The Uptake and Membrane Transport of Cesium in Human Erythrocytes
LU Jing HUANG Jian-Feng(Key Laboratory of Auxiliary Chemistry&Technology for Chemical Industry,Ministry of Education,Shaanxi University of Science&Technology,Xi′an 710021)
The Uptake and Membrane Transport of Cesium in Human Erythrocytes
LU Jing*HUANG Jian-Feng
(Key Laboratory of Auxiliary Chemistry&Technology for Chemical Industry,Ministry of Education,Shaanxi University of Science&Technology,Xi′an 710021)
The uptake of Cs+by human erythrocytes in vitro was investigated via atomic absorption spectrometry.The results indicate that the metal ions concentration,incubation temperature,incubation hours and media pH all have a positive effect on this cellular uptake process.The mechanism of Cs+transport into human erythrocytes was briefly discussed.There are mainly four pathways involve in the Cs+transmembrane activity as far as we concerned:(1)an active transport system mediated by the Na+/K+-ATPase;(2)“leak” process through pores in the membrane proteins;(3)Na+-Cs+countertransport systems;(4)Cl-/CsCO3-exchanger pathway stimulated by bicarbonate through anion channel(band 3 protein).No inhibition but a little stimulation by Nifedpine in the Cs+uptake demonstrates that the transport of Cs+through Ca2+channel is unavailable.
cesium;human erythrocytes;uptake;membrane transport
0 Introduction
Cesium plays an important role in many biological and chemical circulations in nature,and related closely with some food chain of ecological systems both in water and on land[1].Although Cs+occurs in the body at trace levels only,it was reported that Cs+takes a necessary part in some physiological processes.For example,cesium is found to be able to induce the self-assembly of protein clusters[2]and can effect charge translocation by the Na+/K+-ATPase[3].On the other hand,it is well known that cesium is one of toxic metals,and its toxicity depends on the accumulation in cells.The administration of high doses of cesium underexperimental conditions in mammals can cause ectopic ventricular beats, ventricular tachyarrhythmias,ventricularfibrillation,yperkalemia,and profound systolic and diastolic hypertension[4-5].It is inferred that the toxicity of cesium lies in that it can partly take the site instead of potassium in cell,and results in the damage ofsome activity orfunction in organism.For example,the accumulation of cesium in organism may induce falling sickness of cerebral nerve,because Cs+blocks the K+channel of glia,and the abnormal accumulation of extracellular K+cause the epilepsy[6-8].
There have been some studies carried out to investigate the interaction between cesium and biomembranes.Dynamic research of CsCl transportation in human erythrocytes at 38℃showed that if alkali ions in the ion channel of the cell membrane are separated by theirelectric-current-induced inward flows against an electro-osmotic outward flow of water,the logarithms of the stationary cell/medium distributions of these ions should be proportional to the inverse of their diffusion mobilities[9].After acute and chronic administrations,cesium can be widely found in the body,with differential organ distribution and apparent concentration in the liver and blood[10].The trans-erythrocyte membrane behavior of three organocesium compounds revealed that the saturated kinetics characteristic appeared from cesium aspartate,cesium 3,5-dinitrosalicylate and cesium 5-aminotetrazole[11].However,there is still few papers can be found to deal with the uptake process and transporting mechanism offree Cs+in plasma membrane.
In this paper,the cellular uptake of Cs+in human erythrocytes was mainly investigated under varies incubation conditions in vitro.As well as five primary pathways of Cs+across human erythrocytes membranes were briefly concerned.It is expected to provide detailed information about the cellular uptake ability and characteristics for rare alkali metal ions,which help us to know more about the transmembrane mechanism of cesium in human erythrocytes.Furthermore,the overall results may also help to understand the cesium-induced functional changes in or ganisms.
1 Experimental
1.1 Materials
Human red blood cells,provided by the Red Cross Blood Center,Xi′an,China;ouabain,choline chloride,and 4,4-diisothiocyanatostilbene-2,2-disulfonic acid disodium salt hydrate(DIDS),obtained from Sigma;Nifedipine,from Zhejiang anglikang medicine Crop.China;CsCl(mass fraction≥0.999),made from Cs2CO3in our laboratory, and the purification and determination has been described elsewhere[12].Other reagentsare allanalyticalgrade,obtained from Shanghai Chem.Co.Deionized and doubly distilled water was used throughout this work.
