Synthesis of a New Cu(Ⅱ)Complex with in situ Generated 3-Hydroxy-2,4,6-pyridinetricarboxylate Ligand and Analysis of the Reaction Mechanism
LI Cui-Jin GUO Qing-Bing YING Guo-Qiang ZHOU Hong-Jun(Department of Chemistry and Chemical Engineering,Zhongkai University of Agriculture and Engineering,Guangzhou 510225,China)
Synthesis of a New Cu(Ⅱ)Complex with in situ Generated 3-Hydroxy-2,4,6-pyridinetricarboxylate Ligand and Analysis of the Reaction Mechanism
LI Cui-Jin*GUO Qing-Bing YING Guo-Qiang ZHOU Hong-Jun
(Department of Chemistry and Chemical Engineering,Zhongkai University of Agriculture and Engineering,Guangzhou 510225,China)
A new Cu(Ⅱ) complex[Cu2(2,4,6-opyta)(H2O)2]·2H2O wasprepared hydrothermally through 2,4,6-trimethylpyridine(2,4,6-tmpy)and Cu(NO3)2,where 2,4,6-opyta3-is 3-hydroxy-2,4,6-pyridinetricarboxylate.X-ray analysis indicates that Cu1 and Cu2 centers in this complex both adopt a five-coordinated square-pyramidal geometry.Adjacent[Cu4(2,4,6-opyta)2(H2O)4]units link each other through Cu1-O5 coordination interaction and form a wave-like 2D layer.These 2D layers further form a 3D supermolecular network through abundant intra-,intermolecular hydrogen-bonding interactions between water molecules and carboxylate oxygen atoms.Furthermore,the new ligand 2,4,6-opyta3-is derived from copper-mediated oxidation and hydroxylation of 2,4,6-tmpy by utilizing HNO3as an oxidant.Crystal data of[Cu2(2,4,6-opyta)(H2O)2]·2H2O:Mr=422.24,Monoclinic,P21/c,a=1.122 15(11)nm,b=0.671 53(7)nm,c=1.715 29(18)nm, β=107.109(2)°,V=1.235 4(2)nm3,Z=4,R1=0.039 1,wR2=0.098 0(I>2σ(I)).CCDC:783944.
in situ reaction;oxidation and hydroxylation;hydrothermal synthesis;Cu(Ⅱ)complex
Hydro(solve)thermal in situ ligand synthesis is important not only in the crystal engineering of coordination complexes but also in synthetic organic chemistry[1-2].Up to now,more and more important and interesting in situ ligand reactions,such as oxidative hydroxylation of aromatic ring[3-6],dehydrogenative carbon-carbon coupling[7-9],cycloaddition of organic nitriles with azide and ammonia[10-12],and transformationof inorganic and organic sulfur[13-16],have been documented.We once reported a one-step oxidation of 2,9-dimethyl-1,10-phenanthroline ligands (dmphen)to 1,10-phenanthroline-2,9-dicarboxylic acid(phendaH2)in the presence of Cu(NO3)2[17].Recently,we successfully oxidized 2,3,5-trimethylpyridine and 2,4,6-trimethylpyridine and generated four different pyridinecarboxylates in the presence of copper nitrate under controlled hydrothermal conditions.Moreover,the new generated ligand 3-hydroxy-2,4,6-pyridinetricarboxylic acid (2,4,6-opytaH3)have been separated from Cu(Ⅱ)-complex[18].
As an extension of our previous work,we report here our further investigation on generating new 2,4,6-opyta3-contained Cu(Ⅱ)-complexes.The reaction mechanism of in situ generated 2,4,6-opyta3-is also discussed here.
1 Experimental
1.1 Reagents and measurements
The reagents and solvents employed were commercially available and used as received without further purification.The C,H,and N microanalyses were carried out with an Elemental Vario-EL CHNS elemental analyzer.The FTIR spectra were recorded from KBr pellets in the range 4000~400 cm-1on a Bio-Rad FTS-7 spectrometer.
1.2 Synthesis of[Cu2(2,4,6-opyta)(H2O)2]·2H2O
A mixture of 2,4,6-tmpy (1 mmol,0.121 g),Cu(NO3)2·4H2O(3.5 mmol,0.720 g)and distilled H2O(10 mL)was sealed in a 23 mL Teflon-liner autoclave.The mixture was heated in an oven to 180℃for 72 h,then cooled to room temperature at a rate of 5℃·h-1,and finally yielded green sheet-like crystals of title complex (yielding 25% based on 2,4,6-tmpy).Elemental analysis calcd.for C8H9N1O11Cu2(%):C 22.76,H 2.15,N 3.32;Found(%):C 22.46,H 2.31,N 3.10;IR(cm-1):3400s,1603vs,1628vs,1460m,1420 s,1 340s,1 293m,1 150m,970m,940w,870m,830m,750m,720s,610m.
