Suppression of supercooling of PCM-water emulsions using nano-additives
ZHANG Xiyao,NIU Jianlei,WU Jianyong,ZHANG Shuo
(1Department of Building Service Engineering,The Hong Kong Polytechnic University,Kowloon,Hong Kong,China;2Department of Applied Biology&Chemical Technology,The Hong Kong Polytechnic University,Kowloon,Hong Kong,China)
1 Introduction
1Thermal energy storage (TES), as it deals with energy saving and the optimum use of renewable energy, has obtained considerable attention in the last 20 years. In particular, the energy storage materials applied in TES system is a key factor to maximize the use of the energy sources. Generally,the energy storage materials have a high energy storage density which can store or release a large amount of latent heat during their phase transformation processes, so they are also named phase change material(PCM).
However, as the thermal conductivity of most PCM is comparatively low, they generally have a low heat transfer rate which is unfavorable in the heat exchange processes. For solving this problem, PCM emulsion and microencapsulated PCM are often used to improve the heat transfer between the PCM and the ambient by increasing the surface to volume ratio of the PCM[1-2].
Besides, the PCM liquid usually can be cooled below its melting point without occurring crystallization. This phenomenon is called supercooling which is unexpected in the latent heat storage as it leads to a shift of the controlling temperature range of the systems and even an increase of the energy consumption of the chiller resulted from a low COP. Furthermore, from the nucleation theory,supercooling of PCM in small volume is expected to be more severe than in large volumes, which predicts that the supercooling in emulsion will be more serious than that in bulk PCM as their small droplet size.Günther et al. [3] studied in detail the supercooling in hexadecane emulsion with droplet size in the range of 0.1~20 μm.An increased supercooling of at least 10 K for small droplets were observed. For decreasing the supercooling, an effective approach is the addition of liquid or solid nucleating agents to the PCM liquid asthe seeds and catalysts for nucleation and crystal growth [4], such as 1-tetradecanol for microencapsulated n-tetradecane [5], paraffin wax for tetradecane and hexadecane paraffin-in-water emulsion [6] and TiO2[7]or α-Al2O3[8]for the pure water.
Our previous study [9]has investigated the effects of multi-wall carbon nano-tube (MWCNTs) particles as nucleating agent to inhibit supercooling in the PCM liquid, which showed a good performance at low concentration. This study is to evaluate the effectiveness of the MWCNTs nano-particles as nucleating agent in emulsion. Hence, in this study, a kind of n-hexadecane emulsion was designed and prepared and the MWCNTs particles were well dispersed in emulsions at various concentration. The effectiveness of the nucleating agent for emulsion was evaluated by using the DSC technique.
2 Materials and methods
2.1 Materials
In this study, n-hexadecane C16H34(99%) was chosen as the PCM liquid (purchased from International Laboratory USA), and the MWCNTs was used as raw material of the nucleating agent, with an outer diameter 10~20 nm, length 0.5~2 μm and 95%purity (purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences, China). The HNO3(70%) and H2SO4(98%) were obtained from Aldrich. 1-decanol and Tween-20 were used as surfactants,which are all of analytical grade.
2.2 Modification of MWCNTs particles
The original MWCNTs particles were oxidized by the concentrated H2SO4(98%) and HNO3(70%)mixture at 3︰1 volume ratio in a flask with reflux at 65 ℃ for 4 h. After cooling down to room temperature, the particles were separated from acid and rinsed with water until pH was above 5. Final rinsing was done by using ethanol and the resulting solid particles were dried at 80 ℃ in an oven for about one day.
2.3 Preparation of PCM slurry and PCM-water emulsion
After furbished, the surface-modified MWCNTs particles were re-dispersed in hexadecane with 1-decanol as the surfactant. In this step, an ultrasound probe (sonics vibra-cell, model VCX130)was used to help the well dispersion with 30%amplitude of power for 20 min. By repeating the above steps,the PCM slurry with the nucleating agent at various concentration from 0.1%to 0.8%(by mass)was yielded for the following studies.
