Induced defense responses in rice plants against small brown planthopper infestation
Canxing Duan*,Jiaojiao Yu,Jianyu Bai,Zhendong Zhu,Xiaoming Wang
1.Introduction
The small brown planthopper(SBPH),Laodelphax striatellus Fallén,is a serious sap-sucking pest of rice(Oryza sativa L.)in China and other parts of East Asia extending to Indonesia,the Philippines and Vietnam.Leaves infested by SBPH turn yellow,become wilted,and even die,resulting in yield loss and quality reduction.Furthermore,the SBPH also transmits rice viral diseases such as Rice stripe virus (RSV) and Rice black-streaked dwarf virus (RBSDV),which often cause major additional yield losses apart from just the damage by the insect itself [1–3].Currently,pesticides are widely used to control the SBPH,but this leads to the death of natural enemies,environmental pollution,chemical resistance and resurgence [4].Therefore,host-plant resistance has been recognized as one of the most economic,effective and environmentally-friendly measures for controlling SBPH[5,6].
Plant responses to herbivores are regulated through a complex network of signaling pathways that involve three signaling molecules: salicylic acid (SA),jasmonic acid (JA) and ethylene(ET)[7,8].Generally,the JA pathway is considered to be required for defense against necrotrophic pathogens and chewing insects,while the SA pathway is involved in a wide range of plant defense responses [9–11].Herbivore feeding behaviors primarily involve chewing and sucking.The beet armyworm (Spodoptera exigua Hübner) is a typical chewing pest,whose herbivory can cause large scale leaf damage.Some elicitors such as volicitin from beet armyworm oral secretions can provoke defense reactions to wounding mediated by the JA signaling pathway [12,13].Sucking insects such as phloem-feeding whiteflies and aphids that cause little injury to plant foliage are perceived as pathogens and primarily activate SA-dependent and to a certain extent JA/ET-dependent signaling pathways[7,14,15].
Plant defense is usually induced when subjected to pathogens,insects or wounding.Induced resistance can be split broadly into systemic acquired resistance (SAR) and induced systemic resistance(ISR).SAR develops systemically in response to,for example,pathogen infection or treatment with certain chemicals (e.g.,2,6-dichloroisonicotinic acid).This acquired resistance is effective against a wide range of pathogens and is mediated by a SA-dependent process[16].For SAR,many plant enzymes are involved in defense reactions against biotic stresses.Phenylalanine ammonia-lyase(PAL)is the first enzyme of the phenylpropanoid pathway and is involved in the biosynthesis of phenolics,phytoalexins,and lignins,which increase plant resistance [17,18].Oxidative enzymes such as peroxidase(POD) and polyphenol oxidase (PPO) catalyze the formation of lignin and other oxidative phenols that contribute to the formation of defense barriers for reinforcing the cell structure[19].Therefore,defense enzymes such as PAL,PPO and POD are tightly correlated with resistance to pests[20].
Currently,information about rice defense response mechanisms to SBPH,a typical phloem sap-sucking pest,is very limited.Therefore,elucidating the interaction between rice and SBPH would be helpful to understand the molecular basis for plant resistance to sap-sucking insects.In this paper,real-time PCR was used to analyze differential expression of genes involved in the SA-and JA/ET-mediated defense pathways at different time points when resistant and susceptible rice plants were infested by SBPH.Defense enzyme activities were also assayed after SBPH feeding.
2.Materials and methods
2.1.Rice varieties and insect infestation
An indica rice variety,Kasalath,and a japonica cultivar,Wuyujing 3,were selected for their high resistance and susceptibility to SBPH with the resistance scales of 2.0 and 9.0,respectively[21].Seeds for these varieties were provided by the Institute of Crop Science at the Chinese Academy of Agricultural Sciences.
The SBPH population used for infestation was originally collected from a rice field in Nanjing,China,and had been maintained on barley in a greenhouse for four generations before being transferred to Wuyujing 3 rice in the greenhouse of the Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing,China.The SBPH population was confirmed to be non-viruliferous by dot-immunobinding assay and PCR detection[21].
Twenty-five germinated seeds were sown in a plastic pot of 10 cm-diameter and 9 cm-height with a hole in the base.A total of 24 pots were randomly placed in a 65 cm ×44 cm × 14 cm plastic seed-box.All seeds and seedlings for testing were incubated at 26 ± 1 °C with sunlight and natural ventilation.About 2-cm of water level was maintained in the seed-box.
