N3/Al2O3/N749交替组装结构拓宽准固态染料敏化太阳能电池光响应范围和界面修饰效果
高 瑞 牛广达 王立铎 马蓓蓓 邱 勇
(清华大学化学系,有机光电子及分子工程教育部重点实验室,北京100084)
1 Introduction
Dye-sensitized solar cell(DSC)was first reported by Grätzel et al.in 1991.1It was considered as an alternative to the traditional silicon soar cell due to its lower productive cost and easy fabrication process.Up to now,much attention had been focused on both the efficiency2-5and stability6-11of DSCs.Conversion efficiency up 12%had been achieved up to now.12
Photoresponse of sensitizers was a key element in improving performance of DSCs.To enhance the spectrum response over a wider wavelength range,multi-layer sensitized TiO2electrode with different dyes have been used since 1997.13These methods of fabricating multi-layer sensitized TiO2electrodes were always carried out through adsorbing different dyes onto the TiO2films layer by layer through dipping them in different dye solutions successively.14,15However,these methods suffered from competition adsorption or mismatch of energy level of different dye molecules.And excessive adsorption could cause the dye aggregation,which increased the electron quenching and charge recombination in DSCs.As a result,the light-capture efficiency of the cells could be enhanced,but the devices'conversion efficiency was not improved obviously.16-18
To avoid the problems mentioned above,a secondary metal oxide layer was applied for two-layer or multi-layer sensitization of TiO2electrode to separate different dye layers.In this way,the second layer of dye was adsorbed on the metal oxide interlayer.After being sensitized with the first layer of dye,a secondary Al2O3layer was deposited on the sensitized TiO2film.Then the second layer of dye was adsorbed.19Through this method,a significant enhancement of conversion efficiency was achieved.20Besides the sensitizer,the interface of sensitized TiO2/electrolyte in DSCs is also a vital factor for performance of DSCs.21,22Several important reactions in DSCs occurred at this interface,such as the dye electron injection,charge transfer,charge recombination,and dye regeneration.Accordingly,interface modification was considered as a useful method of improving the performance of DSCs.23-29Many metal oxides,such as Al2O3,30,31MgO,32Nb2O5,33SiO2,34ZnO,35or ZrO2,36have been used to make the interface modification between TiO2film and dye.Furthermore,many other insulating materials were also found to be effective in blocking recombination and increasing the conversion efficiency of DSCs.Al2O3was an excellent modification material for TiO2photoanode as it could retard the charge recombination in DSCs obviously.37,38Durrant et al.39obtained a 30%efficiency enhancement using Al2O3modification of DSCs in 2002.In the previous work,40,41the modification with Al2O3after sensitization could improve the conversion efficiency and stability of DSCs by prohibiting the aggregation of N3 dyes and spacing the TiO2and the electrolyte.Furthermore,Al2O3also could adsorb the second layer of dye to form an alternating assembly structure by the interaction of oxygen in Al2O3and hydrogen in the carboxyl group in the dye molecule.40,41
The previous studies about the multi-layer sensitizing mainly focused on the extended photoresponse.20However,the effects of Al2O3,which was used as the carrier layer,had not been studied systemically.To investigate the interface effect of Al2O3in the multi-layer sensitizing structure,this paper introduced an alternating structure with different dyes,which was N3/Al2O3/N749 assembled.The effects of Al2O3and the interface electron processes were discussed systemically.Furthermore,the electron process and internal resistance were analyzed and the mechanism of device based on the alternating assembly structure was simulated by establishing an equivalent circuit model.This structure could combine the advantages of broadening spectrum response of sensitizer and interface modification to retard the charge recombination.
2 Experimental
2.1 Materials
Poly(ethylene oxide)(PEO,Mw=2×106,Aldrich),iodine(I2,Guangdong Xilong Chemicals,China,analytically pure),lithium iodide(LiI,Acros Organics,99%),4-tertbutylpyridine(TBP,Sigma,99%),aluminium isopropoxide(Al(OC3H7)3,Alfa Aesar,99%),3-methoxypropionitrile(MePN,Alfa Aesar,99%),cis-dithiocyanate-N,N'-bis(4,4'-dicarboxylate-2,2'-bipyridine)ruthenium(II)(N3,Solaronix,Switzerland).{(C4H9)4N}3·[Ru(Htctpy)(NCS)3](tctpy=4,4',4ʺ-tricarboxy-2,2',6',2ʺ-terpyridine)(N749,Solaronix,Switzerland).
2.2 Preparation of the photoanode
The TiO2colloid was prepared with a hydrothermal method,which has been well documented in the previous report.42To prepare porous TiO2film,transparent conductive F-doped SnO2(FTO)glass(12 Ω·□-1)was completely cleaned and then a thin compact TiO2film(about 8 nm in thickness)was deposited on the FTO by dip coating in order to improve ohmic contact and adhesion between the following porous TiO2layer and the conductive FTO glass.The doctor blade technique was then adopted to prepare the porous TiO2layer with the thickness of the porous layer being controlled by an adhesive tape.Afterwards,the film was thermo-treated at 450°C for 30 min.When cooled to 110°C,the TiO2electrode was sensitized by immersion in 0.3 mmol·L-1N3 absolute ethanol solution for 2 h and cleaned with absolute ethanol.
Coating of Al2O3was performed as follows:the sensitized TiO2film was dipped into a solution of Al(OC3H7)3for 30 s,then hydrolyzed in air for 30 min to make produced isopropanol during the hydrolysis reaction volatilize.Then the TiO2film was sensitized with 0.3 mmol·L-1N749 absolute ethanol solution dye for 2 h,then the TiO2/N3/Al2O3/N749 structure was assembled.
