固定床反应器上挤条小晶粒TS-1催化丙烯环氧化反应
左 轶 宋万仓 王梦丽 徐永海 王祥生 郭新闻
(大连理工大学化工与环境生命学部催化化学与工程系,精细化工国家重点实验室,辽宁大连116024)
1 Introduction
Propylene oxide(PO)is an important derivative of propylene,only second to polypropylene and acrylonitrile,which is a chemical intermediate for producing polyether polyol polymers.The current processes for PO manufacture include the chlorohydrin route and the Halcon route.1The chlorohydrin route,using chloride as the reactant,leads to an environmental pollution,while the Halcon route is capital intensive and its economics depends on those of its co-products.2Therefore,researchers began to explore a new route which is environmentally friendly and would free PO from its co-products.
Titanium silicalite-1(TS-1),which was first reported by Taramasso et al.in 1983,3showed an excellent catalytic performance of selective oxidation with hydrogen peroxide,such as oxidation of alkanes,epoxidation of alkenes,hydroxylation of aromatics,and oxidation of ammonia to hydroxyamine,attracting many researchers.4-10BASF and Dow Chemical established a hydrogen peroxide to propylene oxide(HPPO)process in Antwerp,Belgium,which had a PO capacity of 300000 tons per year.11However,the cost is always one of the most serious obstacles on the application of TS-1 in industry,mainly due to the expensive template tetrapropyl ammonium hydroxide(TPAOH).Therefore,a cheaper template tetrapropyl ammonium bromide(TPABr)was adopted to substitute for TPAOH.12,13However,using TPABr enlarged the crystal size of TS-1,due to the introduction of Br-and the weaker basicity.The larger crystals had longer channels,causing the diffusion limitation of substrates and exothermal,thus,the catalytic performances of TS-1 would be less than desired.14It was reported that the addition of crystallization seeds would decrease the period of nucleation,giving a smaller crystal size and a higher crystallinity.15,16The shaping method was the other obstacle for TS-1 using in industry.There are mainly two methods for shaping the powdered TS-1 in application.One is spraying or in-situ synthesizing TS-1 on inert supports,of which the active component loading is low,so that the output of PO is very low.The other shaping method is extruding the powdered TS-1 with supports,producing TS-1 extrudates.17The TS-1 extrudates have a much higher active component loading than that shaped with the first method,so that the output of PO is improved significantly.One of the disadvantages of TS-1 extrudate is that the diffusion path is too long for products and reaction heat,so that the PO may react with solvents and H2O2may decompose,lowering the selectivity of PO and the utilization of H2O2.Moreover,the supports may block the pores of TS-1,which will affect the diffusion of substrates.18Therefore,eliminating the diffusion limits was the key of this shaping method.One of the ways was to decrease the crystal size of TS-1.The smaller the crystal is,the shorter the channels are.Thus,the diffusion limit was improved.
We have reported the synthesis of small-crystal TS-1 with a crystal size of 600 nm×400 nm×250 nm in a TPABr-ethylamine hydrothermal system by using the mother liquor of nanosized TS-1 as seeds.15The small-crystal TS-1 powder shows an excellent activity for the epoxidation of propylene in the batch reactor.Therefore,in the present work,the small-crystal TS-1 was extruded after mixing with silica gel and the obtained TS-1 extrudate was introduced into a fixed-bed reactor to test its catalytic performance.The reaction conditions,including temperature,pressure,molar ratio of propylene/H2O2(n(C3H6)/n(H2O2)),WHSVs of propylene,methanol and H2O2,and concentration of NH3·H2O,were systematically studied to identify the most suitable one for the application in industry.
2 Experimental
2.1 Preparation of small-crystal TS-1 extrudate
Small-crystal TS-1 was hydrothermally synthesized as described previously,19using colloidal silica(30%,mass fraction,technical grade)and titanium tetrachloride(analytical reagent)as silicon and titanium sources,respectively.TPABr(technical grade,home-made)was template,aqueous ethylamine(65%,mass fraction,analytical reagent)was the base,and the mother liquor of nano-sized TS-1 was seeds.The mother liquor of nanosizedTS-1 was prepared according to the literature.20Molar composition of the gels was as follows:n(SiO2):n(TiO2):n(TPABr):n(C2H5NH2):n(H2O)=1:0.02:0.15:1.5:25.