At the 3-leaf stage,the seedlings were infested with second to third instar SBPH nymphs that were starved for 2 h prior to infestation.The rate of infestation was 20 insects per seedling.Rice leaves were collected for RNA extraction at 12,24,36,48 or 72 h post infestation(hpi).Leaves without SBPH infestation were used as a control.
2.2.Isolation of total RNA and first-strand cDNA synthesis
Total RNA was extracted with RNAprep Plant kits (Tiangen Corporation,China),and then treated with RQ1 RNase-Free DNase (Promega,USA) before reverse transcription (RT).First-strand cDNA was synthesized using M-MLV Reverse Transcriptase kits(Promega).
2.3.Real-time quantitative PCR
Real-time quantitative PCR was performed using an ABI PRISM 7300 cycler (Bio-Rad Corporation,USA) with a SYBR Premix(SYBR Green)PCR kit(Tiangen).The primer pairs listed in Table 1 were used to amplify the corresponding 11 genes of interest.Amplification reactions were carried out in a 20 μL volume mixture containing 10 μL of 2 × SuperReal Premix,0.2 μmol L-1of each primer,20 ng of DNA template,2 μL of 50 × ROX Reference Dye and 6.2 μL of RNase-Free ddH2O.Template denaturation was conducted for 15 min at 95 °C,followed by 40 cycles of denaturation at 95 °C for 10 s,annealing at 60 °C for 30 s and extension at 72 °C for 40 s.Each sample was repeated three times.Fluorescence signals were measured at each polymerization step.The relative expression of genes was calculated by the 2-ΔΔCTmethod[22]using the equation ΔΔCT= (CT,Target-CT,Actin)Timex-(CT,Target-CT,Actin)Time0,where x represents the time points of 12,24,36,48 and 72 h for SBPH infestation[23].
2.4.Defense enzyme activity assay
PAL activity assays were conducted according to the method of Qin and Tian[24].Three grams of rice leaf was homogenized with 30 mL of 50 mmol L-1sodium borate buffer (pH 8.8,containing 5 mmol L-1β-mercaptoethanol) and 0.5 g of polyvinyl pyrrolidone (PVP) and ground using a polytron tissue grinder at 4 °C.The mixture was centrifuged at 15,000 × g for 30 min at 4 °C,and the supernatant was collected for enzyme analysis.One milliliter of enzyme extract was incubated with 2 mL of borate buffer (50 mmol L-1,pH 8.8) and 0.5 mL of L-phenylalanine(20 mmol L-1) for 60 min at 37 °C.The reaction was stopped with 0.1 mL of 6 mol L-1HCl.The PAL activity was determined by the production of cinnamate,measured by the absorbance change at 290 nm with a spectrophotometer (UV-160,Japan).PPO and POD were extracted according to the method of Chen et al.[20].Rice samples (3 g) from each treatment were homogenized with 30 mL of 0.1 mol L-1sodium phosphate buffer(pH 6.4)containing 0.5 g of PVP and ground at 4 °C.The homogenate was centrifuged at 15,000 × g for 30 min at 4 °C,and the supernatant was used for enzyme assays.The PPO activity was determined by adding 1 mL of enzyme preparation to 2 mL of catechol as a substrate,and the change was measured immediately in absorbance at 398 nm(A398).The activity was expressed as A398per minute per milligram of protein.The POD activity was determined using guaiacol as a substrate.The reaction mixture consisted of 2 mL of crude extract,1 mL of guaiacol,and 1 mL of buffer.The reaction mixture was incubated at 30 °C for 30 min before 1 mL of H2O2was added.Absorbance was measured at 460 nm(A460).The activity of POD was defined as A460per minute per milligram of protein[24].
2.5.Statistical analysis
Statistical analysis was performed with SPSS10.0 software for multiple comparisons and correlation analyses.A value of P <0.05 was considered to be statistically significant.
3.Results
3.1.Detection of RNA and reverse transcription products
1% Agarose gel electrophoresis and UV spectrophotometry were used to detect the quality of the total RNA,and indicated that the extracted RNA was suitable for reverse transcription.The PCR amplified fragments of the target gene PAL showed that the cDNA was specific without background bands or false positive amplification(Fig.1).