1.2 Preparation of human erythrocytes
Freshly drawn blood from healthy human donors was treated by centrifugation(2 500 r·min-1,5 min)at 4℃.Plasma and buffy coat were aspirated and the red blood cells were washed three times with a buffer“A”containing 130 mmol·L-1NaCl,10 mmol·L-1glucose,and 20 mmol·L-1Tris,the pH of this solution is controlled at7.4.Two different hematocrits of erythrocyte suspensions were prepared with buffer“A”.The 3%hematocrit suspension was used for hemolysis examination,while the 50%hematocrit suspension was for transmembrane determination.
1.3 Hemolysis examination
The 3%erythrocytes suspension was incubated in different concentrations of CsCl isotonic solution at 37℃for 1.5 h.After incubation and centrifugation,the supernatant was pulled out to test the absorbance at 540nm by a 721 spectrophotometer(Shanghai No.3 Analytical Instrument Factory)and expressed as A540.Meanwhile, A0represented the absorbance of supernatant obtained from erythrocytes incubated with pure buffer “A”(ce=0 mol·L-1);A100%represented the absorbance of completely hemolysis sample which came from erythrocytes incubated in doubly distilled water.
1.4 Measurement of Cs+uptake
The erythrocytes suspension was incubated with different amount of Cs+at 33,35,37,39,41,43℃respectively for 1.5 h.To keep the solution osmolarity,differentconcentration ofCsClwere added in replacement of NaCl.After incubation,the suspensions were put into an ice bath to stop the entry of Cs+for 1 min,then centrifuged at 2 500 r·min-1immediately[13].The pellets were washed five times with chilly buffer to remove extracellular Cs+,followed by diluting with deionized water at 4℃overnight.After centrifugation at 10 000 r·min-1for 30 mins at 4 ℃ by Sigma 3K-18 centrifuge, the supernatant was used for the determination of Cs+uptake amount by TAS-986 atomic absorption spectrometry(Beijing Purkinje general instrument CO.,LTD.).
The influence of media pH on the Cs+uptake amount was investigated from 6.2 to 9.2 with the initial extracellular Cs+concentration ce=6 mmol·L-1,and erythrocytes suspension-Cs+mixture incubated at 37℃.The influence of incubation hours was examined at time intervals of 1.5,2,3,5,8,12,16,18 h,and with ce=6 mmol·L-1incubated at 37℃.
1.5 Inhibition experiment
In order to know the elementary mechanism of Cs+transport in human erythrocytes,five transmembrane pathways were considered to be investigated.Ouabain,DIDS and Nifedipine were added in the sample respectively.For testing Na+-Cs+countertransport system,Choline chloride was isotonically replaced NaCl to form a no Na+-exist media.In the experiments where NaHCO3was required,the solution was kept being gassed with 5%CO2.The inhibitors were pre-incubated at 37℃for 30 min before mixed with erythrocytes-Cs+suspension.
2 Results and discussion
2.1 Hemolysis
As a determination method of metal toxicity,Hemolysis is generally examined by the release of hemoglobin(Hb)form erythrocytes at its characteristic absorption of 540 nm spectrophotometrically.Table 1 shows that all of the A0and A540are very little and much less than A100%.With the extracellular concentration of Cs+increased,the A540which came from different samples appeared almost unchanged.Cs+isotonic solution has very weak toxicity which could hardly induce hemolysis.It suggested that red blood cells can perform normal state within the Cs+isotonic solution.Therefore,the intracellular Cs+we detected should be transported through the membrane by particular ways.