The crystallization of the catalyst was carried out in a Teflonlined autoclave at 170°C for 48 h.After crystallization,the solid product was washed with distilled water,dried at 100°C for12 h,and calcined at 540°C for 6 h.
The TS-1 extrudate catalyst was prepared by mixing the powdered TS-1 with 30%of silica sol(m(TS-1)/m(SiO2)=4/1),followed by extruding the mixture.The resulting extrudate was dried at room temperature and calcined at 540°C for 6 h.The TS-1 extrudate was then cut into the cylindrical pieces of 1.5 mm×1.5 mm for use in a fixed-bed reactor.21
2.2 Preparation of other TS-1
Nano-sized TS-1 powder with a crystal size of~200 nm was synthesized according to literature,22while micro-sized TS-1 powder with a crystal size of ~1 μm to literature.21The extrusion method of the above powder was the same as that of small-crystal TS-1.
2.3 Characterization of TS-1
The X-ray powder diffraction(XRD)was performed on a Japan Rigaku D/MAX-2400 using Cu Kαradiation.The Fouriertranrform infrared(FTIR)spectrum was recorded on a German Bruker EQUINOX55 spectrometer,and KBr pellet technique was adopted;the wavenumber was from 4000 to 400 cm-1.The ultraviolet-visible(UV-Vis)diffuse reflectance spectrum was obtained on a Japan Jasco UV-550 spectrometer,and pure BaSO4was used as a reference.The wavelength was from 190 to 800 nm.The nitrogen physisorption isotherm was performed at liquid nitrogen temperature on a United States QuantachromeAUTOSORB-1 physical sorption apparatus.
The initial and residual H2O2were measured by iodometric titration.The products of the reaction were analyzed on a 7890F gas chromatography with a flame ionization detector(FID)and a polyethylene glycol(PEG)-20M column(30 m×0.25 mm×0.4 μm).PO was the main product.Propylene glycol monomethyl ether(MME)and propylene glycol(PG)were the by-products.The conversion of H2O2(X(H2O2)),selectivity of PO(S(PO)),utilization of H2O2(U(H2O2)),and yield of PO(Y(PO))are given using the criteria as follows:

where the n0(H2O2)and n(H2O2)represent the initial and final molar contents of H2O2,respectively.The n(PO),n(MME),and n(PG)stand for the molar contents of PO,MME,and PG,respectively.
2.4 Epoxidation of propylene
The epoxidation of propylene was carried out in a fixed-bed reactor,which had a catalyst loading of 7.0 g.The reaction conditions were:35-50°C;1.0-3.0 MPa;methanol as the solvent;the initial concentration of H2O2was in the range of 1.2-2.4 mol·L-1;the molar ratio of propylene to H2O2was 1-4;the WHSV of propylene was 0.4-0.75 h-1;and the concentration of NH3·H2O was 0.8-1.6 mmol·L-1.The details of the reaction conditions are shown in Table 1.
3 Results and discussion
3.1 Characterization of small-crystal TS-1 extrudate
Fig.1 shows the results of characterization of small-crystal TS-1 extrudates.The five characteristic XRD peaks of MFI topology,which are sited at 7.8°,8.8°,23.0°,23.9°,and 24.4°,23are observed in the XRD pattern(Fig.1(a)).The crystallinity,which was calculated by comparing the total intensity of the five characteristic peaks with that of the standard samples,decreases slightly,due to the introduction of amorphous silica by extruding.
