无溶剂一锅法合成N-歧化松香基-1,4-二氢吡啶
曹 宇,邵 宇,王金收,魏世勇,史伯安*
1湖北民族学院化学与环境工程学院;2 湖北民族学院生物资源保护与利用湖北省重点实验室,恩施 445000
1,4-二氢吡啶类化合物具有抗糖尿病、神经保护及抗血小板凝聚的生理活性,在生物和医药中广泛地被用作钙离子通道阻滞剂、抗结核剂及神经肽YY1 受体拮抗剂[1,2]。在临床应用中不仅可以用于治疗心绞痛、充血性心衰、高血压、局部缺血和动脉粥状硬化等心脑血管疾病[3-5],还可以用于肠胃病和治疗肺动脉高压的辅助药物,因此,对该类化合物的研究引起了国内外的药物工作者的极大兴趣。近年来,关于该类化合物的合成研究报道较多,主要集中于通过改变胺的结构、醛的结构和1,3-二羰基化合物的结构来对其结构进行修饰;特别是合成方法的改进更是诸多见于文献,其中尤以无溶剂一锅法合成将产率提高到了90%的高度[6,7]。
松香是一种廉价的天然产物,在其骨架中具有多个手性中心和二萜结构,不仅具有抗癌、抗病毒和抗菌活性,还具有抗氧化、消炎、抑制胃酸分泌、钾离子通道开发、抗惊厥、防止血吸虫感染等生物活性,因此被广泛用于医药和农药领域特别是表面活性剂等精细化学品的制备中[8-10]。本文利用松香的下游产品歧化松香胺,以它作为胺源与醛及1,3-二羰基化合物以无溶剂一锅煮的方法发生Hantzsch 反应,以较高产率得到了松香的改性产品;并且实验中以拼合原理,首次将松香和1,4-二氢吡啶两个活性亚结构拼接在一起,以期得到新的先导化合物。这对于充分利用松香这一天然资源,开发松香资源产品和研究松香深度加工方法具有一定的理论意义。有关合成路线如图1 所示。

图1 1,4-二氢吡啶的合成路线Fig.1 Synthetic route of 1,4-dihydropyridines
1 材料与方法
1.1 原料、试剂与仪器
歧化松香胺(工业级)购自广西梧州松脂厂,其他试剂均为国药集团分析纯。
XL 400MHz 核磁共振仪(德国Bruker 公司);Bruker Esquire 3000 质谱仪;PE-983 型红外光谱仪(KBr 压片,美国Nicolet 公司);Vario EL ⅢCHNSO元素分析仪(德国Elementar 公司);X-6 型显微熔点测定仪(北京泰克仪器有限公司)。
1.2 目标化合物的合成
取0.1 mol 芳香醛和0.22 mol 1,3-二羰基化合物,充分搅拌,然后加入0.1 mol 歧化松香胺,加热至80 ℃,搅拌回流2 h。冷却至室温,用大量蒸馏水冲洗3 次,固体用无水乙醇重结晶,有大量淡黄色结晶析出,抽滤,得产品a~n。
2 结果与分析
2.1 目标化合物a~n 的结构表征
对目标化合物进行了熔点测定、红外光谱、质谱、元素分析和核磁氢谱表征。物理化学常数见表1。
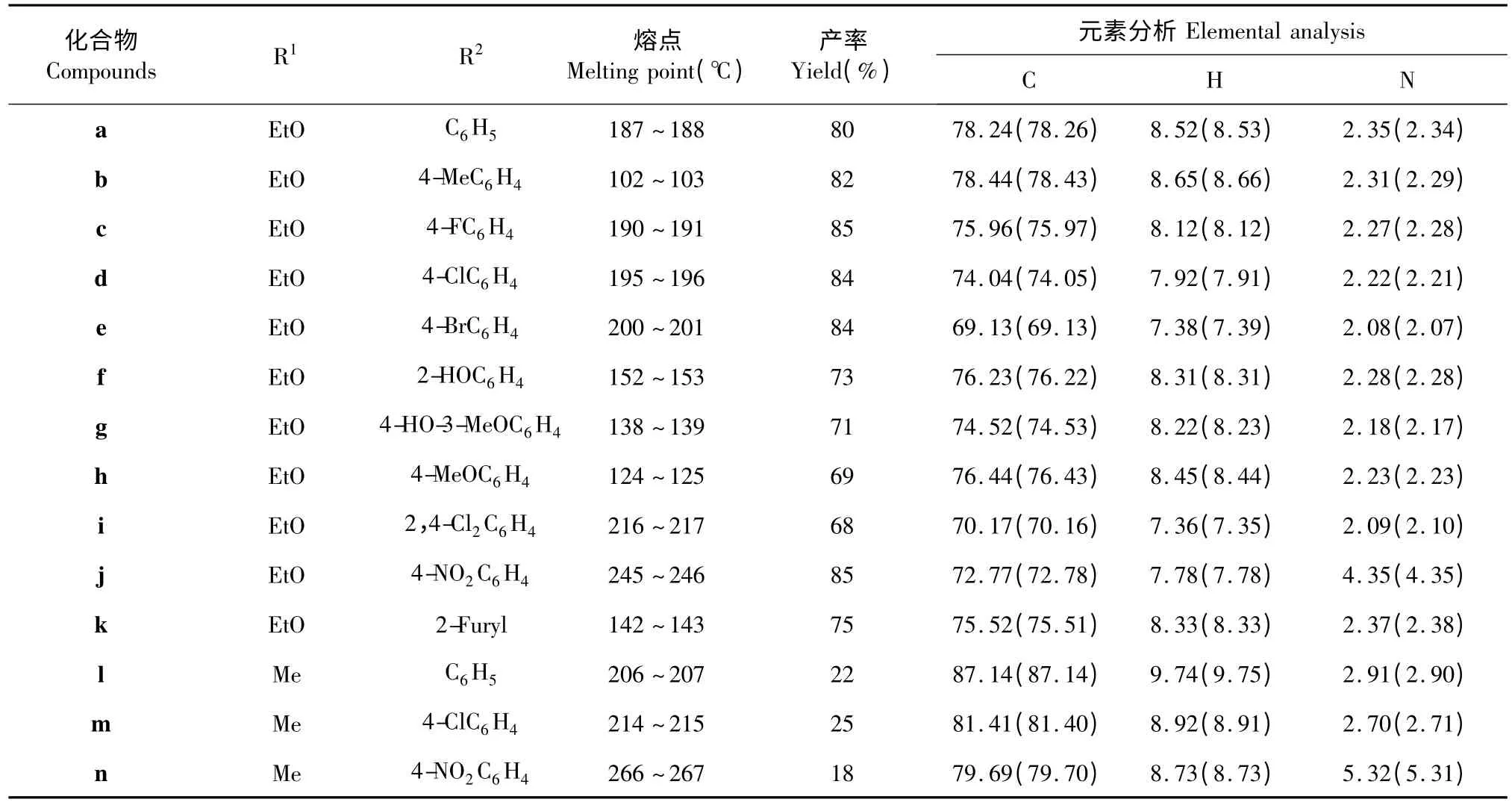
表1 化合物a~n 的结构及物理化学常数Table 1 Structure,physical constants and elemental analysis data of compounds a~n
化合物a 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.26~7.31 (2H,m,3,5-C6H5),7.21(1H,d,J=8.2 Hz,H-11),7.11~7.15 (3H,m,2,4,6-C6H5),7.02 (1H,d,J=8.2 Hz,H-12),6.87(1H,s,H-14),5.01 (1H,s,ArCH),4.05 (4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.41 (1H,d,J=12.4,H-1),2.34 (6H,s,C=CNCH3),1.68~1.96 (6H,m,H-2,H-3,H-5,H-6),1.52~1.55 (5H,m,H-1,H-6,H-19),1.32(6H,t,OMeCH3),1.25 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:599([M +H]+);IR (KBr)ν:2987,1635,1518,1496,1382,1361 cm-1。
化合物b 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.25~7.28 (2H,m,3,5-C6H4),7.21(1H,d,J=8.2 Hz,H-11),7.11~7.15 (2H,m,2,6-C6H4),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05 (4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),2.28 (3H,s,PhCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:613 ([M+H]+);IR(KBr)ν:2990,1636,1515,1493,1386,1364 cm-1。
