以草酸及咪唑基配体构筑的二维网状锌(Ⅱ)配位聚合物的合成、晶体结构及荧光性质
战佩英 纪建业 牛艳玲 王志涛 李秀梅
(通化师范学院化学系,通化 134002)
0 Introduction
Recently, there has been much interest in the construction of coordination compounds due to their versatile structures and interesting topologies[1-4]as well as their potential applications as functional materials in the fields of molecular magnetism,catalysis, gas sorption and optoelectronic devices[5-9].The most useful building blocks for constructing organic-inorganic hybrid coordination compounds are carboxylate and N-donor ligands. Aromatic carboxylate ligands have been extensively employed in the construction of various dimensional coordination compounds because of their abundant coordination modes and high structural stability for functional materials applications[10-16].
Imidazole and its derivatives are typically heterocyclic ligands with nitrogen as the donor atom.It exhibits a wide variety of pharmacological activities as antihelmintics, anticancer, antifungal and antiinflammatory agent[17]. Because of this, the coordination chemistry of related ligands has been the subject of numerous investigations. As a good candidate, the conformational flexible ligand 1,3-bis (imidazol-1-yl)butane has been used as a auxiliary ligand because of the free rotation of the two imidazole planes, which results in cis or trans conformations as required by the metal coordination geometry in the assembly process[18-20].
To explore the combination effects of this neutral di-imidazole ligand (1,3-bix) and anionic squarate ligands, we synthesize coordination polymers of Zn(Ⅱ)containing these ligands. Herein, we report the synthesis, characterization of a new zinc framework,[Zn(C2O4)(1.3-bix)]nunder hydrothermal conditions.
1 Experimental
1.1 General procedures
All reagents were purchased commercially from Jinan Camolai Trading Company and used without further purification. Elemental analyses (C, H and N)were measured on a Perkin-Elmer 2400 CHN Elemental Analyzer. IR spectrum was recorded in the range of 4 000~400 cm-1on an Alpha Centaurt FT/IR Spectrophotometer using a KBr pellet. TG studies were performed on a Perkin-Elmer TGA7 analyzer.The fluorescent studies were carried out on a computer-controlled JY Fluoro-Max-3 spectrometer at room temperature.
1.2 Synthesis
The title complex was prepared from a mixture of Zn(ClO4)2·6H2O (0.2 mmol, 0.074 g), H2C2O4·2H2O(0.4 mmol, 0.05 g), 1,3-bix (0.2 mmol, 0.048 g) and H2O (18 mL) in a 30 mL Teflon-lined autoclave under autogenous pressure at 150 ℃for 7 d. After cooling to room temperature, pale yellow block crystals were collected by filtration and washed with distilled water in 45% yield (based on Zn). Anal. Calcd. for C16H14N4O4Zn (%): C, 49.06; H, 3.60; N, 14.30. Found(%):C,48.86;H,3.25;N,13.95.IR (KBr,cm-1):3 133 w, 3 007w, 1 668m, 1 609s, 1 520m, 1 446w, 1 434m,1 407w,1 364w,1 318m,1 309w,1 229m,1 105w,1 086 m,1 037w,1 029w, 975w, 941m, 827w, 814w, 795m,774w, 745m, 732w, 658m, 638w, 497m.
1.3 Structure determination
A single crystal of the title complex with dimensions of 0.36 mm×0.27 mm×0.22 mm was mounted on a Bruker Smart Apex ⅡCCD diffractometer equipped with a graphite-monochromatic Mo Kα (λ=0.071 073 nm) radiation using an φ-ω scan mode at 292(2) K. In the range of 4.32°<2θ<52.18°, a total of 4 318 reflections were collected and 3 036 were independent with Rint=0.010 2, of which 2 760 were observed with I>2σ(I). The correction for Lp factors was applied.The structure was solved by direct methods with SHELXS-97 program[21]and refined by full-matrix least-squares techniques on F2with SHELXL-97[22].All non-hydrogen atoms were refined anisotropically and hydrogen atoms isotropically. All H atoms were placed in calculated positions and refined as riding with Uiso(H)=1.2Ueq(C). The final R=0.025 5 and wR=0.060 9 (w=1/[σ2(Fo2)+(0.028 7P)2+0.333 9P], where P=(Fo2+2Fc2)/3). S=1.053, (Δρ)max=397 e·nm-3, (Δρ)min=-245 e·nm-3and (Δ/σ)max=0.000. The selected important bond parameters are given in Table 1.