1.3 X-ray crystallography
Diffraction intensities oftitle complex was collected on a Bruker Smart Apex CCD area-detector diffractometer(Mo Kα,λ=0.071 073 nm).Absorption corrections were applied by using multi-scan program SADABS[19].The structure were solved with direct methods and refined with a full-matrix least-squares technique with the SHELXTL program package[20].Anisotropic thermal parameters were applied to all nonhydrogen atoms.The organic hydrogen atoms were generated geometrically (C-H 0.096 nm);the aqua hydrogen atoms were located from difference maps and refined with isotropic temperature factors.Crystal data as well as details of data collection and refinements for title complexe are summarized in Table 1.Selected bond distances and bond angles are listed in Table 2.
CCDC:783944.
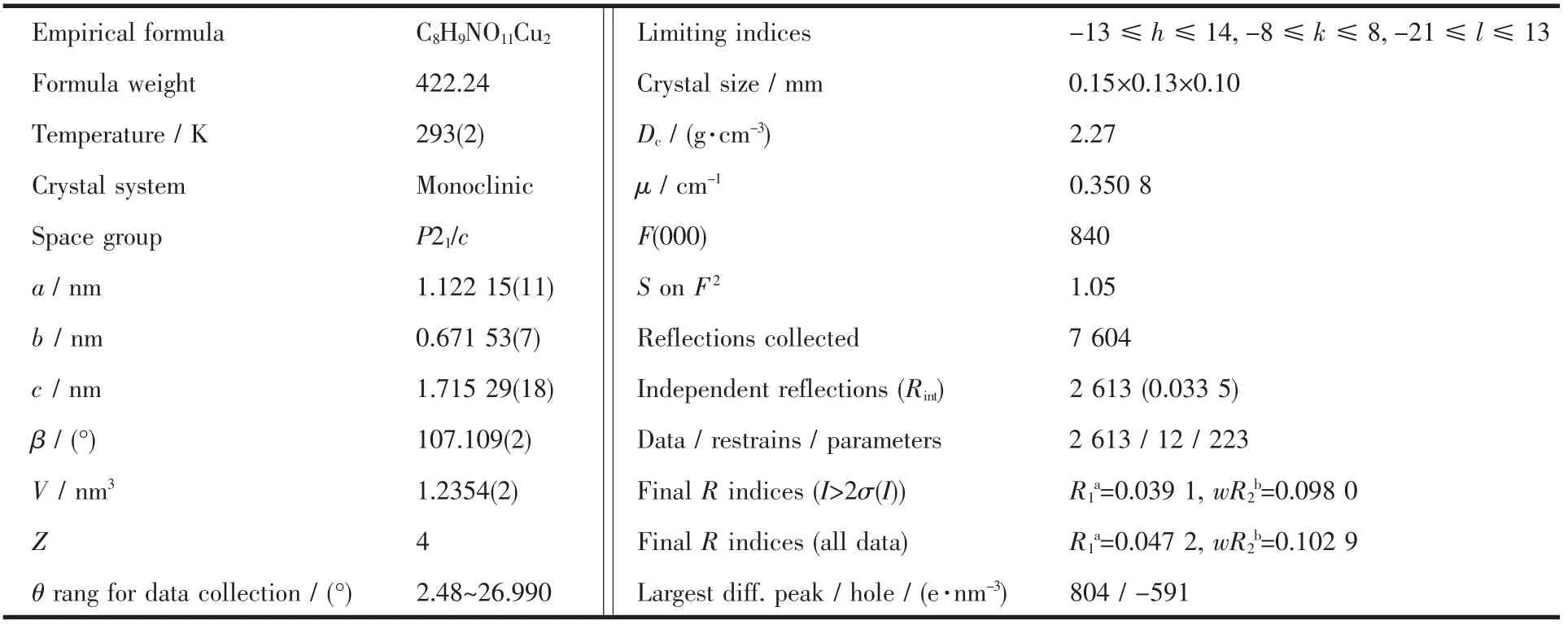
Table 1 Crystallographic data and structure refinement for title complex

Table 2 Bond length(nm)and bond angle(°)of title complex
2 Results and discussion
2.1 Crystal structure of[Cu2(2,4,6-opyta)(H2O)2]·2H2O
X-ray analysis revealed the asymmetrical unit of this complex contains one crystallographically independent μ4-2,4,6-opyta3-molecules,two five-coordinated Cu(Ⅱ),two coordinated water molecules and two solvent water molecules,as shown in Fig.1.Cu1 and Cu2 both adoptdistortedsquare-pyramidalcoordinationgeometry,and Cu1 is surrounded with NO4coordination sphere while Cu2 surrounded with four oxygen atoms from 2,4,6-opyta3-and one water molecule.