2.3 Preparation of the electrolyte
The preparation procedure for the polymer gel electrolytes includes two steps.First,liquid electrolyte was prepared.Second,poly(ethylene oxide)(PEO)was slowly added into the liquid electrolyte and heated under strong stirring until the polymer gel electrolyte became homogeneous.The composition of the liquid electrolyte is as follows:0.1 mol·L-1LiI,0.1 mol·L-1I2,0.6 mol·L-11,2-dimethyl-3-propyl imidazolium iodide(DMPII),and 0.45 mol·L-1N-methyl-benzimidazole(NMBI).The solvent was 3-methoxypropionitrile(MePN);43the mass fraction(versus liquid electrolyte)for the PEO in the electrolyte was 10.0%.
2.4 Fabrication of the DSCs
A chemically platinized conductive glass was used as the counter electrode.When assembling the DSCs,the polymer gel electrolyte was sandwiched by a sensitized TiO2electrode and a counter electrode with two clips;the space between the two electrodes was controlled by an adhesive tape with a thickness of 30 μm.Finally,the DSCs were baked at 80 °C to ensure that the polymer could penetrate into the TiO2film.
2.5 Characterization
The UV-Vis reflectance absorption spectra were measured with a Hitachi U-3010 spectroscope.Photocurrent-voltage(IV)and dark current measurements were performed using a Keithley Model 4200-SCS semiconductor characterization system with real-time plotting and analysis with an active area of 0.25 cm2.EIS,IMVS,and IMPS were investigated by ZAHNER CIMPS electrochemical workstation,Germany.The incident photon-to-current conversion efficiency(IPCE)was measured by using a lab-made IPCE setup in Professor Meng's laboratory inInstituteof Physics,ChineseAcademyof Sciences.
3 Results and discussion
3.1 Photoresponse
Fig.1 showed the UV-Vis absorption spectra of N3,N749,and N3/Al2O3/N749 adsorbed onto TiO2films.The absorption peak of N3 was at about 530 nm,and that of N749 was at 620 nm.When the structure of N3/Al2O3/N749 was applied,a wide absorption peak from 530 to 620 nm could be observed.The results of UV-Vis absorption showed that the structure of N3/Al2O3/N749 combined the absorption spectra of N3 and N749.
To further explore whether the broadened absorption of N3/Al2O3/N749 compared to the photoanode sensitized by the single dye injected into the conductive band of TiO2effectively,the IPCEs of devices based on TiO2/N3,TiO2/N749,and N3/Al2O3/N749 were tested.
IPCE can be expressed by the following formula:44
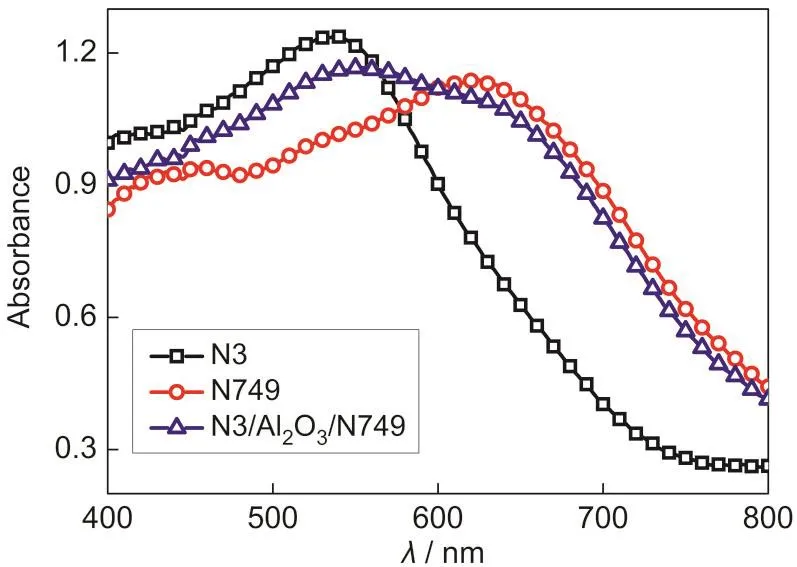
Fig.1 UV-Vis absorption spectra of N3,N749,and N3/Al2O3/N749 adsorbed onto TiO2films

where LHE(λ)is the light-harvesting efficiency for photons of certain wavelength;φinjis the quantum yield for electron injected from the excited sensitizer to the conduction band of TiO2;and ηcis the electron collection efficiency.
As shown in Fig.2,to compare the photoresponse range of N3,N749,and N3/Al2O3/N749 clearly,IPCEs of the three kinds of photoanodes were tested.The results revealed that compared to N3 individually,the IPCE spectrum of N3/Al2O3/N749 was widened in the range from 600 nm to over 700 nm and stronger in the range from 500 to 600 nm,which was corresponding to the results of absorption spectrum.It suggested that most of the electrons leading to the increased absorption of N3/Al2O3/N749 showed in Fig.1 were injected into the conductive band of TiO2.As a result,the short-circuit current density(Jsc)could be increased when using N3/Al2O3/N749 structure,then the conversion efficiency could be enhanced.