The band at 550 cm-1in the FTIR spectrum(Fig.1(b))is considered as the double-frequency vibration band in the framework of TS-1,22while the bands at 790 and 1100 cm-1are assigned to symmetrical and asymmetrical stretching vibrations out of framework,respectively.23The band at 960 cm-1cannot be observed in pure silicalite-1 or ZSM-5,thus,it is considered as a proof of titanium insertion into the framework.However,some researchers believed that it belongs to the Si-OH on the external surface or the defect of TS-1.24In other words,the assignment of this band is still controversial,but it can be seen as a necessary condition of Ti existing in the framework.25
UV-Vis spectroscopy is one of the earliest techniques for the detection of the coordination state of Ti.There are three major bands in the spectrum of small-crystal TS-1 extrudate(Fig.1(c)).The band at~210 nm may be attributed to the transition of the 2p electron from oxygen to the unoccupied 3d orbital of Ti4+,which is framework Ti.26The band at~270 nm is assigned to a charge transfer in isolated[TiO4]or[HOTiO3]units,27while that at~330 nm is the characteristic band of anatase TiO2.The appearance of the band at~330 nm may be due to the introduction of TPAOH by the seed.19
The nitrogen sorption isotherm of small-crystal TS-1 is the typical type IV sorption isotherm(Fig.1(d)).A small hysteresis loop appears between the relative pressures of 0.4 and 0.8,which is likely caused by the inter-crystal mesopores,because there is no mesopore in the crystals of small-crystal TS-1.
3.2 Effect of reaction temperature
The results of propylene epoxidation at different temperatures are shown in Fig.2.The conversion of H2O2increases from 85%to 90%,corresponding to the temperature increasing from 35 to 50°C.However,the selectivity of PO decreases with the increase of temperature,because increasing temperature could accelerate both the main reaction and the side reactions.The reactions in the epoxidation of propylene are all exothermic(see Scheme 1),and the amount of heat released from the main reaction is higher than those from the side reactions,so increasing the temperature will be harmful for the balance ofall the reactions,especially for the main.Therefore,more by-products are formed at a higher temperature,leading to the decrease of PO selectivity.There is no apparent change in the utilization of H2O2with the variation of temperature.The yield of PO,which is a function of several parameters including X(H2O2),S(PO),and U(H2O2),remains constant at about 75%,regardless of the temperature change between 35 and 50°C.In other words,changing the temperature can improve some parameters of propylene epoxidation,but it has little effect on the yield of PO.Therefore,it is not feasible to increase the yield of PO by increasing the temperature.
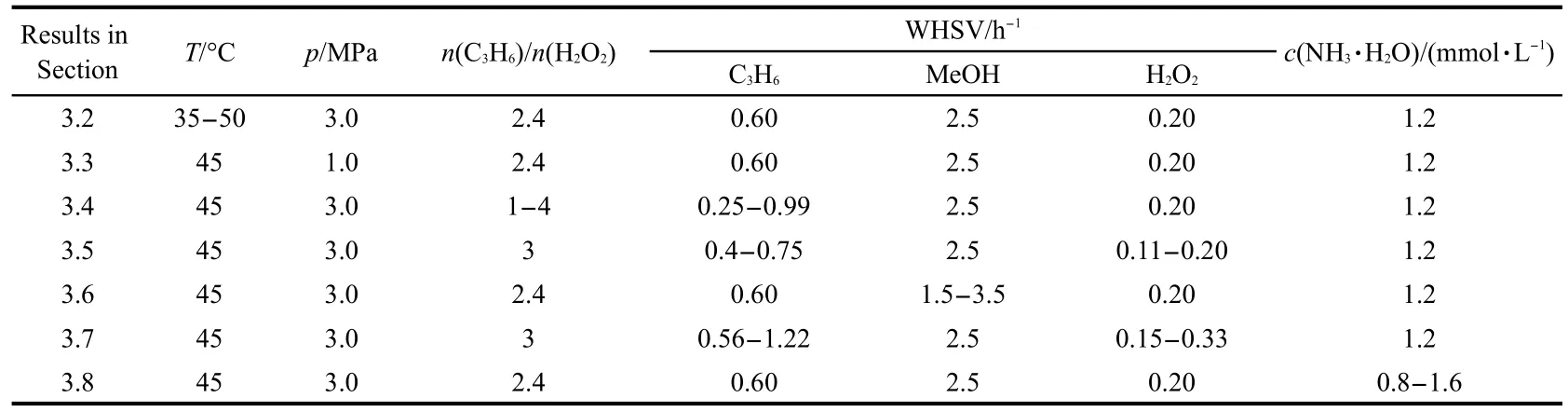
Table 1 Details of the reaction conditions for the epoxidation of propylene
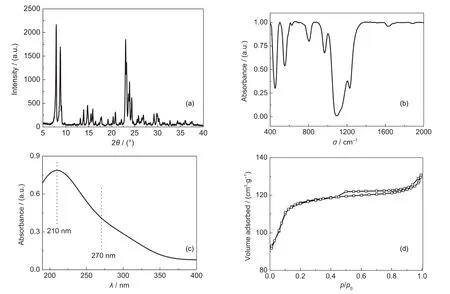
Fig.1 Characterization of small-crystal TS-1 extrudates
3.3 Effect of reaction pressure