(Cu1-O 0.1935(3)~0.2196(3)nm,Cu1-N 0.1912(3)nm,Cu2-O 0.1913(3)~0.2424(4)nm).Two asymmetrical units dimerize into a[Cu4(2,4,6-opyta)2(H2O)4]unit(C in Scheme 2)through the coordination interaction between Cu2 and μ2-O3.Adjacent Cu4units link each other through Cu1-O5 coordination interactions (Fig.2a,b)and form a wavelike two-dimensional(2D)layer(Fig.2c).Adjacent layers further extend into a 3D supermolecular network thoughinter-/intra hydrogen-bonding interactions between carboxylate oxygen atoms and water molecules.(Ow…Ocarboxylate=0.266 1(5)~0.329 7(6)nm,Ow-Hw…Ocarboxylate=137(7)°~173(7)°).All hydrogen bond parameters of this complex are listed in Table 3.
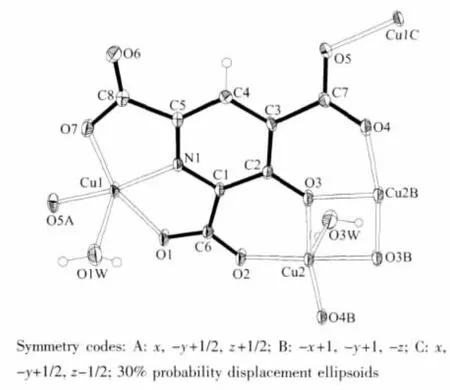
Fig.1 Coordination environments of Cu(Ⅱ) and 2,4,6-opyta3-
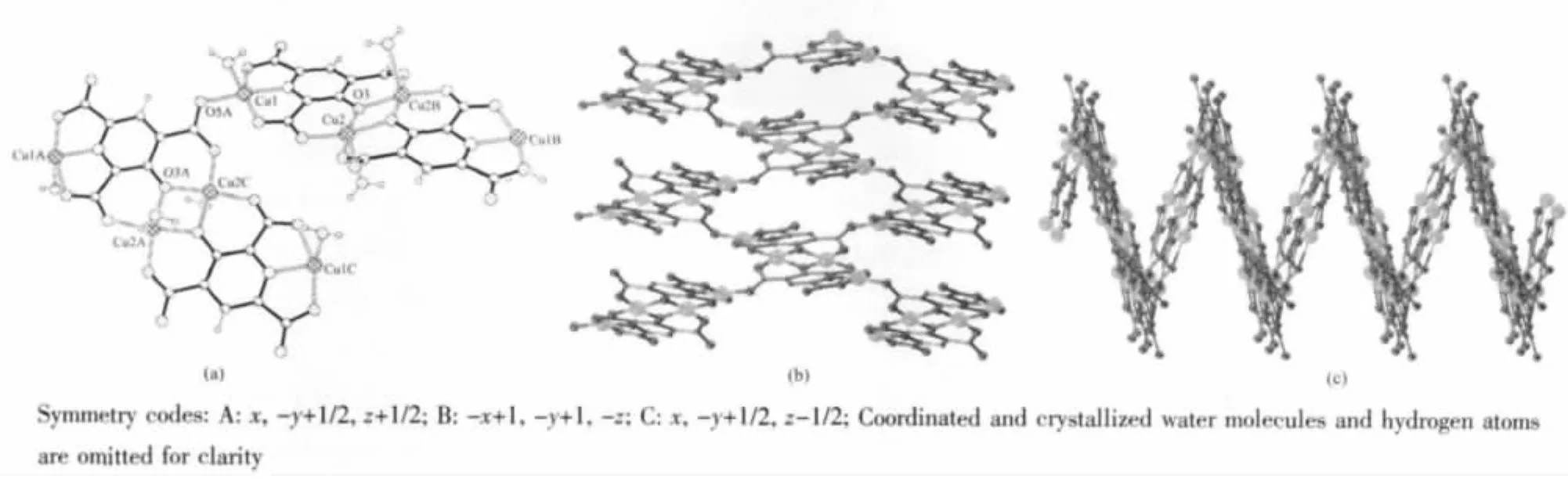
Fig.2 (a)Cu4 units link together through Cu1-O5 coordination interaction;(b)2D layer in title complex perspective viewed from a axis;(c)2D layer perspective viewed from c axis

Table 3 Bond length and bond angle of hydrogen bond
2.2 Mechanistic investigation
Evans and Lin reported that nitrate might act as oxidant for oxidative coupling of methanol to oxalic acid[21].We found in our previous study that the methyl groups in the pyridine ring could be oxidized to carboxylic acids by nitric acid under hydrothermal condition,where HNO3came from the metal salts and the protons in the solution[17].