Table 1 Effect of different extracellular concentrations of Cs+on hemolysis indicated by the supernatant absorbance
2.2 Effects on Cs+uptake
The effect of incubation temperature on the uptake of Cs+by human erythrocytes was tested at 2℃intervals from 33 to 43 ℃ at each(from 0.3 mmol·L-1to 90 mmol·L-1),just shown as Fig.1.The Cs+uptake amount increased linearly with the temperature rising,and the slope of increasing line became bigger at high concentrations.It indicates that high temperature can facilitate the Cs+uptake,and this positive effect appears more obvious at the higher extracellular Cs+concentration area.
The plasma pH ranging from 6.9 to 7.8 is considered to be the endurable extent for normal functions.Erythrocytes will be damaged when the plasma pH out of this extent.Fig.2 shows the dependence of Cs+uptake on media pH at different extracellular Cs+concentrations.The Cs+uptake increased almost linearly with the increase of pH value at lower Cs+concentrations(0.000 3 to 0.03 mol·L-1),however,the dependence became more sensitive when ceat 0.06 and 0.09 mol·L-1.At these two concentration levels,the Cs+uptake amount grew smoothly with pH increase from 6.0 to 7.5 at first,then became much sharper during 7.5 to 8.5,and kept smooth increase again from 8.5 to 9.4.The effect at higher pH value was not examined.
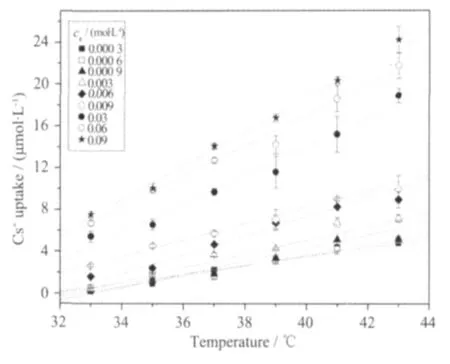
Fig.1 Effect of incubation temperature on the uptake of Cs+by human erythrocytes

Fig.2 Effect of media pH on the uptake of Cs+by human erythrocytes
The dependence ofCs+uptake amounton incubation hours was investigated from 1.5 to 18 h.Fig.3 shows that the Cs+uptake increased with incubation time and reached a maximum at 8 h.After that,the intracellular Cs+concentration almost unchanged and became a constant in the rest hours.No obvious hemolysis was found and it could be postulated that erythrocytes membrane were not damaged throughout the 18 incubation hours.This result is tentatively ascribed to saturated kinetics characteristic of cellular uptake in vitro.

Fig.3 Effect of incubation hours on the uptake of Cs+by human erythrocytes
2.3 Primary Pathways of Cs+transport across the erythrocytes membrane
Ions transport through biomembrane is mainly mediated by two ways:passive transport and active transport.For metal ions,it is difficult to transport across the biomembrane by simple diffusion,because the lipid bilayers is very hydrophobic.The saturated kinetics characteristic(Fig.3)for the uptake of Cs+found in our work also verified that the human erythrocytes membrane is not permeable for metal ions.
Based on the previous investigation on cesium uptake by human red blood cells,five possible mechanisms were investigated which involved in this transportprocess:Na+-K+pump,the Na+-Cs+countertransport, “leak”pathway,anion Cl-/HCO3-exchanger system and the Ca2+channel.
2.3.1 Na+-K+pump
The Na+-K+-ATPase is responsible for the energydependent membrane transport of Na+and K+.To characterize the importance of Na+-K+-ATPase on the membrane transport of cesium in human erythrocytes,Cs+uptake amount was measured from samples in absence(control)and presence of 0.1mmol·L-1ouabain.Just as expected,Fig.4.Ⅰshows that ouabain inhibited the Cs+influx significantly(a>b),which proves that the Na+-K+-ATPase pump can transport Cs+across the erythrocytes membrane.It is assumed that Cs+could take the binding site of Na+-K+-ATPase throughout the membrane instead of K+shown as Fig.5.This is why Cs+can compete with K+in the transmembrane process andpossibly destroy the function of organisms.