化合物c 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.27~7.35 (4H,m,C6H4),7.21 (1H,d,J=8.2 Hz,H-11),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05(4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91(3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:617 ([M+H]+);IR(KBr)ν:2988,1634,1512,1493,1386,1363 cm-1。
化合物d 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.30~7.34 (4H,m,C6H4),7.21 (1H,d,J=8.2 Hz,H-11),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05(4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91(3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:633 ([M+H]+);IR(KBr)ν:2989,1638,1512,1497,1385,1366 cm-1。
化合物e 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.29~7.35 (4H,m,C6H4),7.21 (1H,d,J=8.2 Hz,H-11),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05(4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91(3H,m,H-7,H-15),2.38 (1H,d,J=12.4,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:678([M+H]+);IR(KBr)ν:2985,1633,1515,1496,1385,1365 cm-1。
化合物f 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.21 (1H,d,J=8.2 Hz,H-11),7.02(1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),6.70~6.82 (4H,m,C6H4),5.41 (1H,s,OH),5.01(1H,s,PhCH),4.05 (4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:615 ([M +H]+);IR(KBr)ν:2986,1638,1513,1496,1382,1365 cm-1。
化合物g 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.21 (1H,d,J=8.2 Hz,H-11),7.02(1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),6.79~6.82 (3H,m,C6H3),5.47 (1H,s,OH),5.01(1H,s,PhCH),4.05 (4H,q,OCH2),3.96 (2H,s,H-18),3.82 (3H,s,PhOCH3),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.26(3H,m,H-20),1.32 (6H,t,OMeCH3),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:645([M +H]+);IR(KBr)ν:2984,1632,1514,1493,1384,1365 cm-1。
化合物h 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.21 (1H,d,J=8.2 Hz,H-11),7.02(1H,d,J=8.2 Hz,H-12),6.71~6.80 (4H,m,C6H4),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05 (4H,q,OCH2),3.96 (2H,s,H-18),3.82(3H,s,PhOCH3),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:629 ([M +H]+);IR(KBr)ν:2982,1639,1513,1496,1384,1365 cm-1。