3.2.Effect of SBPH feeding on expression of SA synthesis-related genes in resistant and susceptible rice plants
PAL (phenylalanine ammonia-lyase),EDS1 (enhanced disease susceptibility 1) and PAD4 (phytoalexin deficient 4) are the major genes involved in the SA-synthesis pathway.The relative expression level of PAL was significantly higher in resistant Kasalath rice than in the susceptible Wuyujing 3 cultivar in response to SBPH feeding.The relative expression level of PAL in rice at 12 hpi was 7.52 times greater than that in untreated control rice at the same time point.PAL transcript accumulation was far more rapid and peaked at higher levels in Kasalath; the relative expression level was 49.63,87.18,57.36 and 75.06 times greater than that in Wuyujing 3 at 24,36,48 and 72 hpi,respectively (Fig.2).The relative expression levels of EDS1 and PAD4 were also higher in Kasalath than in Wuyujing 3 at 24 hpi(Fig.2).
Meanwhile,the NPR1(nonexpressor of pathogenesis-related genes 1)is a key regulatory gene in SA-dependent SAR reaction.The relative expression level of NPR1 was remarkably higher in Kasalath than in Wuyujing 3 after SBPH feeding with expression of 6.47,4.84,8.92 and 5.49 times in Kasalath greater than that in Wuyujing 3 at 12,24,36 and 72 hpi,respectively(Fig.2).

Table 1-Primer sequences used in real time-PCR.
Another gene,PR1b,encodes a pathogenesis-related protein that inhibits growth,reproduction and communication of pathogens in plants.The PR1b gene expression level was significantly higher in susceptible Wuyujing 3 than in resistant Kasalath after SBPH feeding.The relative expression of PR1b in Wuyujing 3 was 13.38,89.82,71.01 and 46.66 times greater than that in Kasalath at 24,36,48 and 72 hpi,respectively (Fig.2).The up-regulated PR1b gene expression in the susceptible Wuyujing 3 rice was likely to have been induced by the physical injuries caused by SBPH foraging.The above results showed that SBPH feeding activated the SA-dependent resistance pathway in Kasalath and that the expression levels of PAL and NPR1 played key roles in regulating resistance to SBPH.
Fig.1-Am plification specificity detection by electrophoresis.Lane M:DNA markers;lanes 1-12:am plification products of the PAL gene;lane 13:blank control.
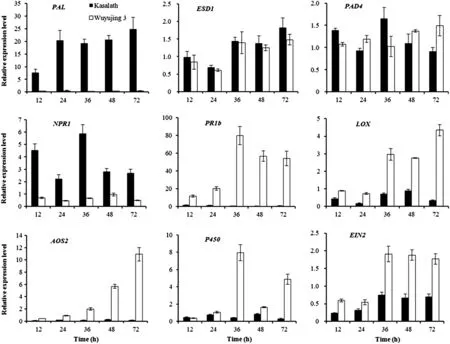
Fig.2-Expression analysis in terms of the relative expression levels of defense-related genes in rice Kasalath and Wuyujing 3 at 12,24,36,48 and 72 hpi by Real-time PCR.The PAL,EDS1 and PAD4 genes are the major loci and corresponding proteins involved in the SA-synthesis pathway.NPR1 is a key regulatory gene in SA-dependent systemic acquired resistance.PR1b is a pathogenesis-related protein.LOX and AOS2 are two JA synthesis-related genes.EIN2 is a receptor gene of the ethylene signaling pathway.
3.3.Effect of SBPH infestation on expression of genes involved in the JA/ET pathway
The expression levels of the JA synthesis-related genes LOX(lipoxygenase)and AOS2(allene oxide synthase 2)were lower in the resistant cultivar Kasalath than in the susceptible cultivar Wuyujing 3 after SBPH feeding.There was a significant difference in transcription level at 36 hpi by the insect when comparing Kasalath and Wuyujing 3.Furthermore,the expression level was substantially lower in Kasalath at subsequent time points.
The relative expression of LOX in Wuyujing 3 was 4.06,4.17,3.06 and 12.43 times greater than that in Kasalath at 24,36,48 and 72 hpi,respectively.AOS2 transcript accumulation was much greater in Wuyujing 3 and the relative expression level was 4.63,12.38,22.72 and 60.72 times greater than that in Kasalath at 36,48 and 72 hpi with SBPH,respectively (Fig.2).Similarly,the relative expression level of P450 was higher in Wuyujing 3 than in Kasalath (Fig.2).
In addition,the expression level of the receptor gene EIN2(ethylene insensitive 2)in the ethylene signaling pathway was higher in Wuyujing 3 than in Kasalath after SBPH feeding.The relative expression of EIN2 in Wuyujing 3 was 2.55,2.81 and 2.53 times greater than that in Kasalath at 36,48 and 72 hpi,respectively,which indicated that SBPH feeding induced defense responses in the susceptible Wuyujing 3 rice associated with a JA/ET-dependent signaling pathway(Fig.2).