Fig.3 shows the influence of pressure on propylene epoxidation.The initial pressure was pressurized with N2.When the pressure was 1.0 MPa,propylene was easy to gasify,and the solubility of propylene in methanol was low,which would lead to the reaction in three phases.Thus,the conversion of H2O2was very low(less than 30%).The epoxidation of propylene and the decomposition of H2O2over TS-1 are competitive reactions.If the amount of propylene is sufficient and the catalytic activity of TS-1 is high,the epoxidation will occur prior to the decomposition of H2O2,and vice versa.Therefore,H2O2which cannot react with propylene,decomposed seriously,when the pressure was low.The low pressure is also beneficial for the forward reaction of H2O2decomposition.These two reasons lead to a low utilization of H2O2.The obtained yield of PO,thus,is only 5%.When the pressure increased to more than 2.0 MPa,the gasification and insolubility of propylene were eliminated.Thus,the conversion of H2O2,selectivity of PO,and utilization of H2O2increased significantly,leading to a high yield of PO(80%).In summary,the reaction pressure limited by the property of propylene,cannot be too low,which should be at least 2.0 MPa.
3.4 Effect of molar ratio of propylene to H2O2
The effect of n(C3H6)/n(H2O2)is shown in Fig.4.When the ratio was 1,the conversion of H2O2was very low(less than 50%),although the stoichiometric ratio of the reaction was 1:1 for propylene:H2O2.Moreover,the decomposition of H2O2was more serious when the ratio was 1 compared to those with the corresponding ratios of 2-4,due to the insufficiency of propylene and the large amount of residual H2O2.When the n(C3H6)/n(H2O2)increased to 2,the conversion of H2O2increased,but the stability was poor.After 120 h reaction,the conversion of H2O2decreased from 90%to 85%.With further increase of the n(C3H6)/n(H2O2),both the activity and the stability of the small-crystal TS-1 were improved.The selectivity of PO decreased with the increase of n(C3H6)/n(H2O2)from 1 to 3,due to the increase of PO content in the products promoting the side reactions of PO.However,when the ratio was 4,the selectivity of PO increased slightly to 94%,because the acid centers,which were able to catalyze the alcoholysis of PO,were covered by excessive propylene.When the ratio was less than 3,the amount of propylene was insufficient to cover the acid centers,thus,the selectivity of PO decreased with the increase of PO content.Furthermore,a very high yield of PO(~82%)was obtained,when the n(C3H6)/n(H2O2)was 4.Nevertheless,adding more propylene beyond the stoichiometric ratio will increase the energy consumption of the separation process in industry.Thus,one needs to achieve a balance between the catalytic property and the cost.
Scheme 1 Reactions in the epoxidation of propylene with H2O2

Fig.3 Effect of pressure on the catalytic property of propylene epoxidation over small-crystal TS-1 extrudates
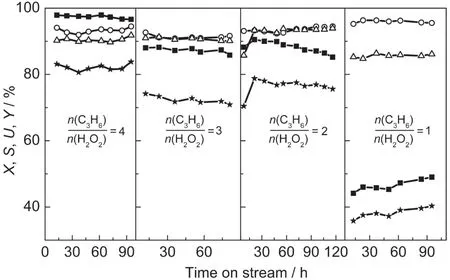
Fig.4 Effect of molar ratio of propylene/H2O2on the catalytic property of propylene epoxidation over small-crystal TS-1 extrudates
3.5 Effect of WHSV of propylene
In the next three sections,we would discuss the influence of the WHSVs of the three major substrates which were propylene(this Section),methanol(Section 3.6),and H2O2(Section 3.7),on the performance of propylene epoxidation.
