield of staygreen wheat Wennong 6 were greater than those of Jimai 20(Table 1).
Application of ABA increased grain weight at all grain filling stages (Fig.1).The final weight of superior kernels was markedly(P <0.05) greater than that of inferior kernels in two cultivars.Meanwhile,the final weight of superior and inferior kernels in staygreen wheat Wennong 6 was significantly(P <0.05) higher than those in Jimai 20,respectively (Fig.1-A and B).Grain-filling rate of all treatments first increased and then decreased,showed a parabolic change.The peak values in grain-filling rate occurred at 15 and 12 DAA for superior and inferior kernels in Jimai 20 and at 18 DAA for superior and inferior kernels in Wennong 6(Fig.1-C and D).
The maximum rate and mean grain-filling rate and duration of ABA-treated Jimai 20 were significantly (P <0.05) increased.However,the maximum rate and mean grain-filling rate for Wennong 6 were increased and the grain filling duration was reduced(Table 2).Grain-filling duration of ABA-treated superior and inferior kernels in Wennong 6 was reduced from 44.56 and 41.19 to 40.76 and 37.93 days,respectively.These results indicate that the improved grain weight of ABA-treated staygreen wheat was due mainly to the positive action of increased grain-filling rate,which compensated for the negative effect of reduced grain-filling duration.ABA application markedly extended the active grain-filling period by 2.39 and 3.53 days for superior and inferior kernels of Jimai 20,respectively.Under ABA treatment,the active grain filling period of Wennong 6 was reduced,but the differences were small(-0.12 d for superior kernels and-0.70 d for inferior kernels).These observations indicated that the effect of exogenous ABA on the active grain filling period was determined by grain position within a panicle and by genotypic differences.
3.2.Distribution and remobilization of dry matter
The dry matter distribution in different organs at maturity is presented in Table 3.Application of exogenous ABA decreased carbohydrate amount and ratio in photosynthetic tissue and stem sheath but increased dry matter assimilation of kernels in both Jimai 20 and Wennong 6.Grain amount of Wennong 6 increased by 0.33 g stalk-1at harvest maturity under exogenous ABA treatment,in contrast to a 13.64% reduction in the amount of leaf dry weight for Jimai 20.No difference was found in total carbohydrate amount of ABA-treated Jimai 20.ABA-treated plants of Wennong 6 showed markedly(P <0.05)enhanced total carbohydrates compared with the control.The total dry matter amount of Wennong 6 was significantly larger than that of Jimai 20.
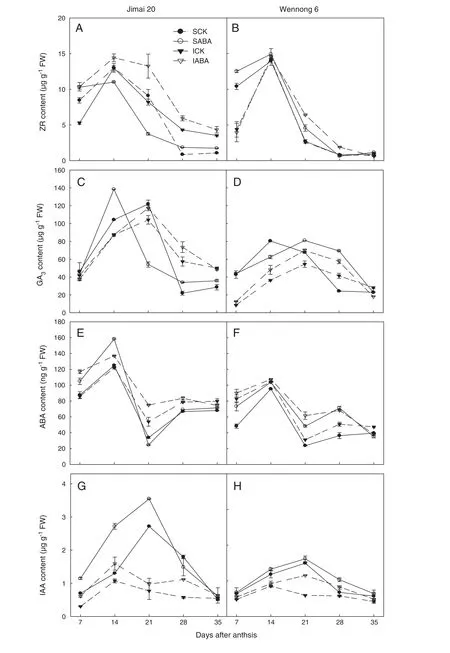
Fig.3-Effects of exogenous ABA on endogenous hormones(ZR:A,B;GA3:C,D;ABA:E,F;and IAA:G,H)contents of two types of kernels(superior kernels and inferior kernels)of the two different types of staygreen wheat.SCK and SABA represent superior kernels spraying water(including Tween-20),ABA(10 mg L-1),respectively.ICK and IABA represent inferior kernels spraying water(including Tween-20),ABA(10 mg L-1),respectively.