3.2 Photovoltaic performance

Fig.2 IPCE spectra of devices based on N3,N749,and N3/Al2O3/N749
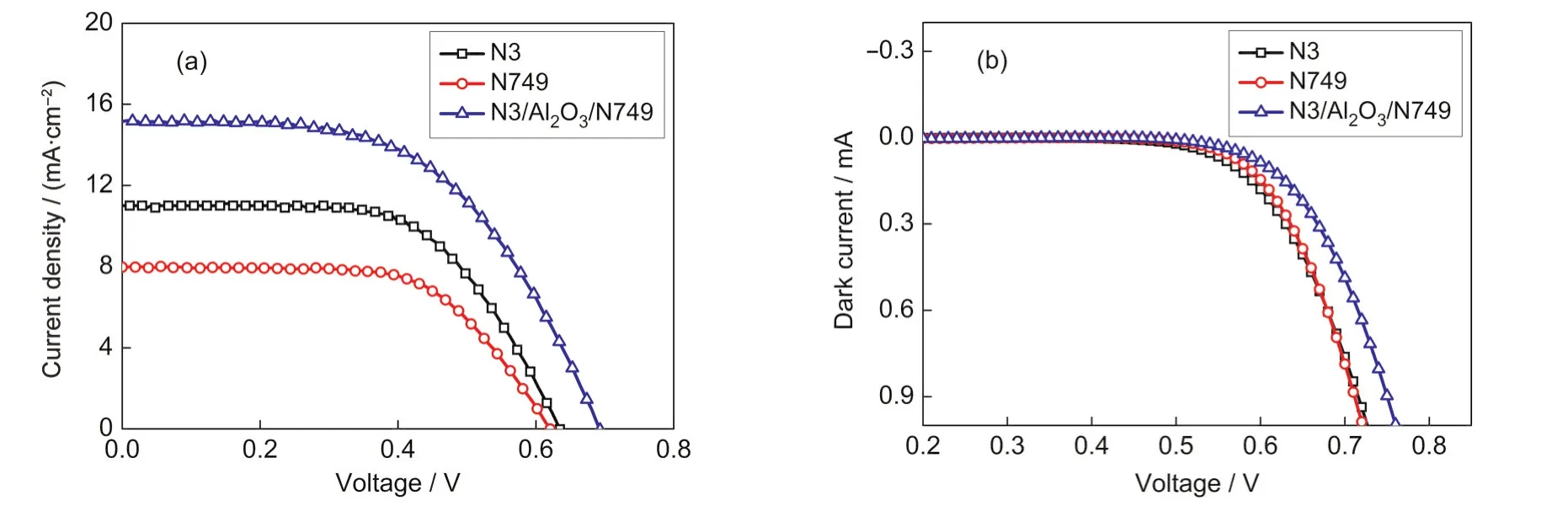
Fig.3 (a)Current density-voltage curves and(b)dark current curves of devices based on N3,N749,and N3/Al2O3/N749
The photocurrent-voltage characteristics of DSCs based on N3,N749,and N3/Al2O3/N749 were tested under AM1.5,100 mW·cm-2.As shown in Fig.3(a)and Table 1,the Jscvalues of devices based on N3 and N749 were 10.95 and 7.97 mA·cm-2,respectively.The Jscvalue increased to 15.14 mA·cm-2when N3/Al2O3/N749 structure was applied.The increasing of Jsccould be explained that the two dyes in the N3/Al2O3/N749 increased the photoresponse range,and then more electrons were injected into the conductive band of TiO2.The increasing of Jsccorresponded to the results of UV-Vis adsorption and IPCE spectra.The open circuit voltage(Voc)of devices based on N3 and N749 were 0.635 and 0.620 V.The Vocof the device based on N3/Al2O3/N749 also increased to 0.690 V.The enhancement of Voccould be explained that as a carrier layer and modification material,besides absorbing more dyes,Al2O3could retard the charge recombination.The decreased recombination caused the enhancement of Voc.As a result,the device based on N3/Al2O3/N749 obtained a conversion efficiency of 5.75%,which was higher than the efficiency of device based on N3 or N749.As show in Fig.3(b),the dark current of device with N3/Al2O3/N749 was also lower than that of device based on N3 or N749.It showed that the back reaction was retarded,which confirmed the charge recombination decreasing caused by interface modification effects ofAl2O3.40
3.3 Electron process and impedance analysis
As shown in Fig.4,the electron transfer processes in DSC based on N3/Al2O3/N749 structure were illustrated.The electron in the lowest unoccupied molecular orbital(LUMO)of the first layer of dye(N3)is injected into the conductive band of TiO2.Besides,electrons in the LUMO of the second layer of dye(N749)also could be injected into the conductive band of TiO2with a quantum tunneling effect.As a result,more electrons could be produced and injected into TiO2than using N3 only,then enhance the photocurrent of the devices.Furthermore,Al2O3retarded the recombination between electrons in the conductive band of TiO2and I-/I-3in electrolyte,showing obvious interface modification effects.Thus the back reactions were reduced and dark current was decreased,which was shown in Fig.3(b).
DSCs could be considered as a leaking capacitor in dark con-dition.45The resistance of the back reaction from TiO2to the I-3ions in the electrolyte could be analyzed through AC impedance technique under dark condition.The resistance at the interface of the sensitized TiO2/electrolyte was presented by the semicircle in intermediate frequency regime of the Nyquist plots.46The bigger the diameter of middle frequency semicircle was,the slighter the electron recombination at the sensitized TiO2/electrolyte interface was.Fig.5(a)showed the Nyquist plots of devices based on N3,N749 and N3/Al2O3/N749 at-0.8 V bias voltage in dark condition.Compared with N3 or N749 individually,the interface resistance of N3/Al2O3/N749 based DSC was much bigger,which meant that the charge recombination was obviously retarded.The decrease of recombination was mainly caused by the interlayer of Al2O3,which acted as a barrier layer besides a carrier layer of the second dye.
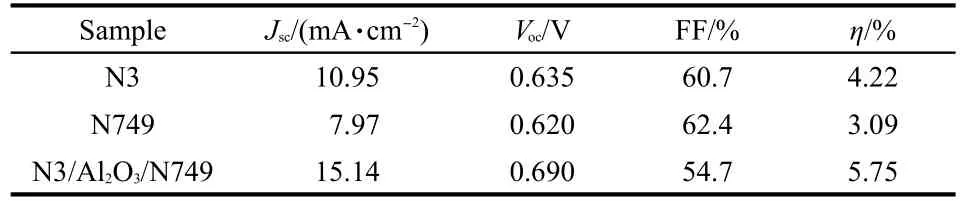
Table 1 Parameters of DSCs based on N3,N749,and N3/Al2O3/N749
Under illumination condition,the DSCs could be taken as diodes.47Resistance at the TiO2/dye/electrolyte interface was also presented by the middle frequency semicircle in the Nyquist plots.The smaller the diameter of middle frequency semicircle was,the faster the electron transfer at the sensitized TiO2/electrolyte interface was.As shown in Fig.5(b),the resistance at TiO2/dye/electrolyte interface of N3/Al2O3/N749 based DSC was similar with that based on N3 or N749 individually,showing that the charge transfer did not deteriorate with such a struc-ture.Thus the increased injection electron could enhance the Jsceffectively,which accorded with the results shown in Fig.3(a)and Table 1.