Fig.4 Characterization of Cs+transmembrane machanism

Fig.5 Membrane transport of Cs+by Na+/K+-ATPase
2.3.2 Na+-Cs+countertransport and “leak”
The Na+-Li+countertransport system has been confirmed several years before.The mechanism has been explained that the countertransportcan be mediated by a “porter”,which can cross the membrane if it has linked Na+or Li+from each side of the membrane,and then can go back again to the starting point[14].We are interested in that whether there is a countertransport system for Cs+entering into cells.Fig.4.I shows that the Cs+uptake in the media containing choline chloride is much higher than the uptake of control sample(c>a).The erythrocytes tend to keep the Na+concentration balance between the different side of membrane when the choline chloride instead of NaCl in the media.So it is assumed that Cs+enters into the cell by countertransportor in exchange of intracellular Na+.This exchange system is passive in nature and independent of energy supply by ATP.The transport mechanism is similar to that of Na+-Li+countertransportand, so we call it Na+-Cs+countertransport system.
When in the media with ouabain in present,there is no Na+can be transferred possibly,the cellular uptake decreased a lot but still a little higher than that of corresponding sample in Na+-exist media(b>d).The difference is quite small in compare with the massive effectofactivetransportway.Erythrocytesafter incubation were viable and no obvious hemolysis was found.So it is postulated that a little part of Cs+influx is probably through some pores in the membrane protein but not the damaged membrane that induced by Cs+.The newly discovered Cs+uptake is assigned as “leak”mechanism,which represents passive permeation,probably through some pores lie in membrane proteins.
2.3.3 The anion Cl-/HCO3-exchanger system
The anion Cl-/HCO3-exchangers(AE)belongs to a multigenic family that comprises AE1,AE2,and AE3.The AEs are ubiquitously expressed in vertebrate tissues[15-16].AE1 is expressed in erythrocytes and encodes the Band3 protein,a major membrane protein that has been extensively studied[17].DIDS could work as a specific inhibitor of Cl-/HCO3-ions transport system.
Fig.4.Ⅱshows that NaHCO3can promote the intracellular Cs+concentration(e<g),whereas DIDS can inhibit the Cs+uptake for a certain extent(f<g).5%CO2was gassed into samples throughout the experiments in order to keep the existence of HCO3-and proper pH value.The results demonstrate that cesium,as otheralkali metals,may be transported via band 3 protein pathway in the presence of
The promotion effectofbicarbonate on the transportofcesium acrosshuman red cellwas investigated(Fig.4.Ⅱ ).It was proposed that the increased Cs+uptake amount is due to the ability of carbonate to form ion pairs with cesium.This negatively charged ion pairs,CsCO3-,can be transported through the membrane by a specific anion exchange system,the band 3 protein.This possible pathway for Cs+has also been proved by the results from pH effect examination(Fig.2).Generally,it is considered that a certain group of protein band 3 will be converted into proton pattern when pH<7,this proton pattern make band 3 lose its activity and difficult for monovalent anion transporting.When pH>7,protein band 3 take a positive charge and is benefit for anion movement[18].It is well known that the acidic media cause K+efflux from cytoplast,whereas,alkaline media have an opposite effect.Fig.2 shows that the effect of media pH on Cs+transport is similar to that of K+,which proved that cesium could mimic the functional role of potassium or compete with it in the metabolic process.This deduction can help us to understand the toxicity of cesium in microcirculations in organism.
2.3.4 The Ca2+channel
Whether the calcium channel can contribute to the cellular uptake of Cs+was investigated by examining the effect of calcium channel blocker Nifedipine on Cs+membrane transport.It appears that Nifedipine has no inhibition but even a little stimulation on Cs+influx(h<i),shown as Fig.4.Ⅲ.So the Ca2+channel is probably prohibited the transport of Cs+.The uptake of Cs+increased a bit in the presence of Nifedipine is quite abnormal.The phenomena may relate to the Na+/Ca2+exchange system or Ca2+-dependent K+-channel.The additional studies are needed to better understand the effect.
3 Conclusion
With the help of atomic absorption spectrometry,the uptake of Cs+by human erythrocyte and their transmembrane mechanisms were systematically investigated in vitro.The effects of extracellular Cs+concentration(0.3~90 mmol·L-1),incubation temperature(33~43 ℃),incubation time(1.5~18 h),and medium pH(6.2~9.2)on the cellular uptake were studied.The results show that every mentioned factor has a positive effect on the uptake of Cs+by human erythrocyte.