3.4.Change in defense enzyme activity after SBPH feeding
The PAL activity in Kasalath was almost identical to that in Wuyujing 3 without SBPH attack and increased in both after SBPH feeding.The PAL activity in Kasalath rose gradually afterSBPH feeding up to the 12 hpi time point,then increased drastically from 12 to 36 hpi,and then increased smoothly and was maintained at a high level.The PAL activity in Wuyujing 3 increased slightly at 12 hpi,significantly increased at 24 hpi,reached its highest value at 36 hpi and then showed a smooth trend of decline.The PAL activity in Kasalath was remarkably higher than in Wuyujing 3 at all of the tested time points in response to SBPH feeding (Table 2).These results indicated PAL activity was induced in both rice accessions by SBPH infestation but the rate and magnitude of increase in activity was significantly higher in Kasalath than in Wuyujing 3.
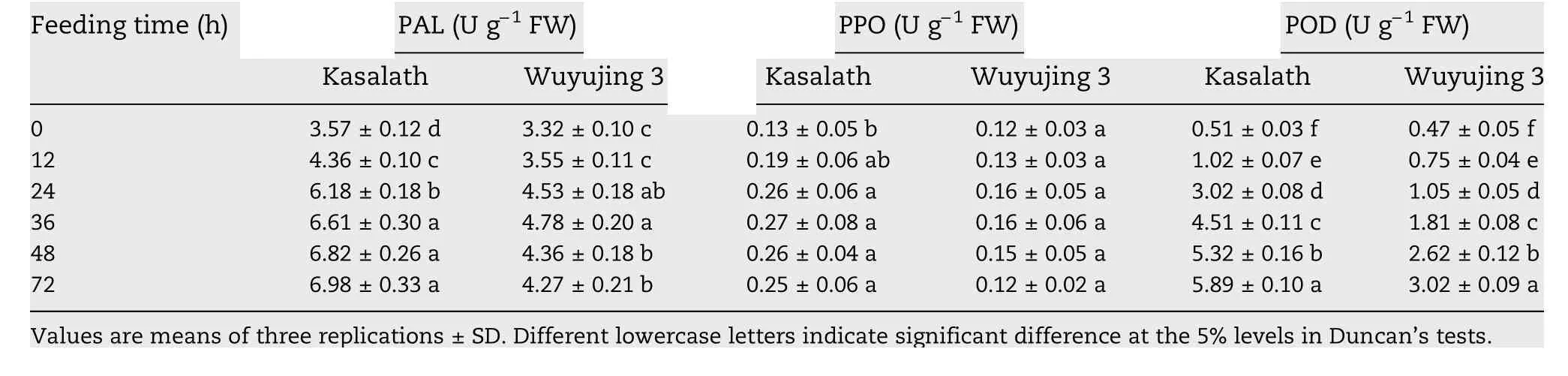
Table 2-Enzyme activities in rice infested by SBPH for different time periods.
SBPH feeding resulted at first in a gradual increase and then a decrease in PPO activity in the two rice varieties.However,PPO activity in Kasalath was significantly higher at 24 hpi than at 0 hpi.This activity reached a peak at 36 hpi and then decreased slightly.Changes in PPO activity in Wuyujing 3 were small after SBPH feeding.There was no significant difference in PPO activity between any of the time points (Table 2).PPO activity in Kasalath was higher than in Wuyujing 3 at all of the time points tested.
For the second enzyme,POD,activity rose significantly in both Kasalath and Wuyujing 3 when infested by SBPH but the rate and magnitude of increase in Kasalath was far greater than in Wuyujing 3.There was no distinct difference in POD activity between Kasalath and Wuyujing 3 before SBPH attack.POD activity increased quickly and maintained an increasing trend in both genotypes when attacked by SBPH.Significant differences in POD activity were detected between every pair of time points (Table 2).The activity of POD in Kasalath was higher than in Wuyujing 3 at every time point after SBPH feeding,indicating that POD accumulation was remarkably responsive and sensitive to SBPH infestation.