Table 4 shows that there was no significant difference in dry matter accumulation amount after anthesis (DMAAA) of ABA-treated Jimai 20,but that that of Wennong 6 was markedly(P <0.05) increased from 1.44 to 1.79 g stem-1by application of ABA.ABA improved dry matter translocation amount(DMTAA)and raised contribution of dry matter translocation amount after anthesis to kernels (CDMTAATG) for Jimai 20 (0.07 g stem-1,4.39%,respectively).The contribution of dry matter assimilation amount after anthesis (CDMAAATG) in Jimai 20 and Wennong 6 was 80.99% and 90.57%,implying that the grain weight gain of Jimai 20 was due to both dry matter translocation and dry matter accumulation after anthesis,whereas that of Wennong 6 was due mainly to dry matter accumulation after anthesis.
3.3.Effects of exogenous ABA on starch content,accumulation,and accumulation rate
Fig.2 displays starch content,starch accumulation,and starch accumulation rate of two types of kernels(superior and inferior).Starch content in both cultivars (Fig.2-A and B) followed a sigmoid curve and increased very slowly at the earlier stage of anthesis(7–14 DAA),but increased rapidly beginning at 14 DAA,reaching its maximum at 35 DAA.At GS60,we applied exogenous ABA in order to evaluate differences in starch content between different kernel positions and genotypes.The final starch contents in both superior and inferior kernels of the two wheat cultivars were significantly (P <0.05) increased,with values of Jimai 20 increasing by 10.2%and 9.6%and those of Wennong 6 by 10.9%and 2.6%respectively,relative to their respective controls.
Starch accumulation of Jimai 20 and Wennong 6 changed slightly at 7 DAA and increased rapidly from 7 DAA to 28 DAA.Starch accumulation rate showed a similar trend with starch accumulation (Fig.2-E and F).The starch accumulation rate of the two cultivars increased gradually,but decreased rapidly after reaching a maximum.The accumulation of total starch was higher in Wennong 6 than in Jimai 20 (Fig.2-C and D),suggesting that the higher starch accumulation in the staygreen wheat was due to higher starch accumulation rate during grain filling.Compared to the control treatment,ABA application increased the starch accumulation rate.This observation may explain the higher starch content of ABA-treated kernels.
3.4.Endogenous hormone levels in kernels
Fig.3(A and B) shows that zeatin riboside levels in superior and inferior kernels in both cultivars rapidly increased during 7 to 14 DAA,reached their highest level at 14 DAA,and then decreased sharply with grain filling.ABA application significantly increased ZR content in superior kernels of Jimai 20 at 7 DAA,but ZR content decreased from 14 to 21 DAA and then increased again from 28 to 35 DAA.Spraying ABA markedly increased the ZR content of inferior kernels of Jimai 20 from 7 to 35 DAA,as well as markedly increasing ZR from 7 to 21 DAA in superior kernels of Wennong 6 and from 14 to 28 DAA for inferior kernels.
GA3contents in kernels of the two cultivars showed a similar trend.GA3content increased rapidly in the early grain filling period,reached its highest value at 14 or 21 DAA,and then declined quickly(Fig.3-C and D).The peak of GA3concentration of ABA-treated superior kernels in Jimai 20 occurred earlier.ABA application increased GA3content from 21 to 28 DAA in superior and 7 to 28 DAA in inferior kernels of Wennong 6.
During the grain filling stage,grain ABA content showed a wavelike up–down–up–down curve,reaching a maximum at 14 DAA (Fig.3-E and F).ABA contents in superior kernels of Wennong 6 and Jimai 20 were higher than those in inferior kernels at 7–14 DAA,but lower than in superior kernels at 21–35 DAA.Endogenous ABA contents were notably increased at 7–14 DAA for superior kernels of Jimai 20 and 7–28 DAA for both superior and inferior kernels of Wennong 6 following exogenous ABA spraying.
Fig.3(G and H) shows that IAA contents in superior and inferior kernels showed a similar trend.IAA content first increased and then decreased,reaching maximum values at 21 DAA for superior kernels and 14 DAA for inferior kernels.Under all the treatments,Jimai 20 showed higher IAA content than Wennong 6.Application of ABA markedly increased IAA content from 7 to 21 DAA for superior kernels and 7 to 35 DAA for inferior kernels of Jimai 20,but significantly increased IAA content from 7 to 35 DAA in both types of kernels of Wennong 6.