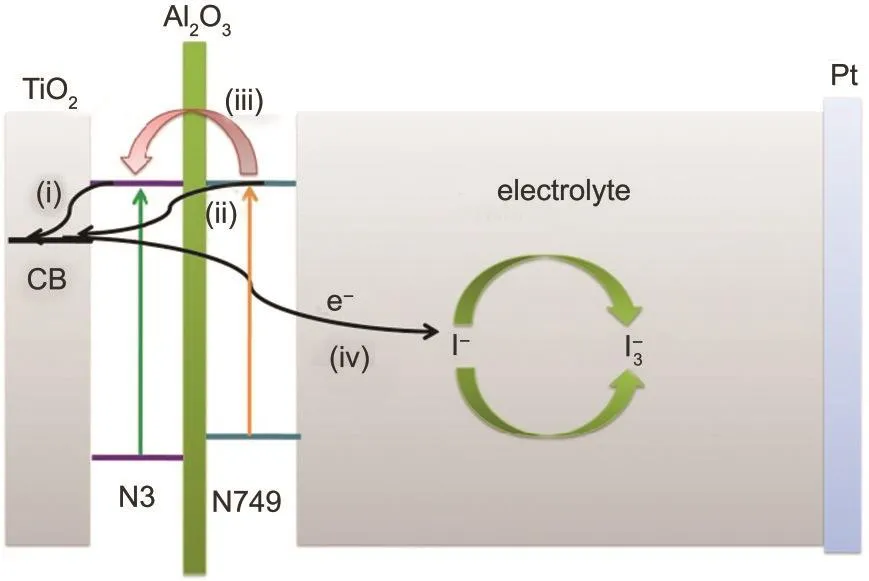
Fig.4 Diagrammatic sketch of electron process in DSC based on N3/Al2O3/N749
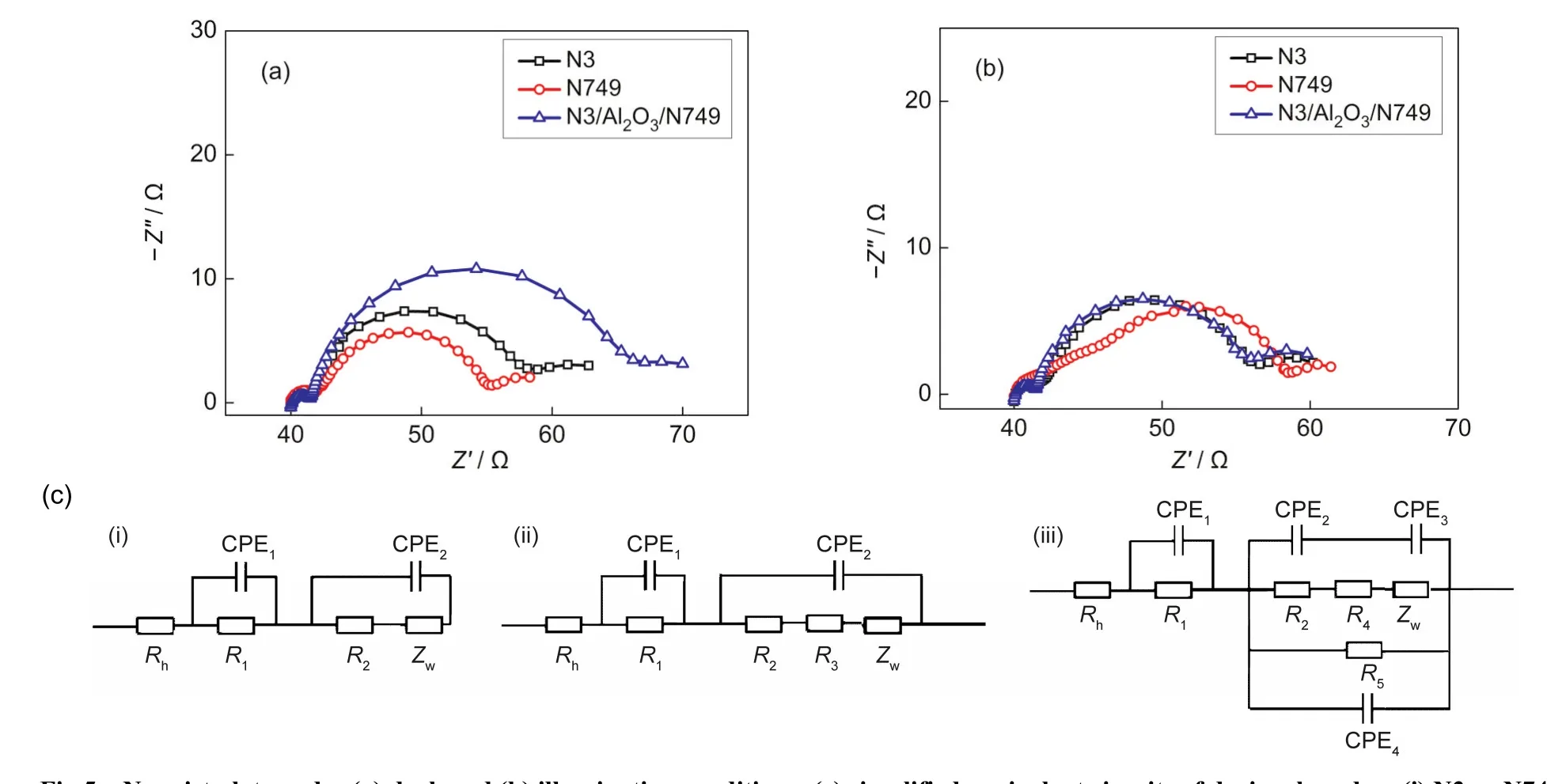
Fig.5 Nyquist plots under(a)dark and(b)illumination conditions;(c)simplified equivalent circuits of devices based on(i)N3 or N749,(ii)N3/Al2O3/N749(dark),and(iii)N3/Al2O3/N749(illumination)
To interpret the mechanism of the dye/Al2O3structure theoretically,a series of equivalent circuits were built based on the EIS results.As shown in Fig.5(c),an equivalent circuit model was built to analyze the influence of N3/Al2O3/N749 on the interface resistance in DSCs.As a conventional sample,the model in Fig.5(c)could interpret the equivalent circuit.47The three semicircles in Nyquist plots represented the redox reaction at the platinum counter electrode(R1),the electron transfer at the TiO2/dye/electrolyte interface(R2),and carrier transport by ions within the electrolyte(Rd),Rdis the resistence part of Zwshowing in Fig.5(c).Rhwas the sheet resistance of FTO and the contact resistance between the FTO and TiO2.When it turned to N3/Al2O3/N749,models on the dark and illumination condition,new models were built to interpret the equivalent circuit.As seen in Fig.5(c),in dark condition,the electron process is only the recombination between electrons and I-/I-3in the electrolyte,which was seen as process(iv)in Fig.4.The Al2O3/N749 could be considered as a resistor in series to R2,which was R3shown in Fig.5(c).
Table 2 showed the calculated values of EIS results of devic-es based on N3,N749,and N3/Al2O3/N749.In dark condition,the interface resistances of TiO2/N3/electrolyte,TiO2/N749/electrolyte,and TiO2/N3/Al2O3/N749/electrolyte were 16.1,12.4,and 23.6 Ω,respectively.The value of latter one was almost the sum of the former two,corresponding to the model of series in Fig.5(c)in dark condition.Furthermore,the interface capacitance value of TiO2/N3/Al2O3/N749 was 607.2 μF,similar to that of TiO2/N3(552.8 μF).It indicated that the second layer of Al2O3/N749 in N3/Al2O3/N749 structure did not influence the interface capacitance.It further confirmed that the equivalent circuit model of dark condition in Fig.5(c)was reasonable.
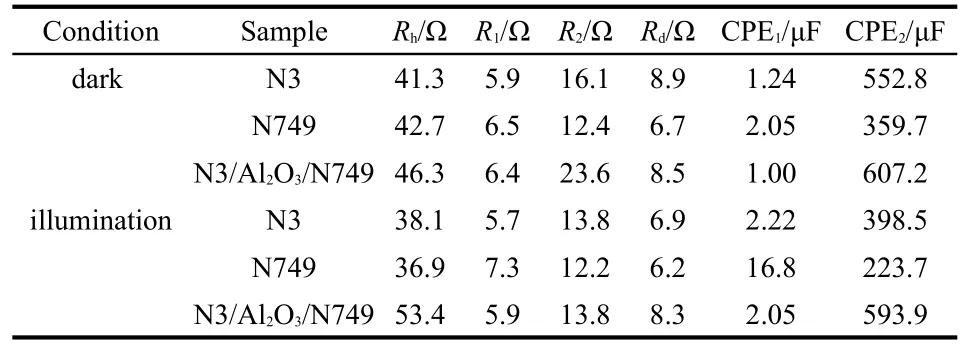
Table 2 Calculated values of EIS results of devices based on N3,N749,and N3/Al2O3/N749
On the illumination condition,there were mainly three processes in DSCs,electron injection from N3 to conductive band of TiO2,electron injection from N749 to conductive band of TiO2,and electron jumping from N749 to N3(an imaginary process,hardly happened as the LUMO energy levels of the two dyes were similar),which were seen in the processes(i),(ii),and(iii)in Fig.4,respectively.The process(i)represented R2and CPE2,and the process(ii)could be considered as a diode in parallel to R2and CPE2,representing R5and CPE4in Fig.5(c).At the same time,the process(iii)could be considered as a diode in series to R2and CPE2,representing R4and CPE3in Fig.5(c).
As shown in Table 2,in illumination condition,the interface resistances of TiO2/N3/electrolyte,TiO2/N749/electrolyte,and TiO2/N3/Al2O3/N749/electrolyte were 13.8,12.2,and 13.8 Ω,respectively.Based on the model in illumination condition,the apparent resistance value(Rapp)of TiO2/N3/Al2O3/N749/electrolyte could be calculated as follow:

Because the LUMO energy levels of N3 and N749 were similar,so the resistance(R5)and capacitance(CPE4)values of process(iii)were rather large.As a result,the value of Rappcould be approximately equal to R6based on equation(2),which was 12.2 Ω,similar to the measured value(13.8 Ω).Similarly,the value of the apparent capacitance(CPEapp)could be calculated as follow:

As a result,the calculated value of CPEappwas 611.2 μF,approximate to the measured value(593.9 μF).
Based on the discussion above,it was shown that reasonable equivalent circuit models were built to explain the effect of N3/Al2O3/N749 structure in DSCs.The error was considered coming from the defect in such a multi-layer structure.
Besides influence on the interface resistance,N3/Al2O3/N749 structure also influenced the fill factor of devices.Series resistance(Rs)was well-known as a key factor that affected the FF of a device.Rsis mainly composed of the resistance of the conductive glass,the resistance of the electron transport within TiO2and the bulk resistance of the electrolyte.The following five equations revealed the relationship between FF and the Rs.47,48In equation(4),Rchrepresented the characteristic resistance of the solar cell.In equation(5),rsrepresented the normalized series resistance.In equation(6),νocwas defined as normalized Voc,k is Boltzman constant,and T is the temperature in Kelvin.41In equation(7),FF0was denoted as the idealized fill factor.

