三七二醇型皂苷元磺酰胺类衍生物的合成及抗肿瘤活性研究
蒲 洪 ,董成梅,邹 澄*,赵 庆,张莲卿,陈艳梅,赵沛基,胡建林
1昆明医科大学药学院暨云南省天然药物药理重点实验室;2 云南中医学院中药学院,昆明 650500;3中国科学院昆明植物研究所 植物化学与西部植物资源持续利用国家重点实验室,昆明 650204
三七二醇型皂苷是三七、人参的主要活性成分,其主要具有抗肿瘤、免疫调节、抗炎镇痛等作用[1]。皂苷经酸水解后主要得到人参二醇,碱水解后主要得到原人参二醇。已报道的脂肪酸类、氨基酸类人参二醇或者原人参二醇的衍生物中均有出现比人参二醇或者原人参二醇更有效的抗肿瘤活性化合物[2-5]。目前国内外对人参二醇类皂苷元的结构修饰主要集中在3 位羟基的酰化修饰,其它类型的结构修饰报道较少。为此笔者利用生物电子等排原理制备3 位氨基,而氨基制备的中间体是人参二醇类皂苷元的氧化产物,之前我们主要是通过先水解成人参二醇,再将3 位羟基氧化成羰基,这一方法需要两步反应,较麻烦。为此笔者试图通过琼斯氧化将三七二醇型皂苷的水解和苷元的氧化两步反应,缩短为一步反应完成,得到人参二醇的降解氧化产物。
本文经还原胺化反应将氧化物的3 位羰基转变成氨基,再与一些磺酰氯等试剂反应,得到一系列人参二醇降解氧化产物的磺酰胺类衍生物10 个,均为未见文献报道的化合物。用1H NMR、13C NMR、MS等鉴定这些化合物的结构,这12 个化合物采用MTS法对人白血病细胞株HL-60、肝癌细胞株SMMC-7721、肺癌细胞株A-549、乳腺癌细胞株MCF-7、结肠癌细胞株SW480 进行活性评价,期望找到一些活性更好的化合物。
1 仪器与材料
AV-500 核磁共振仪(美国Bruker 公司);LCQAdvantage LC-MS(Thermo Finnigan);RE-2000A 旋转蒸发仪(上海亚荣生化仪器厂);FA2004 电子天平(上海舜宇恒平科学仪器有限公司);DF-101S 集热式恒温加热磁力搅拌器(巩义市予华仪器有限公司);KQ-100 型超声清洗器(昆山市超声仪器有限公司);SHZ-D 循环水式真空泵(巩义市予华仪器有限公司)
GF254薄层层析板(青岛海洋化工厂);柱层析用硅胶(青岛海洋化工厂);高效薄层层析板(默克);三七二醇型皂苷(云南红云生物工程技术有限公司);化学合成试剂主要为优级纯,少量分析纯,均购买于上海晶纯实业有限公司(阿拉丁)和上海泰坦科技股份有限公司(阿达玛斯)。
2 实验方法
2.1 化合物1 的合成[6]
称取三七二醇型皂苷20 g,加入250 mL 蒸馏水充分溶解后加入200 mL 丙酮溶解均匀后,加入自制总量140 mL 琼斯试剂(分批加入),接上冷凝装置,置室温下搅拌反应4 h,减压蒸干反应液中的丙酮,加入100 mL 水后,乙酸乙酯萃取(300 mL ×3),合并萃取液,纯化水洗3 次,无水Na2SO4干燥,过滤,减压浓缩,得淡绿色稠状粗产品,经柱色谱纯化得化合物1(1.34 g)。白色粉末,产率6.7%;1H NMR(400 MHz,CDCl3)δ:0.76 (3H,s,H-18),1.03(3H,s,H-19),1.05 (3H,s,H-27),1.09 (3H,s,H-25),1.24(3H,s,H-26),1.24 (3H,s,H-21);13C NMR (CDCl3,100 MHz)δ:216.4 (s,C-3),209.8(s,C-12),176.7 (s,C-24),88.4 (s,C-20),56.7(d,C-13),55.8 (s,C-14),54.8 (d,C-5),53.4 (d,C-9),47.1 (s,C-4),42.5 (d,C-17),40.1 (s,C-8),38.9 (t,C-1),37.1 (t,C-11),33.6 (s,C-10),33.3 (t,C-7),32.8 (t,C-2),32.2 (t,C-22),31.4(t,C-15),28.7 (t,C-23),26.4 (t,C-16),24.7 (q,C-21),20.8 (q,C-25),20.8 (t,C-6),19.5 (q,C-26),16.2 (q,C-27),15.6 (q,C-18),15.2 (q,C-19);ESI-MS (m/z):429.3[M+H]+。
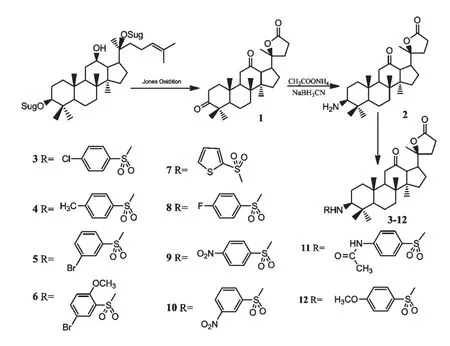