化合物i 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.21 (1H,d,J=8.2 Hz,H-11),7.12~7.17 (3H,m,C6H3),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05(4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91(3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:668 ([M+H]+);IR (KBr)ν:2985,1635,1514,1493,1384,1365 cm-1。
化合物j 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.71~7.79 (2H,m,3,5-C6H4),7.42~7.53 (2H,m,2,6-C6H4),7.21 (1H,d,J=8.2 Hz,H-11),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.05 (4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32(6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (d,6H,J=7.0 Hz,H-16,H-17);ESI-MS m/z:644 ([M+H]+);IR(KBr)ν:2983,1636,1519,1496,1387,1365 cm-1。
化合物k 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.26 (1H,s,5-Fural-H),7.21 (1H,d,J=8.2 Hz,H-11),7.02 (1H,d,J=8.2 Hz,H-12),6.87(1H,s,H-14),6.24 (1H,m,4-Fural-H),6.05 (1H,d,J=3.6 Hz,3-Fural-H),5.01 (1H,s,PhCH),4.05(4H,q,OCH2),3.96 (2H,s,H-18),2.72~2.91(3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.32 (6H,t,OMeCH3),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:589 ([M+H]+);IR (KBr)ν:2986,1634,1518,1496,1381,1365 cm-1。
化合物l 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.26~7.31 (2H,m,3,5-C6H5),7.21(1H,d,J=8.2 Hz,H-11),7.11~7.15 (3H,m,2,4,6-C6H5),7.02 (1H,d,J=8.2 Hz,H-12),6.87(1H,s,H-14),5.01 (1H,s,PhCH),4.23 (6H,s,OCH3),3.96 (2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.26(3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:483 ([M+H]+);IR (KBr)ν:2985,1634,1516,1492,1381,1365 cm-1。
化合物m 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.30~7.34 (2H,m,3,5-C6H4),7.21(1H,d,J=8.2 Hz,H-11),7.04~7.10 (2H,m,2,6-C6H4),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),3.96 (2H,s,H-18),5.01 (1H,s,PhCH),4.23 (6H,s,OCH3),3.96(2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.26 (3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESIMS m/z:517([M+H]+);IR(KBr)ν:2987,1638,1513,1498,1385,1365 cm-1。