The above PCM slurries were then mixed with water at 1︰2 mass ratio respectively,and the Tween 20(5% by mass) was added into the mixture as the surfactant. A disperser (type Ultra-Turrax T25 by IKA-Labor-Technik) was used for the formation of emulsion with a rotate speed at 8 000 r/min for 10 min.The emulsions with different concentration of nano-particles were prepared by the method above.
2.4 Characterization and analysis of emulsion droplets and the nanoparticles
The size distributions of emulsion droplets and the nanoparticles were determined by using a laser diffraction particle size analyzer of type Malven Zetasizer. This analyzer has a broad measuring range from 0.02 μm to 5000 μm. 100 measurements were performed for 20 min at a scattering angle of 90° at 25 ℃. The average particle size (in nm) and the polydispersity index were analyzed by the Zetasizer 3000HSA-advanced software.
The morphology of the PCM-water emulsion droplets were examined with an optical microscope type Leica DM4000 under 400x. The images were processed by Leica LAS AF software.
2.5 Thermal analysis
To determine the melting and nucleation temperatures, a DSC type Mettler Toledo DSC-822e with aluminum crucibles was used. The temperature and sensitivity calibration were carried out by using the standard materials. The temperature program for each material was created in such a way, the heating/cooling rate was 5 ℃/min. The samples were first cooled to the initial temperature of -5 ℃ for at least 15 min for stabilization,and then heated to 30 ℃,held for 5 min at 30 ℃,and finally cooled to-5 ℃.
3 Result and discuss
3.1 The droplet size distribution of the PCMwater emulsion
By a high-speed stirring and different surfactantsparticipated, the emulsion with small droplets of PCM was obtained. As the MWCNTs particles had a similar size distribution to emulsions, they were not added in for avoiding the interference, but the 1-decannol was added for simulating work condition. And measured by the dynamic light scattering analysis at 25 ℃, the average diameter of hexadecane droplets was 2082 nm with a wide distribution (PDI=1) shown in Fig.1 The count rate was at (231.2±31.9) Kc/s (kilo count per second).
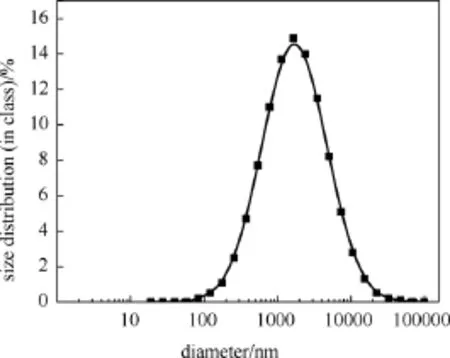
Fig.1 The particle size distribution of PCM-water emulsion
3.2 The morphology of PCM-water emulsion droplets
Optical photomicrographs of the PCM-water emulsion under 400x are presented in Fig.2 The size and shape of droplets can be observed clearly and are consistent with the results from the DLS with a centralized outer diameter of 0.1~10 μm. The modified MWCNTs particles are hardly observed as their diameters were small and concentration was low.
3.3 The supercooling in PCM-water emulsion
We have observed the capacity of the modified MWCNTs as the nucleating agent(NA)to reduce the supercooling of PCM crystallization in previous work[9],especially in relatively light concentration. The optimal concentration of NA was around 0.1%~0.2%(by mass) vs. the n-hexadecane. In this section, a detailed investigation in emulsion was conducted to find out an optimum concentration of nucleating agent.The DSC was used to analyze the thermal property of the n-hexadecane-water emulsion.The melting peak point was defined as the melting temperature Tm.peakand the freezing peak point as the nucleation temperature Tf.peak. The difference between the melting and nucleation temperature was considered as the supercooling degree.Besides,as the heating/cooling rate (5 ℃/min) was higher than that of real working condition of the PCM emulsion, the supercooling degree would also be enhanced. But it did not affect the nucleating agent performance comparison.