图1 化合物1~12 合成路线图Fig.1 Synthetic routes of compounds 1-12
2.2 化合物2 的合成[7,8]
在带有加热、搅拌、回流冷凝管等装置的50 mL二颈瓶中分别加入化合物1(300 mg,0.65 mmol)、过量的氰基硼氢化钠、过量的乙酸铵、甲醇25 mL,保温搅拌反应,24 h 后停止反应,减压蒸干甲醇,加入40 mL 水后,用乙酸乙酯萃取(100 mL ×3),合并萃取液,分别用饱和碳酸氢钠溶液、纯化水洗、饱和食盐水洗,无水Na2SO4干燥,过滤,滤液浓缩,经柱色谱纯化得化合物2 (142 mg)。白色粉末,产率为47.33%;1H NMR (400 MHz,CD3OD)δ:0.77 (3H,s,H-18),0.88 (3H,s,H-19),0.95 (3H,s,H-27),1.00 (3H,s,H-25),1.01 (3H,s,H-26),1.04 (3H,s,H-21),3.80~3.83 (1H,m,3-CH);13C NMR(CD3OD,100 MHz)δ:212.8 (s,C-12),179.6 (s,C-24),90.8 (s,C-20),64.3 (d,C-3),60.9 (d,C-13),57.9 (d,C-5),56.9 (s,C-14),55.5 (d,C-9),43.9 (d,C-17),40.2 (s,C-8),39.4 (t,C-1),39.1(t,C-11),38.1 (s,C-4),37.2 (s,C-10),33.9 (t,C-7),32.9 (t,C-22),31.4 (t,C-15),28.9 (t,C-23),28.1 (q,C-21),24.7 (q,C-25),24.7 (t,C-2),19.9 (t,C-6),18.9 (t,C-16),16.8 (q,C-26),16.2 (q,C-27),16.1 (q,C-18),15.9 (q,C-19);ESI-MS (m/z):430.9[M+H]+。
2.3 化合物3~12 的合成通法[9]
在带有加热、搅拌、回流冷凝管等装置的25 mL二颈瓶中分别加入化合物2(1eq,50 mg,0.12 mmol)、磺酰氯类试剂(6eq)、DMAP(3eq,44 mg)、无水吡啶8 mL,保温反应24 h 后,减压蒸干吡啶,加入30 mL 水后,用乙酸乙酯后萃取(50 mL ×3),合并萃取液,用10%盐酸洗3 次、饱和食盐水洗3 次、无水Na2SO4干燥,过滤,滤液浓缩,经柱色谱纯化得化合物3~12。
化合物3 白色粉末,产率为49.2%;1H NMR(500 MHz,CDCl3)δ:0.74 (3H,s,H-18),0.84(3H,s,H-19),0.85 (3H,s,H-27),1.17 (3H,s,H-25),1.24 (3H,s,H-26),1.42 (3H,s,H-21),4.40-4.42 (1H,m,3-CH),7.47 (1H,d,J=8.5 Hz,H-3',5'),7.80 (1H,d,J=8.5 Hz,H-2',6');13C NMR (CDCl3,125 MHz)δ:210.4 (s,C-12),177.0(s,C-24),139.7 (s,C-1'),138.9 (s,C-4'),129.3(d,C-3'),129.3 (d,C-5'),128.4 (d,C-2'),128.4(d,C-6'),88.6 (s,C-20),62.1 (d,C-3),56.7 (d,C-13),56.6 (d,C-5),55.9 (s,C-14),54.0 (d,C-9),42.6 (d,C-17),40.2 (s,C-8),39.4 (t,C-1),39.1 (t,C-11),38.1 (s,C-4),37.2 (s,C-10),33.9(t,C-7),32.3 (t,C-22),31.4 (t,C-15),28.9 (t,C-23),28.0 (q,C-21),25.9 (t,C-16),24.9 (q,C-25),24.1 (t,C-2),18.7 (t,C-6),16.3 (q,C-26),16.0 (q,C-27),15.8 (q,C-18),15.6 (q,C-19);ESI-MS (m/z):604.3[M+H]+。