When we changed the WHSV of propylene,we kept the n(C3H6)/n(H2O2)as 3,thus,the WHSV of H2O2was changed with that of propylene.Increasing the WHSV of propylene led to an apparent decrease of the conversion of H2O2,due to the limitation of the output of small-crystal TS-1 extrudate(Fig.5).The selectivity of PO and utilization of H2O2increased slightly with the increase of WHSV,until it was 0.6 h-1.When the WHSV of propylene was less than 0.6 h-1,the yield of PO maintained nearly constant,but when the WHSV increased to 0.75 h-1,the yield decreased obviously,because the residual time was too short and the substrates could not get sufficient contact with the active centers.In other words,the needed han-dling capacity was higher than the actual handling capacity of small-crystal TS-1.The more content of H2O2,the more acid centers were introduced in the substrate,which would lead to a lower selectivity of PO.The decomposition of residual H2O2(means not reacting with propylene)caused a lower utilization of H2O2.In summary,0.6 h-1is considered as a proper WHSV of propylene.
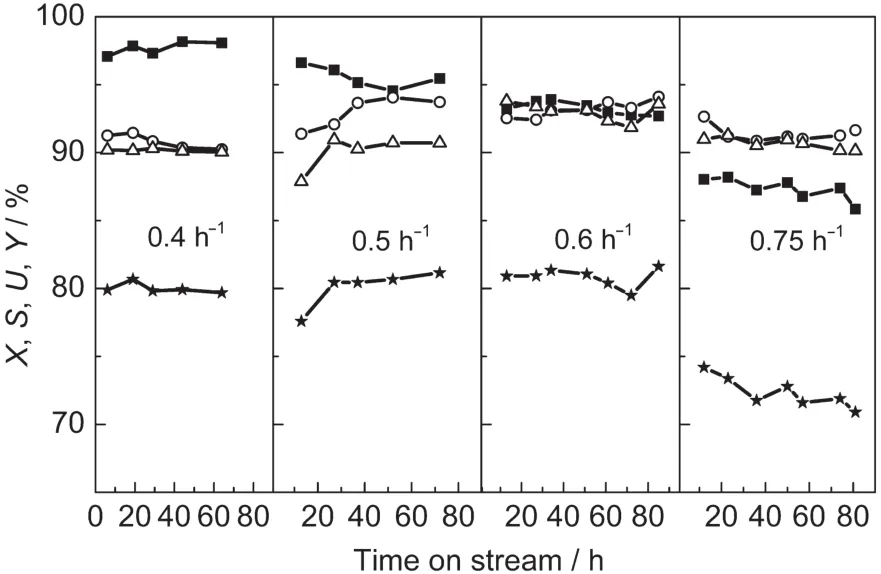
Fig.5 Effect of WHSV of propylene on the catalytic property of propylene epoxidation over small-crystal TS-1 extrudates
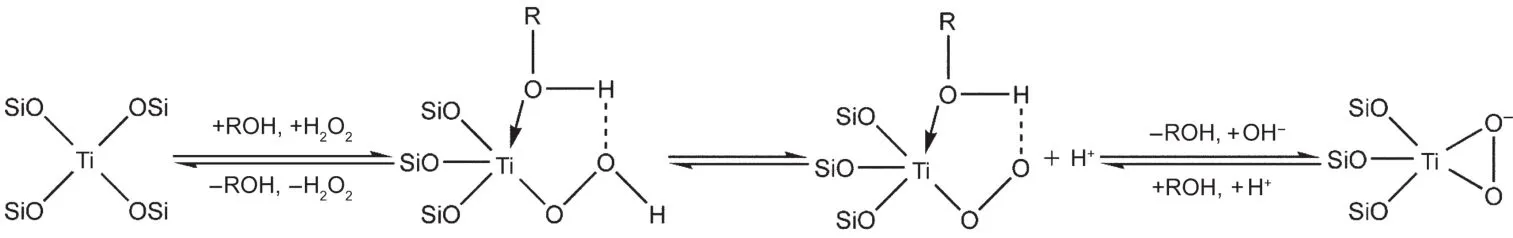
Scheme 2 Catalytic scheme of TS-1 in the TS-1-methanol-H2O2system
3.6 Effect of WHSV of methanol
Methanol is the solvent in the epoxidation of propylene,but it is important for the catalytic activity of the TS-1,according to the five-member-ring mechanism,7the route of which is shown in Scheme 2.Generally,the amount of solvent is expected to be as low as possible in industry,due to the high energy consumption for separation of products.Therefore,we studied the influence of WHSV of methanol on the catalytic properties(see Fig.6).