To gain insight into the mechanism of generating 2,4,6-opyta3-from 2,4,6-tmpy (Scheme 1),we carried out a systematic investigation by controllingthe reaction temperature,time,pH value and stoichiometric proportion of reactants.The results showed that this in situ ligand reaction was copper-mediated and depended markedly upon solvents and anions of cupric salts.No similar metal complexes were obtained by using other transition-metal cations such as Zn(Ⅱ),Ni(Ⅱ) or Co(Ⅱ) in place of Cu(Ⅱ) under similar reaction conditions.If deioned water were replaced by other organic solvents such as acetonitrile,methanol,ethanol or acetone,or the nitrate ion were replaced by other anions,no carboxyl products could be detected.Moreover,autogenerated pressure upon high temperature(T>160℃)via hydrothermal condition played a vital role.

Scheme 1 2,4,6-tmpy was in situ oxidized into the new ligand 2,4,6-opyta3-
Since 2-,4-,6-carboxyl groups always coexist in the final complex,we assume that three methyls of 2,4,6-tmpy are simultaneously oxidized to 2-,4-,6-carboxylgroupsowing totheirsimilarchemical environment such as electronic disposition and steric hindrance.The hydroxylation of pyridine ring may be affected by the pH value of reaction system,and the acidic environment may prevent further hydroxylation of aromatic ring,which is approved by the successfully got complex[Cu2(μ3-OH)(2,4,6-pyta)][18](2,4,6-pyta3-=2,4,6-pyridinetricarboxylate)under acidic environment.It is interesting to note that the in situ generated 2,4,6-pyta3-is oxidized from 2,4,6-tmpy but hydroxylation of pyridine ring is prevented.Notedly,[Cu4(2,4,6-opyta)2(H2O)4]unit(C in Scheme 2)exist in all of the 2,4,6-opyta3-contained Cu(Ⅱ)-complexes[18]whatever reaction condition changes.
社会组织中的各类资产应考虑从资产用途的“公共性”出发,依照“为谁所用”、“由谁受益”的原则,以保障资产的公共受益权为目的,科学界定其资产权属,并通过制定出台《社会公共资产管理办法》,规范资产管理。本文将社会公共资产概念内涵界定为:由社会组织依法所有的,由非财政出资形成的全部资产及权益。该资产及权益主要用于发展社会公益事业或服务特定社会群体。
Considering the aforementioned discussion and our former results[18],the reaction mechanism of in situ generating 2,4,6-opyta3-ligand could be elucidated as follows(Scheme 2):Firstly,three methyls in the 2-,4-,and 6-position are oxidized by HNO3to form 2,4,6-pyta3-,which followed with the coordination of N,O donors to Cu(Ⅱ) cations.Surrounded by strong electron withdraw groups such as carboxyl groups and pyridine nitrogen atom,β-carbon atom of pyridine is electron-defect(A in Scheme 2).Secondly,hydroxyl or water molecules in solution take nucleophilic substituttion reaction to the hydrogen atom of β-carbon.Followed by dehydrogenation of β-hydroxyl,[Cu4(2,4,6-opyta)2(H2O)4]unit generates by chelating with two units of B.In these procedures,Cu(Ⅱ) serves not only as a coordinated metal ion,but also mediated the intramolecular electron transfer.