3.5.Correlation between enzyme activities and gene expression levels
The expression level of the PAL gene was closely related to the activities of the defense enzymes PAL,POD and PPO in the resistant variety of rice,Kasalath,with high correlation coefficients (r) of 0.9051,0.8687 and 0.7504,respectively.Similarly,there was positive correlation between EDS1 gene expression levels and PAL,POD and PPO enzyme activities in Kasalath,with r values of 0.5887,0.7738 and 0.3248,respectively.However,there was no relationship between the PAL expression level and the enzyme activities of PAL,POD and PPO in the susceptible Wuyujing 3 rice(r =-0.0662,-0.1682 and-0.1492,respectively).In addition,there was a close correlation between POD enzyme activity and the expression levels of the AOS2,EIN2 and LOX genes in Wuyujing 3(r = 0.8688,0.7980 and 0.6368,respectively).These data indicated that the increased PAL,POD and PPO activities were in accordance with the PAL gene expression levels in Kasalath after SBPH infestation,which further confirmed that the induction of PAL expression played an important role in the rice defense response against SBPH.
4.Discussion
Plant defense responses against herbivores,pathogens and mechanical wounding involve global changes in gene expression mediated by multiple signaling pathways.These defense pathways are mainly mediated by small molecules such as SA,JA and ET [8,25,26].The genes associated with defense in plants are activated by signaling molecules and then trigger resistance when the plant is subjected to biotic and abiotic stresses [27].SBPH,a typical phloem-feeding insect,sucks rice sap but causes little physical injury to rice foliage and stems [28].The SBPH feeding mode is similar to that of fungal hyphae and nematode mouthparts.Therefore,SBPH,similar to aphid and whitefly,can also be regarded as a pathogen-like insect[7].
Furthermore,rice was considered likely to produce defense responses to sucking insects similar to those induced by fungi and nematodes [29,30].Our results indicate that the expression of defense-related genes was triggered and then SAand JA/ET-dependent signaling pathways were activated when rice was attacked by SBPH.The transcript level of SA synthesis-related genes was significantly higher in the resistant Kasalath than in the susceptible Wuyujing 3.Accumulation of PAL,the key gene in the SA-dependent pathway,was far more rapid in Kasalath and its expression was induced by SBPH challenge.The accumulation of LOX and AOS2,the major genes involved in the JA/ET signaling pathway,was much greater in Wuyujing 3 than in Kasalath.Therefore,we believe that the SA-mediated signaling pathway in resistant Kasalath was activated by SBPH infestation and that PAL played a key role in triggering the signal pathway.
The gene expression patterns involved in the SA-dependent and JA/ET pathways in the resistant Kasalath and the susceptible Wuyujing 3 genotypes used in this study were similar to those in resistant Mudgo and susceptible Kittake,respectively[31],suggesting the same defense mechanisms were likely to be induced by SBPH infestation in these other rice varieties.In another study involving antixenosis and antibiosis tests [21],Kasalath and Mudgo were evaluated for the same resistance reactions against SBPH and the results in these two rice varieties were consistent with our hypothesis of activation of defense gene expression.
Plants have evolved an efficient defense transduction network against insect and pathogen attack.Plant defenses are regulated differentially by cross-communicating signal transduction pathways in which SA and JA play key roles[32,33].Cooperative interactions between signal response pathways may be regarded as a means developed by plant species to increase the number of distinct gene repertoires that can be controlled by a limited set of signaling molecules but in a differential manner and hence to increase behavioral plasticity.In general,the SA-dependent signaling pathway regulates the expression of a wide array of defense-response genes and confers broad-spectrum pest or pathogen resistance[34].
Activation of the SA pathway has been proven to be important in both basal and resistance gene (R)-mediated biotrophic pathogen defense in Arabidopsis thaliana,while the JA/ET pathway is activated in response to necrotrophic pathogens,feeding by tissue-damaging herbivores,and wounding [35].Potato responses to infestation by aphids,a kind of sucking insect whose feeding behavior is similar to SBPH,involve both SA and JA/ET plant defense signaling pathways [36].Another study showed that tomato leaves rapidly accumulated high levels of SA after exposure to the cotton bollworm,a type of chewing pest [10].Plants are usually exposed to insects and pathogens and hence have developed resistance to simultaneous pathogen infection and insect feeding.As insect damage can often increase the risk of pathogen attack this coordination of plant responses seems to make biological sense.In the long-term evolutionary process,the SA-and JA-mediated signal transduction pathways have both been preserved [37].Plants accurately regulate the SA and JA signaling pathways by adjusting SA and JA contents in order to resist stress more efficiently.