Most of extracellular Cs+were transported into erythrocytes by Na+/K+-ATPase,however,the transport of Cs+through Ca2+channel is unavailable.The following transmembrane pathways for Cs+were also found by our experiments: (1)Na+/Cs+-countertransport systems;(2)Cl-/CsCO3-exchanger pathway stimulated by bicarbonate through anion channel(band 3 protein);(3)“leak” process through pores in the membrane.
[1]Wang J L.Nucl.Technol.,2003,26:949-955
[2]Chaput J C,Switzer C.Proc.Nat.Acad.Sci.U.S.A.,1999.96:10614-10619
[3]Pintschovius J,Fendler K,Bamberg E.Biophys.J,1999,76:827-836
[4]Bratter P,Gawlik D,Klingbeil P,et al.Metal Ions in Biology and Medicine.Paris:John Libbey Eurotext,1998.309-314
[5]Hahn F F,Muggenburg B A,Boecker B B.Toxicol.Pathol.,1996,24:281-289
[6]Xiong Z Q,Stringer J L.J.Neurophys.,1999,82(6):3339-3346
[7]Susarla S,Collette T W,Garrison A W,et al.Environ.Sci.Technol.,2000,34(1):224-224
[8]Cowan J A.Chem.Rev.,1998,98:1067-1088
[9]SimoS,AarneE,JussiR.Eur.Biophys.J.,2000,29(7):464-471
[10]Centeno J A,Pestaner J P,Omalu B I,et al.Biol.Trace Elem.Res.,2003,94:97-104
[11]FENG Yun-Xiao(冯云晓),JIANG Yu-Cheng(蒋育澄),HU Man-Cheng(胡 满 成),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(3):421-426
[12]Jiang Y C,Gao S Y,Hu M C,et al.Chem.Anal.,2003,48:37-44
[13]Yang X G,Wang K,Lu J F,et al.Coord.Chem.Rev.,2003,237:103-111
[14]Romano L,Battaglia M,Cordi R,et al.Biochem.Biophys.Res.Commun.,1995,210:119-125
[15]Alper S L.Annu.Rev.Physiol.,1991,53:549-564
[16]Kay M M,Cover C,Schluter S F,et al.Cell Mol.Biol.,1995,41:833-842
[17]Wang D N.FEBS Lett.,1994,346:26-31
[18]Shi Z Y,Chen J W,Huang F.Acta Biophys.Sin.,1994,10(4):243-250
重稀碱金属Cs+跨人红细胞膜行为的研究
卢 靖*黄剑锋
(陕西科技大学教育部轻化工助剂化学与技术重点实验室,西安 710021)
采用原子吸收光谱法检测体外人红细胞摄取Cs+的含量,系统讨论了胞外Cs+浓度,温育时间、温育温度、介质pH值对人红细胞摄取Cs+过程的影响。选用不同离子通道或离子载体的特异性抑制剂进一步探讨Cs+的跨膜途径和机理。结果显示,各实验参数对人红细胞摄取Cs+均有一定的促进作用。Cs+主要借助Na+/K+-泵的主动运输方式跨膜;少量的Cs+能“漏入”细胞,微量的Cs+可以模拟Na+/Li+-反向协同运输的方式跨膜;在允许HCO3-存在的pH环境下,少量Cs+以Cl-/CsCO3-交换的形式通过膜上带3蛋白进入人红细胞;Ca2+通道对Cs+没有通透作用。
铯;人红细胞;摄取;膜运输
O614.115;Q241
:A
:1001-4861(2010)08-1349-06
2009-12-07。收修改稿日期:2010-04-10。
卢 靖,女,28岁,讲师;研究方向:材料物理与化学。
国家自然科学基金(No.50772063)和陕西科技大学研究生创新基金资助项目。
*通讯联系人。 E-mail:lujing@sust.edu.cn
- 无机化学学报的其它文章
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties