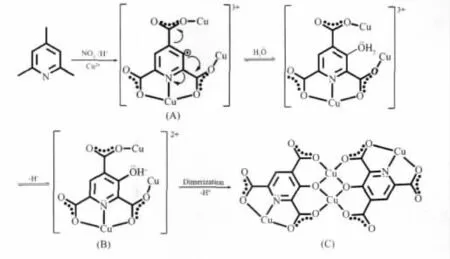
Scheme 2 Possible reaction mechanism of forming 2,4,6-opyta3-
[1]Chen X M,Tong M L.Acc.Chem.Res.,2007,40:162-170
[2]Lin W,Evans O R,Xiong R G,et al.J.Am.Chem.Soc.,1998,120:13272-13273
[3]Zhang X M,Tong M L,Chen X M.Angew.Chem.Int.Ed.,2002,41:1029-1031
[4]Tao J,Zhang Y,Tong M L,et al.Chem.Commun.,2002:1342-1343
[5]Zheng Y Z,Tong M L,Chen X M.New J.Chem.,2004,28:1412-1415
[6]Zheng Y Z,Tong M L,Chen X M.J.Mol.Struct.,2006,796:9-17
[7]Hu S,Chen J C,Tong M L,et al.Angew.Chem.Int.Ed.,2005,44:5471-5475
[8]Liu C M,Gao S,Kou H Z.Chem.Commun.,2001:1670-1671
[10]Xiong R G,Xue X,Zhao H,et al.Angew.Chem.,Int.Ed.,2002,41:3800-3803
[11]Zhang J P,Zheng S L,Huang X C,et al.Angew.Chem.,Int.Ed.,2004,43:206-209
[12]Zhang J P,Lin Y Y,Huang X C,et al.J.Am.Chem.Soc.,2005,127:5495-5506
[13]Li D,Wu T.Inorg.Chem.,2005,44:1175-1177
[14]Li D,Wu T,Zho X P,et al.Angew.Chem.,Int.Ed.,2005,44:4175-4178
[15]Zhang X M,Fang R Q,Wu H S.J.Am.Chem.Soc.,2005,127:7670-7671
[16]Wang J,Lin Z J,Ou Y C,et al.Inorg.Chem.,2008,47:190-199
[17]Fan L L,Li C J,Meng Z S,et al.Eur.J.Inorg.Chem.,2008:3905-39090
[18]LiCJ,LinZJ,YunL,etal.CrystEngComm,2010,12:425-433
[19]Sheldrick G M.SADABS2.05,University of Göttingen.
[20]SHELXTL6.10,Bruker Analytical Instrumentation,Madison,Wisconsin,USA,2000.
[21]Evans O R,Lin W.Cryst.Growth Des.,2001,1:9-11
原位产生的3-羟基-2,4,6-吡啶三酸配位的Cu(Ⅱ)配合物的合成及配体反应机理分析
李翠金*郭清兵 尹国强 周红军
(仲恺农业工程学院化学化工学院,广州 510225)
以 2,4,6-三甲基吡啶(2,4,6-tmpy)和 Cu(NO3)2为原料,采用水热法原位合成一个新的含 3-羟基-2,4,6-吡啶三酸根(2,4,6-opyta3-)的 Cu(Ⅱ)配合物 [Cu2(2,4,6-opyta)(H2O)2]·2H2O,并对其进行了红外、元素分析和X-射线单晶衍射测定。单晶结构分析表明,该配合物中Cu1、Cu2均采取五配位的四方锥几何构型,[Cu4(2,4,6-opyta)2(H2O)4]结构基元间通过Cu1-O5配位作用连成波浪形的层状结构。结晶水、配位水及羧基氧之间存在丰富的氢键作用进一步将二维层拓展成三维超分子网络。新产生的配体2,4,6-opyta3-是由Cu(Ⅱ)诱导HNO3氧化2,4,6-tmpy发生氧化和羟基化反应原位合成的。
原位反应;氧化和羟基化;水热合成;铜配合物
O614.121
:A
:1001-4861(2011)02-0377-05
2010-07-19。收修改稿日期:2010-09-23。
仲恺农业工程学院自然科学基金资助(No.G3100029)。
*通讯联系人。 E-mail:licuijinde@163.com
- 无机化学学报的其它文章
- Synthesis,Crystal Structure and Antibacterial Activity of 2D Hydrogen-bonds Layered Magnesium(Ⅱ)Complex
- The Uptake and Membrane Transport of Cesium in Human Erythrocytes
- Preparation of Functionalized Graphene Sheets via Microwave-Assisted Solid-State Process and Their Electrochemical Capacitive Behaviors
- A New 2D Layer Manganese(Ⅱ)Complex Assembled by Flexible 1,4-Benzenebis(thioacetic acid)Ligand:Synthesis,Crystal Structure and Magnetic Property
- A New Nickel(Ⅱ)Complex Based on Novel Pyridyl-carboxylate Schiff-Base Ligand:Synthesis and Crystal Structure
- Ru掺杂SnO2半导体固溶体的电子结构研究