In this study,the transcription of the key genes PAL for the SA synthesis pathway,as well as LOX and AOS2 for the JA pathway,were significantly up-regulated compared with their basal levels,which indicated two signaling pathways were activated due to SBPH attack.The expression of PAL dramatically increased in Kasalath after SBPH sucking,which promoted synthesis of SA and then increased SA content.Therefore,the SA mediated signaling pathway was the major defense mechanism in resistant Kasalath,which was consistent with the reports mentioned above [7,10,12,15,31].However,the induction LOX and AOS2 in JA responsive pathway in the susceptible Wuyujing 3 was somehow contradictory to the findings reached by Zanate et al.[15]As mentioned above,the JA/ET pathway usually induces genes whose protein products have antimicrobial and antifungal activity and accumulate in response to necrotrophic pathogens [38].In a previous study,we detected that wound healing was probably caused by some substance secreted by a resistance rice variety,which then protected the infected seedling.This substance was observable with a scanning electron microscope(SEM)on epidermis of resistant rice leaves infested by SBPH but not in the leaves of a susceptible variety [39].Non-healing wounds caused by SBPH sucking in the susceptible genotype Wuyujing 3 might have led to a large invasion of bacteria and fungi in this genotype that did not occur in Kasalath which healed its wounds quickly.The massive accumulation of microorganisms in Wuyujing 3 was likely to significantly induce the expression of LOX and AOS2 involved in JA-mediated signal pathway.Therefore,we believed it was reasonable that the expression quantity of LOX and AOS2 in JA pathway was higher in Wuyujing 3 than in Kasalath.
In conclusion,plants defend themselves from insect or pathogen attack through a wide variety of mechanisms and stimulated by many different biotic inducers [40].Our results showed that SBPH feeding induced biochemical defense responses in the rice varieties Kasalath and Wuyujing 3.The activities of PAL,PPO and POD in Kasalath were almost identical to those in Wuyujing 3 when not infested by SBPH.These three enzymes were induced distinctly by SBPH challenge and their activities increased significantly.The combined action of these defense enzymes may account for increased rice resistance to SBPH.PAL is the first enzyme of the phenylpropanoid pathway and is involved in the biosynthesis of phenolics,phytoalexins and lignins[17].Our results indicated the increase in PAL enzyme activity was consistent with the induction of PAL gene expression after SBPH feeding.The resulting phenolics could be oxidized by the action of PPO and POD to produce differently colored phenolic complexes or compounds such as quinines and even tannins[41].PPO usually accumulates upon wounding in plants [20].POD,meanwhile,is involved in lignin-forming plant defense responses and its activity is associated with disease resistance in plants,and increases in host plants following pathogen infection [42].Overall,our results revealed that the expression levels of the SA synthesis-related genes PAL,NPR1,EDS1 and PAD4 and the activities of defense-related enzymes such as PAL,POD,and PPO were highly induced in the resistant Kasalath rice in response to SBPH feeding,suggesting that the biosynthesis of salicylic acid,lignin,phenolic compounds and phytoalexins may contribute greatly to rice resistance mechanisms in the poorly studied rice–SBHP interaction system.
This study was sponsored by the National Nature Science Foundation of China (30971746) and the Major Project for Breeding Genetically Modified Organisms (2009ZX08009-046B).The authors are grateful to the comments of anonymous reviewers and editing from M.Blair.
[1] S.M.Gray,Plant virus proteins involved in natural vector transmission,Trends Microbiol.4(1996) 259–264.
[2] C.X.Duan,J.M.Wan,H.Q.Zhai,Q.Chen,J.K.Wang,N.Su,C.L.Lei,Quantitative trait loci mapping of resistance to Laodelphax striatellus(Homoptera:Delphacidae)in rice using recombinant inbred lines,J.Econ.Entomol.100(2007)1450–1455.
[3] C.X.Duan,Z.J.Cheng,C.L.Lei,H.Q.Zhai,J.M.Wan,Analysis of QTLs for resistance to small brown planthopper in rice using an F2population from a cross between Kasalath and Wuyujing 3,Acta Agron.Sin.35 (2009) 388–394.
[4] K.Tanaka,S.Endo,H.Kazano,Toxicity of insecticides to predators of rice planthoppers:spiders,the mirid bug and the dryinid wasp,Appl.Entomol.Zool.35(2000) 177–187.
[5] H.D.Wang,J.P.Chen,A.G.Wang,X.H.Jiang,M.J.Adams,Studies on the epidemiology and yield losses from rice black-streaked dwarf disease in a recent epidemic in Zhejiang province,China,Plant Pathol.58(2009) 815–825.