The WHSVs of propylene and H2O2were invariant,so the concentration of H2O2decreased with the increase of the WHSV of methanol.When the WHSV of methanol decreased,the conversion of H2O2and yield of PO decreased obviously.Especially when the WHSV was lower than 2.0 h-1,the stability of the catalyst was poor.The conversion of H2O2and selectivity of PO changed but the trend was not well defined,when the WHSV of methanol was more than 2.0 h-1.The concentrations of PO in the product,which could be calculated by multiplying the concentration of H2O2by the yield of PO,were 0.92,1.15,1.14,and 0.84 mol·L-1,corresponding to the WHSVs of methanol of 3.5,2.5,2.0,and 1.5 h-1,respectively.Therefore,it is not feasible to increase the content of PO in the product by decreasing the amount of solvent.
3.7 Effect of WHSV of H2O2

Fig.6 Effect of WHSV of methanol on the catalytic property of propylene epoxidation over small-crystal TS-1 extrudates
Fig.7 shows the effect of WHSV of H2O2on the propylene epoxidation.The WHSVs of methanol and n(C3H6)/n(H2O2)were kept constant,so the WHSV of propylene changed with that of H2O2.It is obvious that increasing the WHSV of H2O2led to the decrease of the conversion of H2O2and yield of PO.When the WHSV of H2O2was 0.33 h-1,the low conversion of H2O2caused a decomposition of H2O2and a low utilization of H2O2.The contents of PO in product were 0.98,1.15,1.32,and 0.84 mol·L-1,corresponding to the WHSVs of H2O2which were 0.15,0.20,0.25,and 0.33 h-1,respectively.Therefore,a proper WHSV of H2O2(0.25 h-1)may lead to a higher content of PO.
3.8 Effect of concentration of NH3·H2O
In the epoxidation of propylene,PO may react with methanol or water in the presence of acid centers to generate by-products.The basic additive can neutralize the acid centers in the substrate and on the TS-1,which will restrain the alcoholysis of PO and improve the selectivity of PO.28Thus,we expected to improve the yield of PO by increasing the selectivity of PO.The concentrations of NH3·H2O from 0.8 to 1.6 mmol·L-1were studied(Fig.8).When the concentration was low,the conversion of H2O2reached 97%,but the selectivity of PO was less than 90%,and the yield of PO was~77%.Increasing the concentration of NH3·H2O,the selectivity of PO increased significantly,but the conversion of H2O2decreased seriously,leading to a slight decrease of the yield of PO.The utilization of H2O2kept constant in the studied concentration scope of NH3·H2O.Thus,a low concentration of NH3·H2O was beneficial for a high PO yield.
3.9 Long term test

Fig.7 Effect of WHSV of H2O2on the catalytic property of propylene epoxidation over small-crystal TS-1 extrudates

Fig.8 Effect of NH3·H2O concentration on the catalytic property of propylene epoxidation over small-crystal TS-1 extrudates
A long term test of propylene epoxidation was conducted at an optimized set of conditions,which was 40°C,3.0 MPa,n(C3H6)/n(H2O2)of 3,the WHSVs of propylene,methanol and H2O2at 0.6,2.5 and 0.16 h-1,respectively,and the concentration of NH3·H2O of 1.2 mmol·L-1.The result of 1000 h run is shown in Fig.9.It is clear that the conversion of H2O2and selectivity of PO can both achieve 95%and are very stable.The utilization of H2O2and yield of PO decreased slightly(<5%)after the long term test.Therefore,the small-crystal TS-1 extrudate as catalyst exhibits a long lifetime in the optimized condition.