Based on the results of Table 2,set n=1,T=300 K,and it was known that the elementary charge q=1.6×10-19C,the Boltzmann's constant k=1.38×10-23J·K-1,after calculating of the equations above,the results were shown in Table 3.Compared to the measured values,the relative errors of calculated FF of devices based on N3,N749,and N3/Al2O3/N749 were only 3.46%,6.41%,and 2.56%,respectively.From equation(7),it was indicated that the idealized fill factor of device based on N3/Al2O3/N749 structure was larger than that of device based on N3 or N749 because enhancement of Vocfrom the retarding of chargerecombination shown in the EIS results.However,the measured results showed a smaller FF of device based on the N3/Al2O3/N749 structure.It could be explained that with the N3/Al2O3/N749 structure,the light harvesting was obviously increased,then Jscenhanced compared to that with N3 or N749 individually.From equation(4),value of Rchdecreased with the increase of Jsc.Besides,the value of Rsincreased caused by the interlayer of Al2O3.Then from equation(5),the value of rsincreased.Thus from equation(8),the value of FF decreased reasonably.And the calculated and measured value confirmed the explanation.

Table 3 Calculated values and measured results of fill factors ofdevices based on N3,N749,and N3/Al2O3/N749
Bode plots of devices based on N3,N749,and N3/Al2O3/N749 were shown in Fig.6.The three peaks in the phase of the spectrum were associated with three transient processes in the DSC.The middle-frequency peak(in the 10-100 Hz range)was determined by the lifetime of the electrons in TiO2,which is shown as following equation:49

As shown in Fig.6,the minimum frequency of device using N3/Al2O3/N749 alternating structure was smaller than that using only one kind of dye.As a result,from equation(9),the lifetime of electrons in the TiO2was enhanced by using such an alternating structure.It was caused by the retarding of charge recombination from the interface modification ofAl2O3.
The Vocof DSCs can be expressed by following equation:50

where R is the molar gas constant,F is the Faraday constant,β is the reaction order for I-3and electrons,A is the electrode area,I is the incident photon flux,n0is the concentration of accessible electronic states in the conduction band,kband krare the kinetic constants of the back reaction and the recombination,respectively.[I-3]and[D+]are concentrations of triodide and oxidized dye,respectively.It could be considered that fminwas the same as the back reaction constant(kb).44The values of Vocincreased with the decreasing of back reaction,which was same as fmin.This result accorded with equation(10),indicating that the enhancement was caused by the increasing of electron lifetime in TiO2due to strengthened retarding effect of charge recombination applying the alternating structure of N3/Al2O3/N749.

Fig.6 Bode plots of devices based on N3,N749,and N3/Al2O3/N749
To explore the influence of N3/Al2O3/N749 alternating structure on the electron diffusion and lifetime in photoanode,IMVS and IMPS of devices based on N3,N749,and N3/Al2O3/N749 were tested.IMVS experiment used the same intensity perturbation but measured the periodic modulation of the photovoltage,giving the information of electron lifetime under open-circuit conditions.51As shown in Fig.7(a),compared to devices using only one kind of dye,the electron lifetime in photoanode of device based on N3/Al2O3/N749 alternating structure was longer,which accorded with the results of EIS test.It could be explained that as an interface modification material,the interlayer of Al2O3retarded the charge recombination effectively,then the electrons in the conductive band of TiO2was difficult to react with redox couple in electrolyte.IMPS measured the periodic photocurrent response of device to a small sinusoidal perturbation of the light intensity superimposed on a larger steady background level,providing information about the dynamics of charge transport and back reaction under short circuit conditions.45As shown in Fig.7(b),compared to devices using only one kind of dye,the electron diffusion coefficient(Dn)of device based on N3/Al2O3/N749 alternating structure obviously increased,which indicated that this structure was beneficial to electron transportation in photoanode of DSCs.This result was also accorded with the value of Jsc.It could be due to the increased electron injection and decreased electron quenching and recombination caused by the interface modification effects.The effective diffusion coefficient of electrons,Deff,determined by the equation(11):52

where nfreeis the density of free conduction band electrons,ntotalis the total density of free and trapped electrons,and D0is the standard electron diffusion coefficient.As shown in the results of IPCE spectra,the electron injected into the conductive band of TiO2increased obviously compared to that using N3 or N749 individually.Using equation(11),it could explain why the electron diffusion coefficient increased using the N3/Al2O3/N749 alternating structure.
To weigh the electron transport and recombination properties,charge collection efficiency(ηcc)derived from IMPS and IMVS measurements was apparently considered as meaningful parameter.In sensitized solar cells,ηcccan be calculated by the following equation:53

where τcis the electron collection time and τdis the electron lifetime.Fig.7(c)showed that the dependence of the charge collection efficiency on the different light intensity.Compared to devices using only one kind of dye,the charge collection efficiency of device based on N3/Al2O3/N749 alternating structure obviously increased,which indicated that this structure was beneficial to charge collection in photoanode of DSCs.According to the following equation:54

where ηlhis the light capture efficiency,ηinjis the electron injection efficiency,ηccis in direct proportion to Jscof the sensitized solar cells,I0is the idea photocurrent.As shown in Fig.7(c),the result of charge collection efficiency also accorded with the results of I-V curve shown in Fig.3(a).
4 Conclusions
In summary,N3/Al2O3/N749 alternating structure widening the photoresponse was introduced and the interface electron processes were discussed.The widened photoresponse increased the Jscof DSCs.Besides,the interlayer of Al2O3retarded the charge recombination obviously,which caused the increase of Vocand decrease of dark current.Thus the conversion efficiency was enhanced.The device based on N3/Al2O3/N749 obtained a 5.75%conversion efficiency,which was higher than that based on N3 or N749,which was 4.22%and 3.09%,respectively.The results of EIS showed that the N3/Al2O3/N749 structure increased the interface resistance in dark condition,indicating that the charge recombination was retarded.To analyze the electron process in DSC based on N3/Al2O3/N749 alternating structure,a series of equivalent circuit models were built based on the EIS results.It could explain the process of electron and the change of parameters of DSCs reasonably.The results of IMVS and IMPS test indicated that the N3/Al2O3/N749 alternating structure increased the electron life time and diffusion coefficient,enhancing the electron transportation.Thus the N3/Al2O3/N749 alternating structure enhanced the photoresponse and remained the interface modification effects at the same time,improving the performance of DSCs effectively.