化合物4 白色粉末,产率为34.7%;1H NMR(400 MHz,CDCl3)δ:0.74 (3H,s,H-18),0.85(3H,s,H-19),0.87 (3H,s,H-27),1.17 (3H,s,H-25),1.23 (3H,s,H-26),1.25 (3H,s,H-21),2.57(3H,s,H-Ar-CH3),4.14-4.16 (1H,m,3-CH),7.29(1H,d,J=8.0 Hz,H-2',6'),7.74 (1H,d,J=8.0 Hz,H-3',5');13C NMR (CDCl3,100 MHz)δ:210.5 (s,C-12),177.0 (s,C-24),143.2 (s,C-1'),138.1 (s,C-4'),129.6 (d,C-3'),129.6 (d,C-5'),126.9 (d,C-2'),126.9 (d,C-6'),88.7 (s,C-20),61.8 (d,C-3),56.7 (d,C-13),56.7 (d,C-5),55.9(s,C-14),54.1 (d,C-9),42.6 (d,C-17),40.2 (s,C-8),39.4 (t,C-1),39.1 (t,C-11),38.1 (s,C-4),37.2 (s,C-10),34.0 (t,C-7),32.3 (t,C-22),31.4(t,C-15),29.6 (t,C-23),28.9 (q,C-21),28.0 (t,C-16),24.8 (q,C-25),24.1 (t,C-2),21.5 (q,C-1″),18.7(t,C-6),16.3(q,C-26),16.0(q,C-27),15.8(q,C-18),15.6(q,C-19);ESI-MS (m/z):584.2[M+H]+。
化合物5 白色粉末,产率为49.6%;1H NMR(400 MHz,CDCl3)δ:0.74 (3H,s,H-18),0.85(3H,s,H-19),0.85 (3H,s,H-27),1.17 (3H,s,H-25),1.22 (3H,s,H-26),2.16 (3H,s,H-21),4.48-4.50 (1H,m,3-CH),7.38 (1H,t,J=6.3 Hz,H-5'),7.68 (1H,d,J=6.6 Hz,H-4'),7.78 (1H,d,J=6.3 Hz,H-6'),8.01 (1H,br.s,H-2');13C NMR(CDCl3,100 MHz)δ:211.3 (s,C-12),177.9 (s,C-24),144.0 (s,C-1'),136.3 (d,C-4'),131.4 (d,C-5'),130.7 (d,C-2'),126.2 (d,C-6'),123.8 (s,C-3'),89.5 (s,C-20),63.0 (d,C-3),57.6 (d,C-13),57.4 (d,C-5),56.8 (s,C-14),54.9 (d,C-9),43.5(d,C-17),41.1 (s,C-8),40.3 (t,C-1),39.9 (t,C-11),39.0 (s,C-4),38.1 (s,C-10),34.8 (t,C-7),33.2 (t,C-22),32.3 (t,C-15),29.7 (t,C-23),28.9(q,C-21),26.8 (t,C-16),25.7 (q,C-25),25.0 (t,C-2),19.6 (t,C-6),17.2 (q,C-26),16.9 (q,C-27),16.7 (q,C-18),16.5 (q,C-19);ESI-MS (m/z):648.2[M+H]+。
化合物6 白色粉末,产率为72.7%;1H NMR(400 MHz,CDCl3)δ:0.72 (3H,s,H-18),0.77(3H,s,H-19),0.86 (3H,s,H-27),0.93 (3H,s,H-25),1.17 (3H,s,H-26),1.24 (3H,s,H-21),3.96(3H,s,OCH3)4.66-4.68 (1H,m,3-CH),6.90(1H,d,J=4.4 Hz,H-3'),7.6 (1H,dd,J=3.4 Hz,6.9 Hz,H-4'),8.00 (1H,br.s,H-6');13C NMR(CDCl3,100 MHz)δ:210.4 (s,C-12),177.0 (s,C-24),155.0 (d,C-6'),136.8 (d,C-4'),132.2 (s,C-2'),130.7 (s,C-1'),113.8 (s,C-5'),112.8 (d,C-3'),88.6 (s,C-20),62.1 (d,C-3),56.7 (d,C-13),56.5 (d,C-5),56.4 (q,C-OCH3),55.9 (s,C-14),53.9 (d,C-9),42.6 (d,C-17),40.2 (s,C-8),39.4(t,C-1),38.9 (t,C-11),38.1 (s,C-4),37.2 (s,C-10),33.9 (t,C-7),32.3 (t,C-22),31.4 (t,C-15),28.8 (t,C-23),27.9 (q,C-21),25.1 (t,C-16),24.9 (q,C-25),24.1 (t,C-2),18.7 (t,C-6),16.5(q,C-26),16.3 (q,C-27),15.8 (q,C-18),15.6(q,C-19);ESI-MS (m/z):678.2[M+H]+。
化合物7 白色粉末,产率为25.4%;1H NMR(400 MHz,CDCl3)δ:0.75 (3H,s,H-18),0.87(3H,s,H-19),0.91 (3H,s,H-27),1.17 (3H,s,H-25),1.23 (3H,s,H-26),1.25 (3H,s,H-21),4.38-4.40 (1H,m,3-CH),7.07 (1H,dd,J=3.0 Hz,3.9 Hz,H-4'),2.94 (1H,d,J=2.94 Hz,H-3'),7.59(1H,d,J=2.1 Hz,H-5');13C NMR (CDCl3,100 MHz)δ:210.5 (s,C-12),177.0 (s,C-24),142.2(s,C-1'),131.7 (d,C-2'),131.6 (d,C-3'),127.3(d,C-4'),88.6 (s,C-20),62.3 (d,C-3),56.7 (d,C-13),56.7 (d,C-5),55.9 (s,C-14),54.1 (d,C-9),42.6 (d,C-17),40.2 (s,C-8),39.4 (t,C-1),39.1 (t,C-11),38.1 (s,C-4),37.3 (s,C-10),34.0(t,C-7),32.3 (t,C-22),31.4 (t,C-15),29.6 (t,C-23),28.9 (q,C-21),27.8 (t,C-16),24.9 (q,C-25),24.2 (t,C-2),18.7 (t,C-6),16.3 (q,C-26),16.1 (q,C-27),15.8 (q,C-18),15.6 (q,C-19);ESI-MS (m/z):576.3[M+H]+。
化合物8 白色粉末,产率为21.2%;1H NMR(400 MHz,CDCl3)δ:0.73 (3H,s,H-18),0.85(3H,s,H-19),0.88 (3H,s,H-27),1.17 (3H,s,H-25),1.22 (3H,s,H-26),1.27 (3H,s,H-21),4.47-4.49 (1H,m,3-CH),7.17 (1H,d,J=10.8 Hz,H-3',5'),7.88 (1H,d,J=10.8 Hz,H-2',6');13C NMR (CDCl3,100 MHz)δ:210.5 (s,C-12),177.0(s,C-24),166.1 (s,C-4'),137.3 (s,C-1'),129.6(d,C-2'),129.5 (d,C-6'),116.3 (d,C-3'),116.1(d,C-5'),88.7 (s,C-20),62.0 (d,C-3),56.7 (d,C-13),56.6 (d,C-5),55.9 (s,C-14),54.0 (d,C-9),42.6 (d,C-17),40.2 (s,C-8),39.4 (t,C-1),39.1 (t,C-11),38.1 (s,C-4),37.2 (s,C-10),33.9(t,C-7),32.3 (t,C-22),31.4 (t,C-15),29.6 (t,C-23),28.9 (q,C-21),28.0 (t,C-16),24.8 (q,C-25),24.1 (t,C-2),18.7 (t,C-6),16.3 (q,C-26),16.0 (q,C-27),15.8 (q,C-18),15.6 (q,C-19);ESI-MS (m/z):588.3[M+H]+。