4.Discussion
Staygreen wheat exhibits delayed leaf senescence and enhanced photosynthetic competence[20].In general,staygreen mutants show increased grain weight and improved yield associated with extended duration of photosynthesis,which results in increased translocation of photoassimilate to the grain [3].Wennong 6,a staygreen wheat cultivar,exhibited a higher grain filling rate and longer grain filling duration than did Jimai 20.Consequently,Wennong 6 accumulated more assimilates,represented by starch,during the filling stage.Grain filling process and grain weight are determined by grain filling rate and filling duration [21].Both 1000-grain weight and yield were higher for Wennong 6 than for Jimai 20,owing presumably to the longer active grain filling period and higher grain filling rate resulting in improved accumulation of starch.
Plant endogenous hormones play important roles in regulating grain filling and are involved in determining sink strength and seed weight during development of the caryopsis[22].Grain development and assimilate accumulation may be regulated by endogenous hormone levels and equilibria that may be influenced by exogenous hormones or plant growth regulators[15,23–25].In this study,the external application of 10 mg L-1ABA changed endogenous hormone contents.Exogenous ABA increased endogenous zeatin content from 7 DAA.In both cultivars,application of ABA resulted in significant increases of endogenous IAA and ABA contents from 7 to 21 DAA.Zeatin promotes endosperm cell division and increases sink capacity,resulting in more assimilates accumulation [24].IAA has been reported to mediate the ATPase activity inducing photosynthate transportation and distribution,thereby improving grain filling [26].IAA is also associated with the regulation of starch synthase activity and involved in promoting starch synthesis [27].Previous studies have indicated that endogenous ABA increased starch content by regulating the activity of starch synthase and sucrose synthase.ABA promoted the accumulation of storage materials such as starch[27,28]and induced stress-related material production [29],via inducing gene expression [30].More recently,Cui et al.[31] found that exogenous ABA enhanced xylem sap at the neck–panicle node,increasing the transport of photosynthetic products from leaves to growing kernels.ABA-treated plants showed increased numbers of vascular bundles and more phloem area in vascular bundles,suggesting that they had greater structural capacity for the conduction of assimilates to kernels [32].In the present study,ABA application markedly increased the grain filling rate of two types of cultivars,extended the active grain filling period and grain filling duration of Jimai 20,but did not significantly affect the active grain filling period of Wennong 6.The two varieties showed similar behavior,with starch content and accumulation both increased by exogenous ABA.Application of ABA strongly affected dry matter accumulation and remobilization.Exogenous ABA decreased carbohydrate amounts in the photosynthetic tissue and stem sheath and increased dry matter assimilation of kernels.Consequently,the dry matter distribution and remobilization ratios of different organs were changed.We referred to a previously described method to calculate dry matter translocation amounts and ratios,so that the resulting numbers represent apparent and not actual translocation amounts and ratios.Further research on exogenous ABA regulation of dry matter translocation is desirable.Based on our results and previous studies,we may summarize the relationship between ABA treatment and grain yield as follows: exogenous ABA (i) accelerated grain carbohydrate accumulation by enhancing starch accumulation and accelerating grain filling and (ii) affected the dry matter distribution and remobilization of different organs,accelerating the transportation and partition of photo assimilates from stem and sheath into the grain sink.
5.Conclusions
Grain filling duration,active grain filling period,and mean and maximum grain filling rate in kernels of Wennong 6 were higher than in those of Jimai 20.Final grain weight differed significantly between Wennong 6 and Jimai 20.ABA increased the grain filling rate and shortened the grain filling period of Wennong 6 but prolonged that of Jimai 20.Starch content and starch accumulation were increased in both cultivars by ABA treatment.Treatment with ABA strongly affected grain yield,dry matter accumulation,and remobilization,resulting from changing the endogenous hormone contents of kernels.
This study was supported by the National Natural Science Foundation of China (31271661),the National Basic Research Program of China (2009CB118602),and the Public Service Sector(Agriculture) Research Program of China(201203100).
[1] X.B.Liu,W.X.Li,Preliminary studies on the accumulation of grain starch and protein during grain filling in wheat,Acta Agron.Sin.22(1996)736–740(in Chinese with English abstract).