(1) O'Regan,B.;Grätzel,M.Nature 1991,353,737.doi:10.1038/353737a0
(2) Kuang,D.B;Klein,C.;Ito,S.;Moser,J.;Baker,R.;Zakeeruddin,S.;Grätzel,M.Adv.Funct.Mater.2007,17,154.
(3) Hu,L.H.;Dai,S.Y.;Weng,J.;Xiao,S.F.;Sui,Y.F.;Huang,Y.;Chen,S.H.;Kong,F.T.;Pan,X.;Liang,L.Y.;Wang,K.J.J.Phys.Chem.B 2007,111,358.doi:10.1021/jp065541a
(4) Hara,K.;Sugihara,H.;Tachibana,Y.;Islam,A.;Yanagida,M.;Sayama,K.;Arakawa,H.Langmuir 2001,17,5992.doi:10.1021/la010343q
(5) Jung,H.S.;Lee,J.K.;Nastasi,M.;Lee,S.W.;Kim,J.Y.;Park,J.S.;Hong,K.S.Langmuir 2005,21,10332.doi:10.1021/la051807d
(6) Nakade,S.;Kanzaki,T.;Kambe,S.;Wada,Y.;Yanagida,S.Langmuir 2005,21,11414.doi:10.1021/la051483t
(7) Sommeling,P.M.;Späth,M.;Smit,H.J.P.;Bakker,N.J.;Kroon,J.M.J.Photochem.Photobiol.A:Chem.2004,164,137.doi:10.1016/j.jphotochem.2003.12.017
(8) Grätzel,M.C.R.Chimie.2006,9,578.
(9) Figgemeier,E.;Hagfeldt,A.Int.J.Photoenergy 2004,6,127.doi:10.1155/S1110662X04000169
(10) Meng,Q.B.;Takahashi,K.;Zhang,X.T.;Sutanto,I.;Rao,T.N.;Sato,O.;Fujishima,A.Langmuir 2003,19,3572.doi:10.1021/la026832n
(11) Sathiya Priya,A.R.;Subramania,A.;Jung,Y.S.;Kim,K.J.Langmuir 2008,24,9816.doi:10.1021/la801375s
(12) Grätzel,M.Accounts Chem.Res.2009,42,1788.doi:10.1021/ar900141y
(13)Fang,J.H.;Mao,H.F.;Wu,J.W;Zhang,X.Y;Lu,Z.H.Appl.Surf.Sci.1997,119,237.doi:10.1016/S0169-4332(97)00195-5
(14)Fang,J.H.;Su,L.Y.;Wu,J.W.;Shen,Y.C.;Lu,Z.H.New J.Chem.1997,21,1303.
(15) Perera,V.;Pitigala,P.;Jayaweera,P.;Bandaranayake,K.;Tennakone,K.J.Phys.Chem.B 2003,107,13758.doi:10.1021/jp0348979
(16) Kuang,D.B.;Walter,P.;Nüesch,F.;Kim,S.;Ko,J.;Comte,P.;Zakeeruddin,S.M.;Grätzel,M.Langmuir 2007,23,10906.doi:10.1021/la702411n
(17) Cid,J.;Yum,J.;Jang,S.;Nazeeruddin,M.K.;Ferrero,E.M.;Palomares,E.;Ko,J.;Grätzel,M.;Torres,T.Angew.Chem.Int.Edit.2007,46,8358.
(18)Liu,B.Q.;Zhao,X.P.;Luo,W.Dyes and Pigments 2008,76,327.doi:10.1016/j.dyepig.2006.09.004
(19) Clifford,J.N.;Palomares,E.;Nazeeruddin,M,K.;Thampi,R.;Grätzel,M.;Durrant,J.R.J.Am.Chem.Soc.2004,126,5670.doi:10.1021/ja049705h
(20) Choi,H.;Kim,S.;Kang,S.O.;Ko,J.;Kang,M.S.;Clifford,J.N.;Forneli,A.;Palomares,E.;Nazeeruddin,K.;Grätzel,M.Angew.Chem.Int.Edit.2008,120,8383.doi:10.1002/ange.v120:43
(21)Bandaranayake,K.M.P.;Senevirathna,M.K.I.;Weligamuwa,P.;Tennakone,K.Coord.Chem.Rev.2004,248,1277.doi:10.1016/j.ccr.2004.03.024
(22)Diamant,Y.;Chen,S.G.;Melamed,O.;Zaban,A.J.Phys.Chem.B 2003,107,1977.doi:10.1021/jp027827v
(23)Gao,R.;Wang,L.D.;Ma,B.B.;Zhan,C.;Qiu,Y.Langmuir 2010,26,2460.doi:10.1021/la902688a
(24)Gao,R.;Ma,B.B.;Wang,L.D.;Shi,Y.T.;Dong,H.P.;Qiu,Y.Acta Phys.-Chim.Sin.2011,27,413.[高 瑞,马蓓蓓,王立铎,史彦涛,董豪鹏,邱 勇.物理化学学报,2011,27,413.]doi:10.3866/PKU.WHXB20110234
(25) Lao,C.F.;Chu,Z.Z.;Zou,D.C.Acta Phys.-Chim.Sin.2011,27,419.[劳春峰,初增泽,邹德春.物理化学学报,2011,27,419.]doi:10.3866/PKU.WHXB20110209
(26)Gao,R.;Wang,L.;Geng,Y.;Ma,B.;Zhu,Y.;Dong,H.;Qiu,Y.Phys.Chem.Chem.Phys.2011,13,10635.