化合物9 淡黄色粉末,产率为48.3%;1H NMR (400 MHz,CDCl3)δ:0.68 (3H,s,H-18),0.78 (3H,s,H-19),0.78 (3H,s,H-27),1.01 (3H,s,H-25),1.14 (3H,s,H-26),1.17 (3H,s,H-21),4.02-4.04 (1H,m,3-CH),7.97 (1H,d,J=1.5 Hz,H-2',6'),8.26 (1H,d,J=1.5 Hz,H-3',5');13C NMR (CDCl3,100 MHz)δ:211.1 (s,C-12),177.6 (s,C-24),149.6 (s,C-4'),147.5 (s,C-1'),128.0 (d,C-2'),128.0 (d,C-6'),124.1 (d,C-3'),124.1 (d,C-5'),89.1 (s,C-20),62.2 (d,C-3),56.7 (d,C-13),56.5 (d,C-5),55.9 (s,C-14),54.0 (d,C-9),42.5 (d,C-17),40.1 (s,C-8),39.3(t,C-1),39.0 (t,C-11),38.2 (s,C-4),37.2 (s,C-10),33.8 (t,C-7),32.1 (t,C-22),31.3 (t,C-15),29.5 (t,C-23),28.8 (q,C-21),28.0 (t,C-16),24.6 (q,C-25),24.1 (t,C-2),18.6 (t,C-6),16.2(q,C-26),15.8 (q,C-27),15.7 (q,C-18),15.4(q,C-19);ESI-MS (m/z):615.3[M+H]+。
化合物10 淡黄色粉末,产率为46.9%;1H NMR (500 MHz,CDCl3)δ:0.74 (3H,s,H-18),0.76 (3H,s,H-19),0.81 (3H,s,H-27),0.86 (3H,s,H-25),1.17 (3H,s,H-26),1.22 (3H,s,H-21),4.57-4.59 (1H,m,3-CH),7.73 (1H,t,J=8.0 Hz,H-5'),8.19 (1H,d,J=7.8 Hz,H-4'),8.42(1H,d,J=8.0 Hz,H-6'),8.72 (1H,s,H-2');13C NMR (CDCl3,125 MHz)δ:210.2 (s,C-12),176.9(s,C-24),148.3 (s,C-3'),143.6 (s,C-1'),132.4(d,C-6'),130.4 (d,C-5'),126.9 (d,C-4'),122.2(d,C-2'),88.6 (s,C-20),62.5 (d,C-3),56.8 (d,C-13),56.6 (d,C-5),55.9 (s,C-14),54.0 (d,C-9),42.7 (d,C-17),40.3 (s,C-8),39.4 (t,C-1),39.0 (t,C-11),38.2 (s,C-4),37.3 (s,C-10),34.0(t,C-7),32.3 (t,C-22),31.5 (t,C-15),28.9 (t,C-23),28.1 (q,C-21),26.3 (t,C-16),24.9 (q,C-25),24.2 (t,C-2),18.7 (t,C-6),16.4 (q,C-26),16.0 (q,C-27),15.8 (q,C-18),15.6 (q,C-19);ESI-MS (m/z):1251.3[2M+Na]+。
化合物11 白色粉末,产率为61.7%;1H NMR(500 MHz,C5D5N3)δ:0.76 (3H,s,H-18),0.78(3H,s,H-19),0.83 (3H,s,H-27),1.03 (3H,s,H-25),1.19 (3H,s,H-26),1.28 (3H,s,H-21),2.17(3H,s,COCH3),3.17-3.19 (1H,m,3-CH),8.49(1H,d,J=9.5 Hz,H-2',6'),8.18 (1H,d,J=9.5 Hz,H-3',5'),11.21 (1H,s,NHCOCH3);13C NMR (C5D5N3,125 MHz)δ:209.9 (s,C-12),176.8(s,C-24),169.5 (s,NHCOCH3),143.9 (s,C-4'),137.4 (s,C-1'),128.5 (d,C-2'),128.5 (d,C-6'),119.4 (d,C-3'),119.4 (d,C-5'),88.5 (s,C-20),62.2 (d,C-3),57.0 (d,C-13),56.7 (d,C-5),55.9(s,C-14),54.2 (d,C-9),43.0 (d,C-17),40.5 (s,C-8),39.6 (t,C-1),39.4 (t,C-11),38.8 (s,C-4),37.5 (s,C-10),34.3 (t,C-7),32.5 (t,C-22),31.7(t,C-15),29.2 (t,C-23),28.6 (q,C-21),25.5 (t,C-16),24.6 (q,C-25),24.6 (t,C-2),24.3 (q,COCH3),19.1 (t,C-6),16.9 (q,C-26),16.5 (q,C-27),16.0 (q,C-18),15.4 (q,C-19);ESI-MS (m/z):1275.4[2M+Na]+。
化合物12 白色粉末,产率为51.8%;1H NMR(500 MHz,CDCl3)δ:0.71 (3H,s,H-18),0.71(3H,s,H-19),0.83 (3H,s,H-27),0.85 (3H,s,H-25),1.14 (3H,s,H-26),1.20 (3H,s,H-21),3.84(3H,s,OCH3),4.59-4.61 (1H,m,3-CH),7.77(1H,d,J=8.8 Hz,H-2',6'),6.94 (1H,d,J=8.8 Hz,H-3',5');13C NMR (CDCl3,125 MHz)δ:210.5 (s,C-12),177.0 (s,C-24),162.6 (s,C-4'),132.8 (s,C-1'),129.0 (d,C-2'),128.9 (d,C-6'),114.0 (d,C-3'),114.0 (d,C-5'),88.7 (s,C-20),61.8 (d,C-3),56.7 (d,C-13),56.7 (d,C-5),55.9(s,C-14),55.5 (q,OCH3),54.1 (d,C-9),42.6(d,C-17),40.2 (s,C-8),39.4 (t,C-1),39.1 (t,C-11),38.1 (s,C-4),37.2 (s,C-10),34.0 (t,C-7),32.3 (t,C-22),31.4 (t,C-15),28.9 (t,C-23),28.0(q,C-21),25.5 (t,C-16),24.8 (q,C-25),24.1 (t,C-2),18.7 (t,C-6),16.3 (q,C-26),16.0 (q,C-27),15.8 (q,C-18),15.6 (q,C-19);ESI-MS (m/z):600.3[M+H]+。
3 MTS 法抗肿瘤活性筛选
采用MTS 法对合成的12 个化合物进行体外抗肿瘤细胞株人白血病细胞株(HL-60)、肝癌细胞株(SMMC-7721)、肺癌细胞株(A-549)、乳腺癌细胞株(MCF-7)、结肠癌细胞株(SW480)的生物活性筛选,以顺铂和紫杉醇作为阳性对照(实验结果见表1)。
实验方法:用含10% 胎牛血清的培养液(DMEM 或者RMPI1640)配成单个细胞悬液,以每孔5000~10000 个细胞接种到96 孔板,每孔体积100 μL,贴壁细胞提前12 h 接种培养;加入待测化合物溶液(固定浓度40 μM 初筛,在该浓度对肿瘤细胞生长抑制达到50%的化合物设5 个浓度进入梯度复筛),每孔终体积200 μL,每种处理均设3 个复孔;37oC 培养48h 后,小心吸弃孔内培养上清液,每孔加MTS 溶液20 μL 以及培养液100 μL,继续孵育4 h,使反应充分进行;选择490 nm 波长,酶联免疫检测仪(Bio-Rad 680)读取各孔光吸收值,记录结果,以浓度为横坐标,细胞存活率为纵坐标绘制细胞生长曲线,应用两点法Reed and Muench 法)计算化合物的IC50值。

表1 化合物1~12 对人肿瘤细胞的半数抑制浓度IC50(μM)Table 1 The IC50values of compounds 1-12 on human cancer cell lines (μM)
4 讨论