CCDC: 908764.
2 Results and discussion
2.1 IR spectrum
The COO-is coordinated with its asymmetric and symmetric stretching appearing at 1 609 cm-1(ν(OCO)assym)and 1 434 cm-1(ν(OCO)sym)[23],respectively.The Δν(ν(OCO)assym-ν(OCO)sym) is 175 cm-1(<200),showing the presence of bidentate linkage of carboxylates in the dianions. Thus the carboxylates coordinate to the metal as bidentate ligands via the carboxylate groups[24]. The absence of the characteristic bands at abound 1 700 cm-1in compound 1 attributed to the protonated carboxylic group indicates that the complete deprotonation of cbba ligand upon reaction with Zn ions[25]. In addition, X-ray diffraction analysis further indicates the existence of bidentate coordination manners of the carboxylate groups and prence deprotonation of C2O42-ligands.
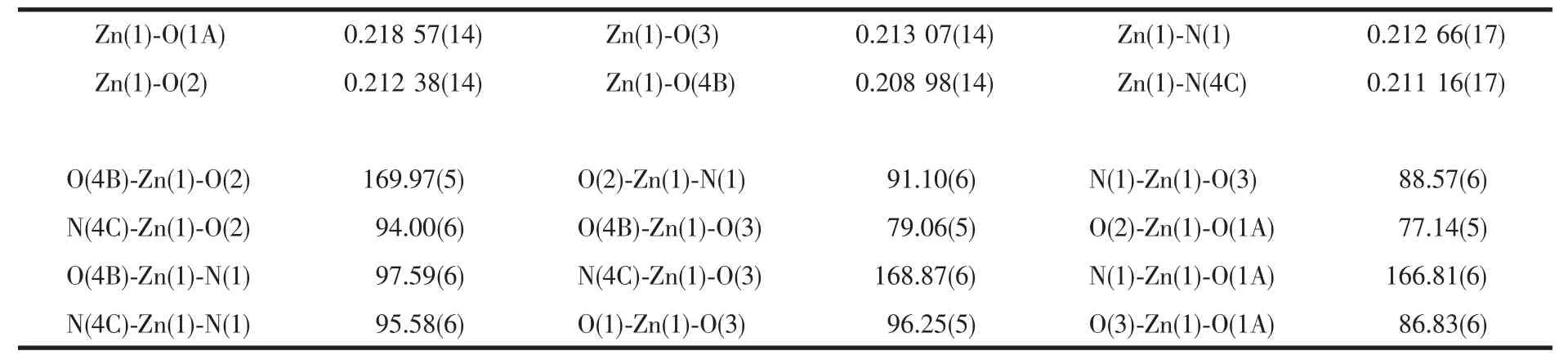
Table 1 Selected bond lengths (nm) and bond angles (°) for 1
2.2 Description of the structure
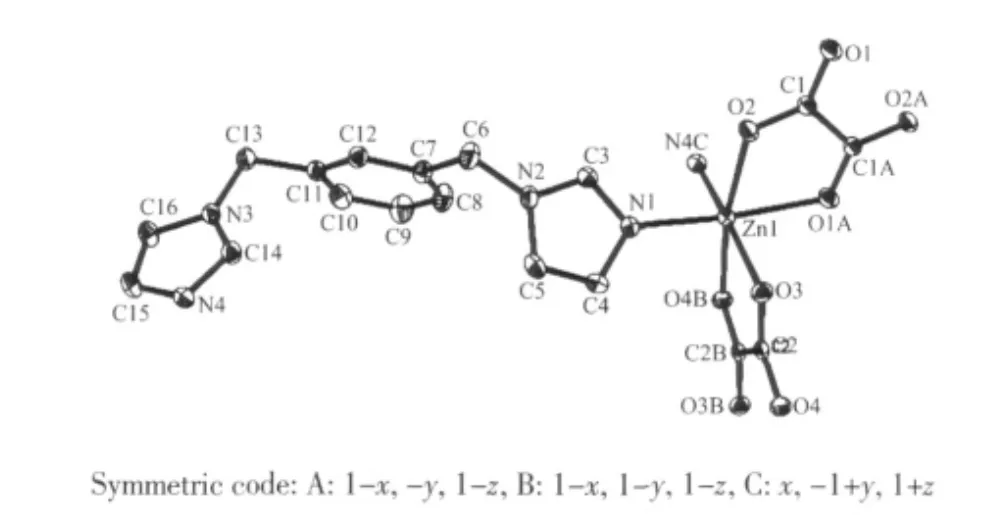
Fig.1 Coordination environment of Zn(Ⅱ)in 1 showing the labling of atoms with thermal ellipsoids at 30% probability
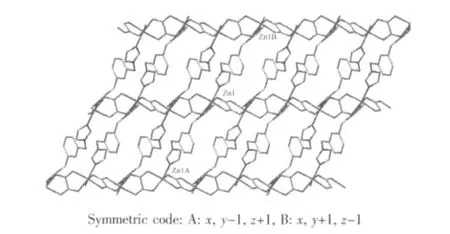
Fig.2 View of the two-dimensioal network construced from [Zn4(C2O4)2(1,3-bix)2] units along a axis