(27)Chen,D.P.;Zhang,X.D.;Wei,C.C.;Liu,C.C.;Zhao,Y.Acta Phys.-Chim.Sin.2011,27,425.[陈东坡,张晓丹,魏长春,刘彩池,赵 颖.物理化学学报,2011,27,425.]doi:10.3866/PKU.WHXB20110222
(28)Gao,R.;Wang,L.;Geng,Y.;Ma,B.;Zhu,Y.;Dong,H.;Qiu,Y.J.Phys.Chem.C 2011,115,17986.doi:10.1021/jp204466h
(29)Gao,R.;Niu,G.D.;Wang,L.;Geng,Y.;Ma,B.;Zhu,Y.;Dong,H.;Qiu,Y.Phys.Chem.Chem.Phys.2012,14,5973.
(30)O'Regan,B.C.;Scully,S.;Mayer,A.C.J.Phys.Chem.B 2005,109,4616.doi:10.1021/jp0468049
(31)Alarcon,H.;Boschloo,G.;Mendoza,P.;Solis,J.L.;Hagfeldt,A.J.Phys.Chem.B 2005,109,18483.doi:10.1021/jp0513521(32)Wu,S.J.;Han,H.W.;Tai,Q.D.;Zhang,J.;Xu,S.;Zhou,C.H.;Yang,Y.;Hu,H.;Chen,B.L.;Sebo,B.;Zhao,X.Z.Nanotechnology 2008,19,215704.doi:10.1088/0957-4484/19/21/215704
(33)Chen,S.G.;Chappel,S.;Diamant,Y.;Zaban,A.Chem.Mater.2001,13,4629.doi:10.1021/cm010343b
(34) Palomares,E.;Clifford,J.N.;Haque,S.A.;Lutz,T.;Durrant,J.R.J.Am.Chem.Soc.2003,125,475.doi:10.1021/ja027945w
(35) Wang,P.;Wang,L.D.;Li,B.;Qiu,Y.Chin.Phys.Lett.2005,22,2708.doi:10.1088/0256-307X/22/10/069
(36) Menzies,D.B.;Cervini,R.;Cheng,Y.B.;Simon,G.P.;Spiccia,L.J.Sol-Gel Sci.Technol.2004,32,363.doi:10.1007/s10971-004-5818-0
(37) Liu,Z.Y.;Pan,K.;Liu,M.;Wang,M.J.;Lu,Q.;Li,J.H.;Bai,Y.B.;Li,T.J.Electrochim.Acta 2005,50,2583.doi:10.1016/j.electacta.2004.11.003
(38) Zhang,X.Y.;Sutanto,I.;Taguchi,T.;Tokuhiro,K.;Meng,Q.B.;Rao,T.N.;Fujishima,A.;Watanabe,H.;Nakamori,T.;Uragami,M.Sol.Energy Mater.Sol.Cells 2003,80,315.doi:10.1016/j.solmat.2003.08.006
(39) Palomares,E.;Clifford,J.N.;Haque,S.A.;Lutz,T.;Durrant,J.R.Chem.Commun.2002,1464.
(40)Luo,F.;Wang,L.D.;Ma,B.B.;Qiu,Y.J.Photochem.Photobiol.A:Chem.2008,197,375.doi:10.1016/j.jphotochem.2008.02.011
(41) Ma,B.B.;Gao,R.;Wang,L.D.;Luo,F.;Zhan,C.;Li,J.L.;Qiu,Y.J.Photochem.Photobiol.A:Chem.2009,202,33.doi:10.1016/j.jphotochem.2008.11.004
(42) Burnside,S.D.;Shklover,V.;Barbé,C.;Comte,P.;Arendse,F.;Brooks,K.;Grätzel,M.Chem.Mater.1998,10,2419.doi:10.1021/cm980702b
(43) Huo,Z.P.;Dai,S.Y.;Wang,K.J.;Kong,F.T.;Zhang,C.N.;Pan,X.;Fang,X.Q.Sol.Energy Mater.Sol.Cells 2007,91,1959.doi:10.1016/j.solmat.2007.08.003
(44) Grätzel,M.Inorg.Chem.2005,44,6841.doi:10.1021/ic0508371
(45) Bisquert,J.J.Phys.Chem.B 2002,106,325.doi:10.1021/jp011941g
(46)Wang,Q.;Moser,J.;Grätzel,M.J.Phys.Chem.B 2005,109,14945.doi:10.1021/jp052768h
(47)Qin,D.;Zhang,Y.D.;Huang,S.Q.;Luo,Y.H.;Li,D.M.;Meng,Q.B.Electrochim.Acta 2011,56,8680.doi:10.1016/j.electacta.2011.07.065
(48) Green,M.A.Solar Cells;Prentice-Hall:Englewood,NJ,1982;Vol.96,pp 85-86.
(49) Kern,R.;Sastrawan,R.;Ferber,J.;Stangl,R.;Luther,J.Electrochim.Acta 2002,47,4213.doi:10.1016/S0013-4686(02)00444-9
(50)Lee,K.;Park,S.W.;Ko,M.J.;Kim,K.;Park,N.G.Nature Materials 2009,8,665.doi:10.1038/nmat2475
(51) Schlichthörl,G.;Huang,S.Y.;Sprague,J.;Frank,A.J.J.Phys.Chem.B 1997,101,8141.doi:10.1021/jp9714126
(52) Dloczik,L.;Ileperuma,O.;Lauermann,I.;Peter,L.M.;Ponomarev,E.A.;Redmond,G.;Shaw,N.J.;Uhlendorf,I.J.Phys.Chem.B 1997,101,10281.doi:10.1021/jp972466i
(53) Hagfeldt,A.;Boschloo,G.;Sun,L.C.;Kloo,L.;Pettersson,H.Chem.Rev.2010,110,6595.doi:10.1021/cr900356p
(54) Zhu,K.;Neale,N.R.;Miedaner,A.;Frank,A.J.Nano Lett.2007,7,69.doi:10.1021/nl062000o