3.10 Comparison of TS-1 with different crystal sizes
The three TS-1 samples with different crystal sizes were studied under the optimized condition(cf.Section 3.9),including nano-sized TS-1(~200 nm),small-crystal TS-1 and microsized TS-1(~1 μm).The results,which are shown in Table 2,are the average data for more than 100 h on stream after the catalytic activity becomes stable.When there was no catalyst added in the reactor,the conversion of H2O2was less than 1%.The highest yield of PO was obtained over small-crystal TS-1.The proper crystal size was considered as the main reason for the high catalytic property.The larger crystal size of microsized TS-1 led to low conversion of H2O2and selectivity of PO.The low conversion of H2O2over nano-sized TS-1 was likely due to the low crystallinity.
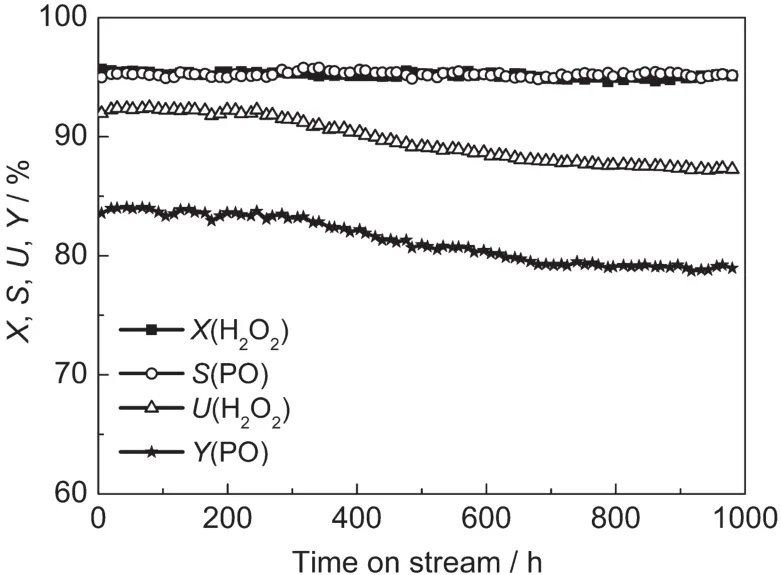
Fig.9 Long term test of propylene epoxidation under the optimized condition

Table 2 Catalytic properties of propylene epoxidation over TS-1 with different crystal sizes
4 Conclusions
The epoxidation of propylene over small-crystal TS-1 extrudates was evaluated in a fixed-bed reactor under different reaction conditions.The temperature had little effect on the yield of PO.The pressure needed to be higher than 2.0 MPa,or the propylene was easy to gasify.The reaction conditions were favorable for PO production when the n(C3H6)/n(H2O2)was 4,the WHSVs of propylene,methanol,and H2O2were 0.93,2.5,and 0.25 h-1,respectively.A low concentration of NH3·H2O led to a high yield of PO.The small-crystal TS-1 extrudate showed a stable and high catalytic performance under the optimized condition.
(1)Kirk,R.O.;Dempsey,T.J.Kirk-Othmer Encyclopedia of Chemical Technology;Wiley:New York,1982;p 246.
(2) Wulff,H.P.;Wattimena,F.Olefin Epoxidation.U.S.Pat.4021454,1977.
(3) Taramasso,M.;Perego,G.;Notari,B.Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides.U.S.Pat.4410501,1983.
(4)Fan,W.B.;Duan,R.G.;Yokoi,T.;Wu,P.;Kubota,Y.;Tatsumi,T.J.Am.Soc.Chem.2008,130,10150.doi:10.1021/ja7100399
(5)Yube,K.;Furuta,M.;Mae,K.Catal.Today 2007,125,56.doi:10.1016/j.cattod.2007.03.017
(6)Zhuang,J.Q.;Ma,D.;Yan,Z.M.;Liu,X.M.;Han,X.W.;Bao,X.H.;Zhang,Y.H.;Guo,X.W.;Wang,X.S.Appl.Catal.A 2004,258,1.doi:10.1016/j.apcata.2003.06.002
(7) Lv,L.;Zhou,J.K.;Su,F.;Zhao,X.S.J.Phys.Chem.C 2007,111,773.doi:10.1021/jp056107w
(8) Laha,S.C.;Kumar,R.J.Catal.2001,204,64.doi:10.1006/jcat.2001.3352
(9) Li,Y.G.;Lee,Y.M.;Porter,J.F.J.Mater.Sci.2002,37,1959.doi:10.1023/A:1015234812360
(10) Barbera,D.;Cavani,F.;D'Alessandro,T.;Fornasari,G.;Guiditti,S.;Aloise,A.;Giordano,G.;Piumetti,M.;Bonelli,B.;Zanzottera,C.J.Catal.2010,275,158.doi:10.1016/j.jcat.2010.07.030
(11)Yu,J.K.;Li,Z.;Liu,Q.W.Chem.Propell.Poly.Mat.2011,9,8.[于剑昆,李 中,刘青炜.化学推进剂与高分子材料,2011,9,8.]