Hydrogen bonding interactions are usually important in the synthesis of supramolecular architectture[26].There are persistent C-H…O hydrogen bonding interactions between carboxylate oxygen atom and carbon atoms of 1,3-bix ligands in complex 1 (Table 2). Moreover, there are π-π interactions in complex 1 between imidazole rings of 1,3-bix ligands. The centroid-to-centroid distance between adjacent ring is 0.364 85(17) nm for N1C3N2C5C4 and N1′C3′N2′C5′C4′ (1-x, 1-y, -z) imidazole rings. The perpendicular distance is 0.334 42 (10) nm for N1C3N2C5C4 and N1′C3′N2′C5′C4′ (1-x, 1-y, -z) imidazole rings.Therefore, through hydrogen bonds and π-π interactions,the two-dimensional networks are further extended into a three-dimensional supramolecular framework(Fig.3).
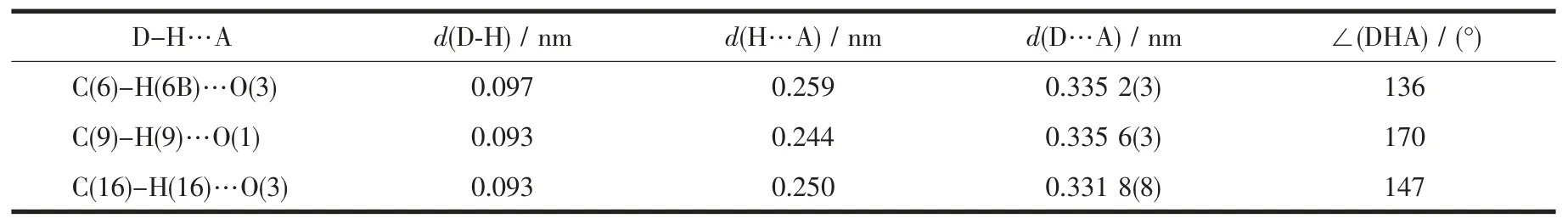
Table 2 Hydrogen bonds for complex 1
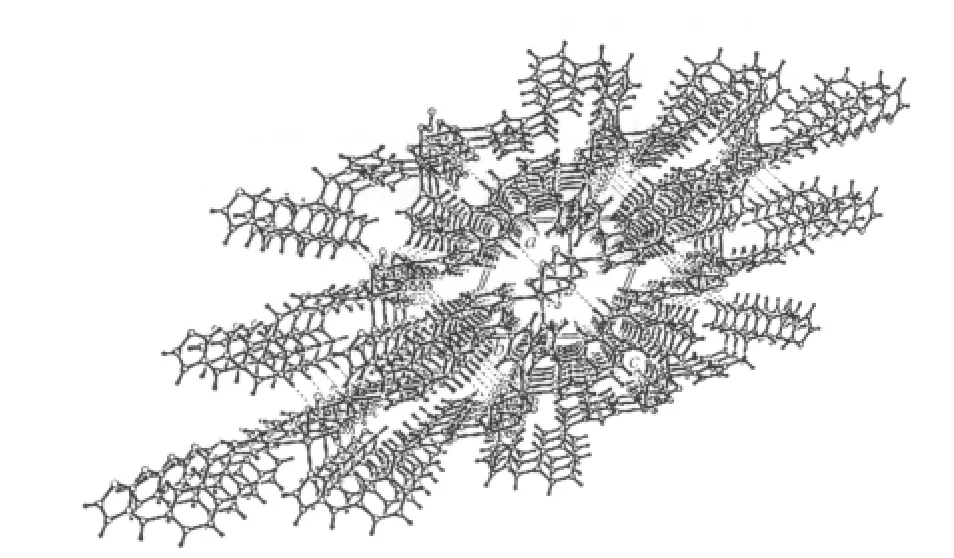
Fig.3 3D Supramolecular framework viewed along the b axis in 1
2.3 Thermal analysis
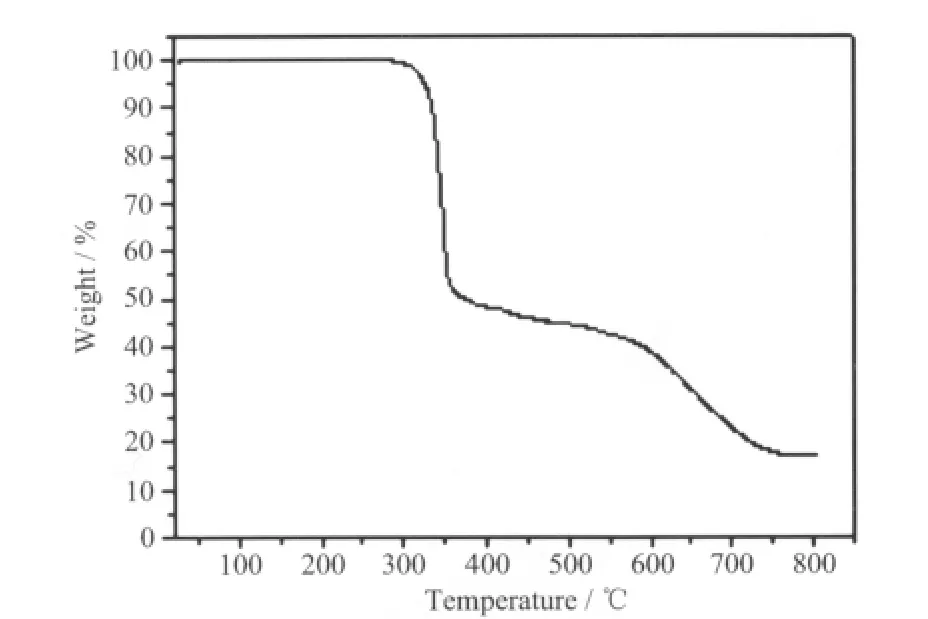
Fig.4 TG curve of the title complex
TG curve of 1 (Fig.4) show that the first weight loss of 61.1% from 285 to 534 ℃corresponds to the removal of 1.3-bix ligand (Calcd. 60.84%). Upon further heating, an obvious weight loss (23.7%) occurs in the temperature range of 534 ~760 ℃, corresponding to the release of C2O42-ligand (Calcd. 22.47%).After 760 ℃no weight loss is observed, which means the complete decomposition of 1. The residual weight should be ZnO.
2.4 Photoluminescent properties
The emission spectrum of complex 1 in the solid state at room temperature is shown in Fig.5. It can be observed that complex 1 exhibits green photoluminescence with an emission maximum at ca. 470 nm upon excitation at 282 nm. In order to understand the nature of these emission bands, we first analyzed the photoluminescence properties of free oxalic acid, and confirmed that it does not emit any luminescence in the range of 400~800 nm. And then we investigated the emission spectrum of 1,3-bix itself and the result indicated that it does not emit any luminescence in the range 400~800 nm, which has also been confirmed previously.Thus,according to the previous literature[27],the emission band could be assigned to the emission of ligand-to-metal charge transfer (LMCT). For possesses strong fluorescent intensity, it appears to be good candidates for novel hybrid inorganic-organic photoactive materials.