Fig.2 The morphology of the PCM-water emulsion droplets
According to the nucleation theory, the supercooling is expected to increase as the dispersed PCM droplets are small[3]. As the DSC heating/cooling curves of the PCM-water emulsions shown in Fig.3, the emulsion without nucleating agent (MWCNTs at 0) did not freeze until the temperature approaches the ice point, and there were only small fluctuations in freezing circle at approximate 15 ℃, thus there was a significantly large extent of supercooling. Two obvious peaks can be observed on the freezing curves at 0.2% (by mass)concentration and the 2nd peak area above 10 ℃increased gradually with the 1st peak going down as the mass ratio of MWCNTs to emulsion reached 0.6%(by mass). At 0.8% (by mass), the nucleation effect was similar to that at 0.6% (by mass). It can be observed that the supercooling degree was controlled to below 4 ℃ at a concentration range of the nucleating agent from 0.4% to 0.8% (by mass). The detailed melting and crystallization properties calculated from the DSC data are presented in Table 1.

Fig.3 The DSC freezing/melting curves of different emulsion samples with MWCNTs as NA(mass concentration vs.the n-hexadecane)
Considering that the mass ratio of PCM and water was 1︰2, the ‘macroscopic’concentration of the nucleating agent in the PCM droplets should be approximately 1/3 of that in the case of bulk PCM liquid,thus the results coincide with the performance of MWCNTs in PCM liquid in our previous work.Moreover, in the microcosmic view,as the PCM was separated into many small volumes, nucleation had to occur in every volume individually. Especially, it is possible that the nucleating agent particles in low concentration only existed in some droplets, and the heterogeneous nucleation was limited to these PCM droplets and the rest PCM volumes could only solidify by homogeneous nucleation, which contributes to the two freezing peaks in some curves. Therefore, there was a minimum effective concentration of the nucleating agent and it was 0.6% (by mass) vs. the n-hexadecane for the emulsion investigated in this work. And it can be concluded that the average phase change enthalpy was approximate 81.49 J/g.
4 Conclusions
In this study,the experimental results show that,as the nucleating agent, a low concentration of modified MWCNTs nano-particles were effective to reduce the supercooling degree in the PCM-water emulsion. Besides, it is clear that there existed a minimum effective concentration of the nucleating agent and it was 0.6% (mass concentration vs. the n-hexadecane) for the emulsion investigated in this work. Eventually, a kind of emulsion which has heat capacity of approximate 81.49 J/g and freezes around 16 ℃was obtained.

Table 1 Melting and crystallization properties of PCM-water emulsion with MWCNTs as NA
[1] KaszaK E,Chen M M. Improvement of theperformance of solar energy or waste heat utilization systemsby usingphase-change slurry as an enhanced heat-transfer storage fluid[J].Sol.Energy Eng.,1985,107:229-236.
[2] Regin A F,Solanki S C,Saini J S. Heat transfer characteristics of thermal energy storage system using PCM capsules:A review[J].Renew.Sust.Energ.Rev.,2008,12(9):2438-2458.
[3] Günther E,Schmid T,Mehling H,et al. Subcooling in hexadecane emulsions[J].Int.J.Refrig.,2010,33:1605-1611.
[4] SangwalK. Additives and Crystallization Processes : From Fundamentals to Applications[M]. West Sussex:John Wiley & Sons Ltd.,2007.
[5] Yamagishi Y , Sugeno T , Ishige T , et al. An evaluation of microencapsulated PCM for use in cold energy transportation medium[C]//Washington:Proceedings of the 31st Intersociety Energy Conversion Engineering Conference,1996:2077-2083.
[6] Huang L,Günther E,Doetsch C, et al. Subcooling in PCM emulsions—part 1:Experimental[J]. Thermochimica Acta, 2010,509:93-99.
[7] He Q,Tong W,LiuY.Experimental study on super-cooling degree of nanofluids for cryogenic cool storage[J]. Journal of Refrigeration,2007,28:33-36.
[8] Zhang X J,Wu P,Qiu L M,et al. Analysis of the nucleation of nanofluids in the ice formation process[J]. Energ. Convers. Mange.,2010,51:130-134.
[9] Zhang S,Wu J Y,Tse C T,et al.Effective dispersion of multi-wall carbon nano-tubes in hexadecane through physiochemical modification and decrease of supercooling[J]. Sol.Energ.Mat.Sol. C,2012,96:124-130.

