Purification and Identification of a Clotting Protein from the Hemolymph of Chinese Shrimp (Fenneropenaeus chinensis)
WANG Baojie1), 2), PENG Hongni3), LIU Mei1), JIANG Keyong1), ZHANG Guofan1),and WANG Lei1),*
Purification and Identification of a Clotting Protein from the Hemolymph of Chinese Shrimp ()
WANG Baojie, PENG Hongni, LIU Mei, JIANG Keyong, ZHANG Guofan,and WANG Lei
1),,266071,2),100049,
The clotting protein (CP) plays important and diverse roles in crustaceans, such as coagulation and lipid transportation. A clotting protein was purified from the hemolymph of Chinese shrimp(named as Fc-CP) with Q sepharose HP anion-exchange chromatography and phenyl sepharose HP hydrophobic interaction chromatography. Fc-CP was able to form stable clotsin the presence of hemocyte lysate and Ca, suggesting that the clotting reaction is catalyzed by a Ca-dependent transglutaminase in shrimp hemocytes. The molecular mass of Fc-CP was 380 kDa under non-reducing conditions and 190 kDa under reducing conditions as was determined with SDS-PAGE. CP exists as disulfide-linked homodimers and oligomers. The N-terminal amino acid sequence of Fc-CP was identical to that of shrimps including,and; and similar to that of other decapods. The purified Fc-CP was digested with trypsin and verified on an ABI 4700 matrix-assisted laser desorption/ionization tandem time-of-flight (MALDI-TOF/TOF) mass spectrometry. Our results will aid to better understanding the coagulation mechanism of shrimp hemolymph.
; clotting protein; purification; proteomic identification; MALDI-TOF/TOF MS
1 Introduction
The defense mechanisms of crustaceans depend completely on the innate immune system, and hemolymph coagulation is an essential immune mechanism for them with an open circulatory system (Vazquez., 2009). Crustaceans hemolymph generally form clots rapidly while injured, which is necessary for preventing hemolymph loss and further intrusion of invading pathogens into hemocoel (Theopold., 2004). The coagulation reaction has been characterized at the molecular level in crustaceans. The reaction involves a specific clotting protein from hemolymph, which covalently crosslinks to form stable clots with the catalysis of transglutaminase (TGase) from hemocytes (Fagutao., 2012; Wang., 2001). Clotting protein (CP) has been purified and characterized from the hemolymph of several crustaceans including freshwater crayfish(Kopacek., 1993), white shrimp(Montano-Perez., 1999), tiger shrimp(Yeh., 1998), kuruma shrimpand pink shrimp(Perazzolo., 2005). All these CPs are homodimeric glycoproteins with molecular masses ranging from 340 to 420kDa. The Nterminal amino acid sequence of these CPs is highly homologous . The CP genes have been isolated and characterized from some crustaceans. Several full length cDNAs of CP gene transcripts have been isolated and sequenced. The abundance of CP gene transcript has also beendetermined for different shrimp tissues with RT-PCR (Cheng., 2008; Yeh., 2007). Based on its molecular weight, subunit composition and N-terminal amino acid sequence, a very high- density lipoprotein (VHDL) found in hemolymph of white shrimpwas demonstrated to be highly similar to CP (Yepiz-Plascencia., 2002). Thus, crustacean CP might also be involved in lipid transportation, and multifunctional.
The Chinese shrimpis one of cultured aquatic animals with high values, which is an important aquatic protein resource for Chinese. To our knowledge, the coagulation mechanism and the characterization of CP of in Chinese shrimp.were not reported. In present study, we attempted to purify and characterize the CP from the hemolymph of Chinese shrimp in order to better understand the coagulation mechanism of shrimp hemolymph.
2 Materials and Methods
2.1 Hemocyte Lysate Supernatant (HLS) and Plasma/Serum Preparation
Adult Chinese shrimp,., was obtained from local markets in Qingdao, China and kept in a PVC tank with aerated sea water for at least 7d prior to experiments. To collect hemolymph, the shrimp individuals were anesthetized by cooling in ice-water. Hemolymph, 1.0mL each individual was obtained by inserting a 2.5mL syringe containing 1.0mL shrimp anticoagulant (450mmolLNaCl, 10mmolLKCl, 10mmolLHEPEs, 10mmolLEDTA.Na, pH7.4) into the ventral sinus at the first abdominal segment. The pooled hemolymph was immediately centrifuged at 800×g and 4℃ for 10min to pellet hemocytes with the supernatant collected as plasma which was used for purifying CP. The hemocytes was pooled and resuspended in 10mmolLsodium cacodylate, pH7.5, 0.4molLNaCl and sonicated with lysate supernatant (HLS) collected as described earlier (Perazzolo., 2005) .