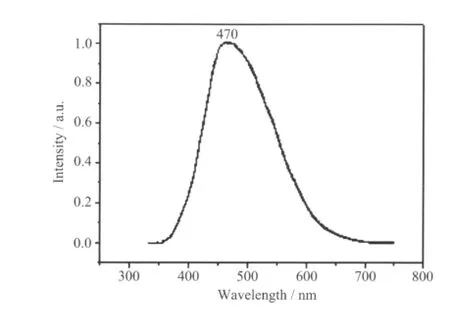
Fig.5 Solid-state emission spectrum of 1 at room temperature
Reference:
[1] Hill R J, Long D L, Champness N R, et al. Acc. Chem. Res.,2005,38:335-348
[2] Friedrichs O D, O′Keeffe M, Yaghi O M. Acta Crystallogr.A, 2003,59:22-27
[3] Ye B H, Tong M L, Chen X M. Coord. Chem. Rev., 2005,249:545-565
[4] Inoue K, Imai H, Ghalsasi P S, et al. Angew. Chem., Int.Ed. Engl., 2001,40:4242-4245
[5] Evans O R, Lin W B. Acc. Chem. Res., 2002,35:511-522
[6] Janiak C J. Chem. Soc. Dalton. Trans., 2003:2781-2804
[7] Seo J S, Whang D, Lee H, et al. Nature, 2000,404:982-986
[8] Sato O,Lyoda T,Fujishima A,et al.Science,1996,271:49-51
[9] Lin W, Evans O R, Xiong R, et al. J. Am. Chem. Soc.,1998,120:13272-13273
[10]Anokhina V, Vougo-Zanda M, Wang X Q, et al. J. Am.Chem. Soc., 2005,127:15000-15001
[11]Wang X Q, Liu L M, Jacobson A J. Angew. Chem. Int. Ed.,2006,39:6499-6503
[12]Chen W, Wang J Y, Chen C, et al. Inorg. Chem., 2003,42:944-946
[13]Pan L, Parker B, Huang X Y, et al. J. Am. Chem. Soc.,2006,128:4180-4181
[14]Reineke T M, Eddaoudi M, Fehr M, et al. J. Am. Chem.Soc., 1999,121:1651-1657
[15]He J H, Yu J H, Zhang Y T, et al. Inorg. Chem., 2005,44:9279-9282
[16]Go Y B, Wang X Q, Anokhina E V, et al. Inorg. Chem.,2005,44:8265-8271
[17]Bhatnagar A, Sharma P K, Kumar N. Int. J. Pharm., 2011,3(1):268-282
[18]Liu W L, Yu J H, Jiang J X, et al. CrystEngComm, 2011,13:2764-2773
[19]Chen Z Y, Lin Z E, Stips B, et al. Inorg. Chem. Commun.,2011,14(1):137-139
[20]Liu Y Y, Ma J F, Jin Y, et al. Inorg. Chem., 2007,46(8):3027-3037
[21]Sheldrick G M. SHELXS 97, Program for the Solution of Crystal Structure, University of Göttingen, Germany, 1997.
[22]Sheldrick G M. SHELXS 97, Program for the Refinement of Crystal Structure, University of Göttingen, Germany, 1997.
[23]Devereux M, Shea D O, Kellett A, et al. Inorg. Biochem.,2007,101:881-892
[24]Farrugia L J, Wing X A. Windows Program for Crystal Structure Analysis,University of Glasgow,Glasgow,UK,1988.
[25]Bellamy L J. The Infrared Spectra of Complex Molecules.New York: Wiley, 1958.
[26]Krische M J, Lehn J M. Struct. Bonding, 2000,96:3-29
[27]Mohamed G G, El-Gamel N E A. Spectrochim. Acta, Part A,2004,60:3141-3154