(12) Müller,U.;Steck,W.Stud.Surf.Sci.Catal.1994,84,203.doi:10.1016/S0167-2991(08)64115-4
(13) Shibata,M.;Gabelica,Z.Zeolites 1997,19,246.doi:10.1016/S0144-2449(97)00078-X
(14) Zhang,H.J.;Liu,Y.M.;Jiao,Z.;He,M.Y.;Wu,P.Ind.Eng.Chem.Res.2009,48,4334.doi:10.1021/ie8016253
(15)Xu,F.;Dong,M.;Gou,W.Y.;Li,J.F.;Qin,Z.F.;Wang,J.G.;Fan,W.B.Microporous Mesoporous Mat.2012,163,192.doi:10.1016/j.micromeso.2012.07.030
(16)Chen,Y.;Wu,Y.L.;Zhang,Y.R.;Long,L.;Tao,L.;Yang,M.D.;Tang,N.J.Mol.Catal.A-Chem.2012,352,102.doi:10.1016/j.molcata.2011.10.020
(17) Bellussi,G.;Buonomo,F.;Esposito,A.;Clerici,M.;Romano,U.;Notari,B.Catalyst of Silicon and Titanium Having High Mechanical Strength and a Process for Its Preparation.U.S.Pat.4701428,1987.
(18)Zuo,Y.;Wang,M.L.;Song,W.C.;Wang,X.S.;Guo,X.W.Ind.Eng.Chem.Res.2012,51,10586.doi:10.1021/ie300581z(19)Zuo,Y.;Wang,X.S.;Guo,X.W.Microporous Mesoporous Mat.2012,162,105.doi:10.1016/j.micromeso.2012.06.016
(20)Wang,L.Q.;Wang,X.S.;Guo,X.W.;Li,G.;Xiu,J.H.Chin.J.Catal.2001,22,513.[王丽琴,王祥生,郭新闻,李 钢,修景海.催化学报,2001,22,513.]
(21)Li,G.;Wang,X.S.;Yan,H.S.;Liu,Y.H.;Liu,X.W.Appl.Catal.A 2002,236,1.doi:10.1016/S0926-860X(02)00288-0
(22) Thangaraj,A.;Kumar,R.;Mirajkar,S.P.;Ratnasamy,P.J.Catal.1991,130,1.doi:10.1016/0021-9517(91)90086-J
(23) Zhang,X.J.;Wang,Y.;Xin,F.Appl.Catal.A 2006,307,222.doi:10.1016/j.apcata.2006.03.050
(24) Notestein,J.M.;Solovyov,A.;Andrini,L.R.;Requejo,F.G.;Katz,A.;Iglesia,E.J.Am.Soc.Chem.2007,129,15585.doi:10.1021/ja074614g
(25) Bordiga,S.;Damin,A.;Berlier,G.;Bonino,F.;Ricchiadi,G.;Zecchina,A.;Lamberti,C.Int.J.Mol.Sci.2001,2,167.doi:10.3390/i2050167
(26) Fraile,J.M.;Garcia,J.I.;Mayoral,J.A.;Vispe,E.J.Catal.2005,233,90.doi:10.1016/j.jcat.2005.04.018
(27) Perego,C.;Carati,A.;Ingallina,P.;Mantegazza,M.A.;Bellussi,G.Appl.Catal.A 2001,221,63.doi:10.1016/S0926-860X(01)00797-9
(28) Clerici,M.G.;Ingallina,P.J.Catal.1993,140,71.doi:10.1006/jcat.1993.1069