本研究对三七二醇型皂苷氧化降解产物进行结构修饰,经还原胺化反应将3 位羰基转变为氨基,再进行磺酰化反应得到一系列磺酰胺类衍生物。细胞毒性实验结果表明,所得的化合物9 对SMMC-7721、A-549 细胞增殖有一定的抑制活性,可能与分子中含有硝基有关,因为硝基是一类NO 供体化合物,而高浓度的一氧化氮(NO)能够抑制肿瘤细胞的生长[11];化合物1 与经转化成氨基后的化合物2 显示无活性,可能与人参二醇的六元醚环被破坏,变成五元内酯环有关。这些实验结果为人参二醇的结构改造和构效关系的进一步探讨提供了一定依据,为进一步寻找更加理想抗肿瘤的人参二醇衍生物提供了思路。
1 Liu XK,Ye BJ,Wu Y,et al.Synthesis and anti-tumor evaluation of panaxadiol derivatives.Eu J Med Chem,2011,46:1997-2002.
2 Zhang CH(张春红),Zhang LX(张连学),Li XG(李向高),et al.Primary research on anti-tumor activity of panaxadiol fatty acid esters.J Chin Med Mater (中药材),2006,11:1200-1203.
3 Wei Y,Ma CM,Hattori M.Synthesis of dammarane-type triterpene derivatives and their ability to inhibit HIV and HCV proteases.Bioorgan Med Chem,2009,17:3003-3010.
4 Zhang CH(张春红),Li XG(李向高),Zhang LX(张连学),et al.Preliminary results of anti-cancer activity comparison of panaxadiol derivatives.Chin J Nat Med(中国天然药物),2004,6:369-371.
5 Bi Y,Tian JW,Wang L,et al.Synthesis,structural determination and protective effects on cultured anoxia/reoxygen injury myocardiocytes of ocotillol-type derivatives.J Med Plant Res,2011,5:2424-2429.
6 Shao RF(邵日凤),Zhao Q(赵庆),Zou C(邹澄),et al.Jones’oxidation of protopanaxadiol type crude saponin from Panax notoginseng.Chin J Ethnomed Ethnopharm (中国民族民间医药杂志),2009,17:33-34.
7 Csuk R,Schwarz S,Siewert B,et al.Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives.Eu J Med Chem,2011,46:5356-5369.
8 Nie W,Luo JG,Wang XB,et al.Synthesis of new a-Glucosidase inhibitors based on oleanolic acid incorporating cinnamic amides.Chem Phar Bull,2011,53:1051-1056.
9 Xu GY(许国友),Peng SX(彭司勋),Hua WY(华维一).Synthesis of N-Aeylated/Sulphonylated tetrahydro-isoquinoline compounds.J China Pharm Univ (中国药科大学学报),1993,24:1-6.
10 Deng X,Kong LM,Zhao Y,et al.Exploring of drug leads from diversity-oriented Michael-acceptor library derived from natural products.Nat Prod Bioprospect,2012,2:210-216.
11 Xie KP,Huang SY.Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency.Free Radic Biol Med,2003,34:969-986.