[6] C.X.Duan,N.Su,Z.J.Cheng,C.L.Lei,J.L.Wang,H.Q.Zhai,J.M.Wan,QTL Analysis for the resistance to small brown planthopper (Laodelphax striatellus Fallén) in rice using backcross inbred lines,Plant Breed.129 (2010) 63–67.
[7] L.L.Walling,The myriad plant responses to herbivores,J.Plant Growth Regul.19(2000) 195–216.
[8] B.N.Kunkel,D.M.Brooks,Cross talk between signaling pathways in pathogen defense,Curr.Opin.Plant Biol.5(2002)325–331.
[9] G.W.Felton,K.L.Korth,J.L.Bi,S.V.Wesley,D.V.Huhman,M.C.Mathew,J.B.Murphy,C.Lamb,R.A.Dixon,Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory,Curr.Biol.9(1999)317–320.
[10] J.Y.Peng,X.J.Deng,J.H.Huang,S.H.Jia,X.X.Miao,Y.P.Huang,Role of salicylic acid in tomato (Lycopersicon esculentum) plant defense against cotton bollworm,Helicoverpa armigera Hubner,Z.Naturforsch.C 59(2004) 856–862.
[11] Y.F.Li,Z.H.Zhang,Y.F.Nie,L.H.Zhang,Z.Z.Wang,Proteomic analysis of salicylic acid-induced resistance to Magnaporthe oryzae in susceptible and resistant rice,Proteomics 12(2012)2340–2354.
[12] H.T.Alborn,T.C.J.Turlings,T.H.Jones,G.Stenhagen,J.H.Loughrin,J.H.Tumlinson,An elicitor of plant volatiles from beet armyworm oral secretion,Science 276 (1997) 945–949.
[13] H.T.Alborn,T.V.Hansen,T.H.Jones,D.C.Bennett,J.H.Tumlinson,E.A.Schmelz,P.E.Teal,Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana,elicitors of plant volatiles,Proc.Natl.Acad.Sci.U.S.A.104(2007) 12976–12981.
[14] Q.Li,Q.G.Xie,J.Smith-Becker,D.A.Navarre,I.Kaloshian,Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades,Mol.Plant Microbe Interact.19(2006) 655–664.
[15] S.I.Zarate,L.A.Kempema,L.L.Walling,Silverleaf whitefly induced salicylic acid defenses and suppresses effectual jasmonic acid defenses,Plant Physiol.143 (2007) 866–875.
[16] D.R.Walters,A.C.Newton,G.D.Lyon,Induced resistance:helping plants to help themselves,Biologist (Lond.) 52(2005)28–33.
[17] L.Pellegrini,O.Rohfritsch,B.Fritig,M.Legrand,Phenylalanine ammonia-lyase in tobacco,Plant Physiol.106(1994) 877–886.
[18] A.Polle,T.Otter,F.Seifert,Apoplastic peroxidases and lingnification in needles of Norway spruce (Picea abies L.),Plant Physiol.106 (1994) 53–60.
[19] S.A.Avdiushko,X.S.Ye,J.Kuc,Detection of several enzymatic activities in leaf prints cucumber plant,Physiol.Mol.Plant Pathol.42(1993) 441–454.
[20] C.Chen,R.Bélanger,N.Benhamou,T.C.Paulitz,Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum,Physiol.Mol.Plant Pathol.56(2000) 13–23.
[21] C.X.Duan,S.X.Zhang,C.L.Lei,Z.J.Cheng,Q.Chen,H.Q.Zhai,J.M.Wan,Evaluation of rice germplasm for resistance to small brown planthopper (Laodelphax striatellus Fallén) and analysis of resistance mechanism,Rice Sci.15(2008) 36–42.
[22] K.J.Livak,T.D.Schmittgen,Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod,Methods 25(2001) 402–408.
[23] J.B.Chen,S.M.Wang,R.L.Jing,X.G.Mao,Cloning the PvP5CS gene from common bean(Phaseolus vulgaris) and its expression patterns under abiotic stresses,J.Plant Physiol.166 (2009) 12–19.
[24] G.Z.Qin,S.P.Tian,Enhancement of biocontrol activity of Cryptococcus laurentii by silicon and the possible mechanisms involved,Phytopathology 95(2005) 69–75.
[25] C.A.Ryan,Protease inhibitors in plants: genes for improving defenses against insects and pathogens,Annu.Rev.Phytopathol.28(1990) 425–449.