化合物n 淡黄色固体,1H NMR (400 MHz,CDCl3)δ:7.71~7.79 (2H,m,3,5-C6H4),7.42~7.53 (2H,m,2,6-C6H4),7.21 (1H,d,J=8.2 Hz,H-11),7.02 (1H,d,J=8.2 Hz,H-12),6.87 (1H,s,H-14),5.01 (1H,s,PhCH),4.23 (6H,s,OCH3),3.96 (2H,s,H-18),2.72~2.91 (3H,m,H-7,H-15),2.38 (1H,d,J=12.4 Hz,H-1),2.34 (6H,s,C=CNCH3),1.68~1.95 (6H,m,H-6,H-2,H-3,H-5),1.51~1.55 (5H,m,H-1,H-6,H-19),1.26(3H,m,H-20),1.20 (6H,d,J=7.0 Hz,H-16,H-17);ESI-MS m/z:528 ([M+H]+);IR (KBr)ν:2983,1636,1519,1496,1387,1365 cm-1。
2.2 目标化合物的结构解析
目标化合物的IR 中出现苯环和歧化松香的特征伸缩震动吸收峰;1H NMR 中各氢的化学位移都有清晰的归属;质谱数据中分子离子峰和对应化合物的分子量相符;元素分析数值与计算值吻合良好。
3 结论
无溶剂条件下,以歧化松香胺、芳香醛和1,3-二羰基化合物为原料,一锅法合成了N-歧化松香基-1,4-二氢吡啶化合物。其中选乙酰乙酸乙酯为1,3-二羰基化合物合成时产率达到68%~85%。对合成的目标化合物均进行了元素分析、质谱、红外和核磁共振氢谱表征。
1 Chen GH (陈国华),Wang L (王丽),Yao XM (姚秀梅).Synthesis and biological activity of dihydropyridine calcium antagonists.Chin J Org Chem(有机化学),2010,30:997-1004.
2 Deng L (邓兰),Xu MX (徐鸣夏).Study on synthesis and Ca2+antagonizing activity of piperazine substituted new dihydropyridine derivatives.Chin J Pharm(中国医药工业杂志),2002,33:472-474.
3 Zeng RJ (曾荣今),Yao F (姚飞),Wang H (王慧),et al.Synthesis of novel 1,4-dihydropyridine tailed porphyrin compounds and investigation of their antibacterial activity.Chin J Org Chem (有机化学),2012,32:1270-1277.
4 Du JY (杜江燕),Wang BX (王炳详),Xu F (徐飞),et al.Voltammetric and circular dichroism studies on interaction between 1,4-dihydropyridine derivatives and DNA.Chem J Chin Univ(高等学校化学学报),2005,26:663-666.
5 Wu XY (伍小云),Hu AX (胡艾希),Xie YL (谢艳丽).Synthesis and characterization of 4-aryl-1,4-dihydropyridine-3,5-dicarboxylates.Chin J Org Chem (有机化学),2006,26:93-98.
6 Cai XH(蔡小华),Zhang GL(张国林).One-pot synthesis of dihydropyridines under solvent-free condition.Chin J Org Chem (有机化学),2006,26:93-98.
7 Liu DM (柳冬梅),Du LT (杜丽婷),Sun J (孙晶),et al.Efficient synthesis of alkylenedioxy-bridged bisdihydropyridines.Chin J Org Chem (有机化学),2008,28:930-933.
8 Tong BH (童碧海),Li FY (李芳耀),Pan YM (潘英明).Study progress of dehydroabietic acid modifications.Technol Dev Chem Ind (化工技术与开发),2004,33(6):20-27.
9 Mo QJ(莫启进),Duan WG (段文贵),Ma XL (马献力),et al.Syntheses and fungicidal activities of 2-sustituted acylamino-5-dehydroabietyl-1,3,4-thiadiazole derivatives.Chemistry (化学通报),2012,75:160-165.
10 Chen Y(陈泳),Lin ZX(林中祥).Bioactivity of resin acids and their derivatives.Nat Prod Res Dev (天然产物研究与开发),2009,21:712-718.