[2] A.Masoni,L.Ercoli,M.Mariotti,I.Arduini,Post-anthesis accumulation and remobilization of dry matter,nitrogen and phosphorus in durum wheat as affected by soil type,Eur.J.Agron.26(2007) 179–186.
[3] Z.H.Xu,J.Y.Li,Plant hormones research in China: past,present and future,Chin.Bull.Bot.23 (2006) 433–442(in Chinese with English abstract).
[4] J.C.Yang,J.H.Zhang,Y.X.Ye,Z.Q.Wang,Q.S.Zhu,L.J.Liu,Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling,Plant Cell Environ.27(2004) 1055–1064.
[5] J.H.Zhang,W.S.Jia,J.C.Yang,Abdelbagi M.Ismail,Role of ABA in integrating plant responses to drought and salt stresses,Field Crops Res.97(2006) 111–119.
[6] H.Y.He,L.F.He,M.H.Gu,X.F.Li,Nitric oxide improves aluminum tolerance by regulating hormonal equilibrium in the root apices of rye and wheat,Plant Sci.183(2012)123–130.
[7] G.H.Zhu,N.H.Ye,J.C.Yang,X.X.Peng,J.H.Zhang,Regulation of expression of statch synthesis genes by ethylene and ABA in relation to the development of rice inferior and superior spikelets,J.Exp.Bot.62 (2011) 3907–3916.
[8] B.H.Zhao,H.X.Zhang,Q.S.Zhu,J.C.Yang,Causes of poor grain plumpness of two-line hybrids and their relationships to contents of hormones in the rice grain,Sci.Agric.Sin.39(2006) 477–486 (in Chinese with English abstract).
[9] G.Spano,N.D.Fonzo,C.Perrotta,C.Platani,G.Ronga,D.W.Lawlor,J.A.Napier,P.R.Shewry,Physiological characterization of ‘stay green'mutants in durum wheat,J.Exp.Bot.54(2003) 1415–1420.
[10] D.R.Jordan,C.H.Hunt,A.W.Cruickshank,A.K.Borrell,R.G.Henzell,The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments,Crop Sci.52(2012) 1153–1161.
[11] P.G.Luo,K.J.Deng,X.Y.Yu,L.Q.Li,X.Li,J.B.Chen,H.Y.Zhang,Z.X.Tang,Y.Zhang,Q.X.Sun,F.Q.Tan,Z.L.Ren,Chloroplast ultrastructure regeneration with protection of photosystem II is responsible for the functional‘stay-green'trait in wheat,Plant Cell Environ.36(2013) 683–696.
[12] A.P.Derkx,S.Orford,S.Griffiths,M.J.Foulkes,M.J.Hawkesford,Identification of differentially senescing mutants of wheat and impacts on yield,biomass and nitrogen partitioning,J.Integr.Plant Biol.54(2012) 555–566.
[13] S.L.Yu,Wheat in Shandong Province,China,China Agriculture Press,Beijing,China,1990.(in Chinese).
[14] J.C.Zadoks,T.T.Chang,C.F.Konzak,A decimal code for the growth stages of cereals,Weed Res.14(1974) 415–421.
[15] W.B.Yang,Z.L.Wang,Y.P.Yin,W.Y.Li,Y.Li,X.G.Chen,P.Wang,E.Y.Chen,J.X.Guo,T.Cai,Y.L.Ni,Effects of spraying exogenous ABA or GA on the endogenous hormones concentration and filling of wheat grains,Sci.Agric.Sin.44(2011) 2673–2682 (in Chinese with English abstract).
[16] Q.S.Zhu,X.Z.Cao,Y.Q.Luo,Growth analysis on the process of grain filling in rice,Acta Agron.Sin.14(1988) 182–192(in Chinese with English abstract).
[17] X.L.Luo,Q.F.Huang,Relationships between leaf and stem soluble sugar content and tuberous root starch accumulation in cassava,J.Agric.Sci.3 (2011) 64–72.
[18] Q.Zou,Guidebook of Plant Physiology Experiments,China Agriculture Press,Beijing,2000.110–112 (in Chinese).
[19] K.P.Despo,A.A.Gagianas,Nitrogen and dry matter accumulation,remobilization,and losses for Mediterranean wheat during grain filling,Agron.J.83(1991) 864–870.