[26] D.Y.Qiu,J.Xiao,X.H.Ding,M.Xiong,M.Cai,Y.L.Cao,X.H.Li,C.G.Xu,S.P.Wang,OsWRKY13 mediates rice disease resistance by regulating defense related genes in salicylate-and jasmonate-dependent signaling,Mol.Plant Microbe Interact.20(2007)492–499.
[27] J.Y.Peng,Y.P.Huang,The signaling pathways of plant defense response and their interaction,J.Plant Physiol.Mol.Biol 31(2005) 347–353.
[28] Y.C.Wang,M.Tang,P.Y.Hao,Z.F.Yang,L.L.Zhu,G.C.He,Penetration into rice tissues by brown planthopper and fine structure of the salivary sheaths,Entomol.Exp.Appl.129(2008) 295–307.
[29] D.G.Cai,M.Kleine,S.Kifle,H.J.Harloff,N.N.Sandal,K.A.Marcker,R.M.Klein-Lankhorst,E.M.J.Salentijn,W.Lange,W.J.Stiekema,U.Wyss,F.M.W.Grundler,C.Jung,Positional cloning of a gene for nematode resistance in sugar beet,Science 275 (1997) 832–834.
[30] J.D.Jones,J.L.Dangl,The plant immune system,Nature 444(2006) 323–328.
[31] W.C.Li,J.J.Yu,C.X.Duan,Z.D.Zhu,X.M.Wang,Expression of rice defense genes under small brown planthopper stress,Acta Agron.Sin.38(2012) 1625–1630.
[32] S.H.Spoel,A.Koornneef,S.M.Claessens,J.P.Korzelius,J.A.Van Pelt,M.J.Mueller,J.Buchala,J.P.Métraux,R.Brown,K.Kazan,L.C.Van Loon,X.N.Dong,C.M.J.Pieterse,NPR1 modulates crosstalk between salicylate-and jasmonate-dependent defense pathways through a novel function in the cytosol,Plant Cell 15(2003) 760–770.
[33] K.Zhu-Salzman,R.A.Salzman,J.E.Ahn,H.Koiwa,Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid,Plant Physiol.134 (2004)420–431.
[34] W.E.Durrant,X.Dong,Systemic required resistance,Annu.Rev.Phytopathol.42(2004) 185–209.
[35] J.Glazebrook,Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens,Annu.Rev.Phytopathol.43(2005) 205–227.
[36] O.Martinez de Ilarduya,Q.Xie,I.Kaloshian,Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions,Mol.Plant Microbe Interact.16(2003) 699–708.
[37] J.Li,G.Brader,E.T.Palva,The WRKY70 transcription factor:a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense,Plant Cell 16(2004) 319–331.
[38] E.Rojo,R.Solano,J.J.Sanchez-Serrano,Interactions between signaling compounds involved in plant defense,J.Plant Growth Regul.22(2003) 82–98.
[39] C.X.Duan,G.S.Peng,Z.D.Zhu,H.J.Li,X.M.Wang,Changes in reactive oxygen species and ultrastructure in resistant and susceptible rice leaves infested by small brown planthopper,Acta Agric.Boreali-Sin.26(2011) 207–211.
[40] S.Schneider,W.R.Ullrich,Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic and biotic inducers,Physiol.Mol.Plant Pathol.45(1994) 291–304.
[41] R.Campos-Vargas,M.E.Saltveit,Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce,Physiol.Plant.114(2002)73–84.
[42] J.S.Scott-Craig,K.B.Kerby,B.D.Stein,S.C.Somerville,Expression of an extracellular peroxidase that is induced in barley(Hordeum vulgare)by the powdery mildew pathogen(Erysiphe graminis f.sp.hordei),Physiol.Mol.Plant Pathol.47(1995)407–418.
- The Crop Journal的其它文章
- Effect of nitrogen fertilizer on distribution of starch granules in different regions of wheat endosperm
- Impacts of nighttime post-anthesis warming on rice productivity and grain quality in East China
- Overexpression of GmDREB1 improves salt tolerance in transgenic wheat and leaf protein response to high salinity
- Establishment of the integrated applied core collection and its comparison with mini core collection in soybean(Glycine max)
- Differential microRNA expression between shoots and rhizomes in Oryza longistaminata using high-throughput RNA sequencing
- Genome-wide analysis of the WRKY transcription factor gene family in Gossypium raimondii and the expression of orthologs in cultivated tetraploid cotton