[20] P.G.Luo,Z.L.Ren,X.H.Wu,H.Y.Zhang,H.Q.Zhang,J.Feng,The structure and physiological and biochemical mechanism of delaying senescence of wheat,Chin.Sci.Bull.51 (2006)2154–2460 (in Chinese).
[21] G.H.Xie,J.C.Yang,Z.Q.Wang,Q.S.Zhu,Gain filling characteristics of rice and their relationships to physiological activities of grains,Acta Agron.Sin.27(2001) 557–565(in Chinese with English abstract).
[22] J.C.Yang,J.H.Zhang,Z.Q.Wang,Q.S.Zhu,Hormones in the grains in relation to sink strength and postanthesis development of spikelets in rice,Plant Growth Regul.41(2003)185–195.
[23] J.C.Yang,J.H.Zhang,Z.Q.Wang,Q.S.Zhu,W.Wang,Hormonal changes in the grains of rice subjected to water stress during grain filling,Plant Physiol.127 (2001) 315–323.
[24] J.C.Yang,J.H.Zhang,Z.L.Huang,Z.Q.Wang,Q.S.Zhu,L.J.Liu,Correlation of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice,Ann.Bot.90(2002) 369–377.
[25] S.S.Zheng,C.F.Sun,H.C.Sun,L.T.Liu,J.F.Zhao,C.D.Li,Effects of different exogenous hormones on physiological characteristics of main stem leaves at flower and boll stage in cotton,Sci.Agric.Sin.42(2009)4383–4389(in Chinese with English abstract).
[26] Z.W.Li,J.Xiong,Z.F.Li,X.H.Qi,H.F.Chen,C.H.Shao,J.Y.Wang,Y.Y.Liang,W.X.Lin,Analysis of differential expression of proteins in rice leaf sheath during grain filling,Acta Agron.Sin.34(2008)619–626(in Chinese with English abstract).
[27] S.Saeedipour,F.Moradi,Relationship of endogenous ABA and IAA to accumulation of grain protein and starch in two winter wheat cultivars under post-anthesis water deficit,J.Agric.Sci.4(2012) 147–156.
[28] R.R.Finkelstein,S.I.Gibson,ABA and sugar interactions regulating development:cross-talk or voices in a crowd,Curr.Opin.Plant Biol.5 (2002) 26–32.
[29] L.Yang,C.L.Yu,F.Shi,Y.Q.Wei,C.C.Wang,H.T.Hu,C.G.Cheng,Effects of abscisic acid on growth and dehydration tolerance of Cynanchum komarovii seedlings,Plant Growth Regul.51(2007) 177–184.
[30] Y.Y.Chao,T.S.Chou,C.H.Kao,Involvement of abscisic acid and hydrogen peroxide in regulating the activities of antioxidant enzymes in leaves of rice seedlings under magnesium deficiency,Plant Growth Regul.66(2012)1–8.
[31] Z.Q.Cui,Y.P.Yin,Q.Z.Tian,G.C.Wang,F.Y.Meng,P.Wang,Z.L.Wang,Effects of spraying ABA on bleeding intensity in neck-panicle node,spike traits and grain yields of two different panicle-type winter wheat,Acta Ecol.Sin.31(2011) 1085–1092 (in Chinese with English abstract).
[32] C.Travaglia,G.Balboa,G.Esposito,H.Reinoso,ABA action on the production and redistribution of field-grown maize carbohydrates in semiarid regions,Plant Growth Regul.67(2012) 27–34.
- The Crop Journal的其它文章
- Genome-wide analysis of the WRKY transcription factor gene family in Gossypium raimondii and the expression of orthologs in cultivated tetraploid cotton
- Development of highly glyphosate-tolerant tobacco by coexpression of glyphosate acetyltransferase gat and EPSPS G2-aroA genes
- SSR genetic linkage map construction of pea (Pisum sativum L.) based on Chinese native varieties
- Rank correlation among different statistical models in ranking of winter wheat genotypes,
- Genetic characterization and linkage disequilibrium mapping of resistance to gray leaf spot in maize(Zea mays L.)
- Overexpression of GmDREB1 improves salt tolerance in transgenic wheat and leaf protein response to high salinity