For serum preparation, hemolymph was drawn directly from shrimp without anticoagulant and transferred to a tube and allowed to coagulate at 4℃ for 2h. The clot was repeatedly centrifuged (12000×g) at 4℃ for 20min with the supernatant used as a source of serum. The serum and plasma were divided into aliquots of 1.0mL and immediately frozen at −70℃ until use.
2.2 Purification of CP
The plasma was dialyzed against 50mmolLTris-HCl, pH8.0 (equilibration buffer) at 4℃ overnight. Four microliters of the dialyzed was applied to Q-sepharose HP column (GE Healthcare) coupled with an AKTA™ Purifier chromatography system (GE Healthcare) pre-equili- brated with 10 column volumes of equilibration buffer at a flow rate of 2mLmin. The bound fractions were eluted sequentially with 0.30, 0.45 and0.60molLNaCl. The elution was collected in fractions of 2mL and collection was started automatically when a threshold of 100mAU at 280nm was reached. The CP-enriched fractions, determined by clotting assay (see below), were localized and pooled, into which dry (NH)SOwas added to a final concentration of 0.50molL. The adjusted was reapplied to a column (phenyl sepharose HP; GE Healthcare) equilibrated with buffer A. The CP was eluted with a continuously descending linear gradient of (NH)SO(0.50 to 0molL) buffered with 20mmolLTris-HCl pH7.4 at a constant flow rate of 2mLmin. The UV absorbance of the eluate was read at 280nm.
2.3 SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE of the purified was carried out with Laemmli method using 7.5% polyacrylamide as separating gel and 3.9% polyacrylamide as stacking gel. The molecular weight of CP was determined with SDS-PAGE with or without adding dithiothreitol (DTT). Reduction of CP was performed by heating at 100℃ for 5min in a buffer containing 2% SDS and 2.5% DTT. The protein standards used included myosin (200kDa), calmodulin binding protein (130kDa), rabbit phosphorylase B (97.4kDa), bovine serum albumin (66.2kDa) and rabbit actin (43kDa). Proteins were envisaged by fixing and Coo- massie Blue G-250 staining.
2.4 N-Terminal Amino Acid Sequence Determination
The purified CP was separated in SDS-7.5% polyacrylamide gel and electrophoretically transferred to a PVDF membrane with a BG-blot MINI Transblot System. The membrane was stained with Coomassie brilliant blue R-250, destained and washed extensively to remove any glycine bound to the membrane. The proteins in the blot was N-terminal sequenced with Edman degradation in a PROCISE491 sequencer (Applied Biosystems) equipped in Biology and Life Science College, Beijing University, with sequence compared through NCBI-BLAST network service.
2.5 MALDI TOF/TOF MS and Protein Identification
2.5.1 In-gel digestion
The protein band corresponding to CP was cut out by hand, diced into 1- to 2-mm pieces, and placed into a 0.5 mL Eppendorf tube for in-gel digestion as described earlier (Wojdyla., 2011). Gel pieces were destained twice with 25mmolLNHHCOand 100% ACN, and reduced by 10mmolLDTT and alkylated by 100mmolLiodoacetamide. The protein was subjected to in-gel digestion by incubation with sequencing grade trypsin (Promega) at 37℃ overnight. After digestion, the supernatant was removed, and the gel pieces were extracted with 30μL of 5% formic acid followed by 30μL of 50% ACN and 50μL of 95% ACN. Each of these extractions was combined with the supernatant and dried in a SpeedVac concentrator (Thermo Savant). Dry peptides were dissolved in 5mgmLCAN in 50% ACN containing 0.1% TFA, and manually spotted onto a stainless steel plate for mass spectrometric analysis.
2.5.2 MALDI-TOF/TOF mass spectrometry
A MALDI-TOF/TOF mass spectrometer (ABI 4700 Proteomics Analyzer, Applied Biosystems) equipped with a 200Hz ND: YAG laser (355nm) was used to obtain mass spectrometry (MS) and tandem mass spectrometry (MS/MS) data. All MS survey scans were acquired over the mass range 700–3500m/z in the positive reflection mode and averaged 2000 laser shots with accelerating voltage of 21kV. The most and least intense ions per MALDI spot, with signal/noise ratios >25, were selected for subsequent MS/MS analysis in 1kV mode and 800–1000 consecutive laser shots. A mixture of standard peptides was used to externally calibrate the instrument.
2.5.3 Data analysis
Protein identification was carried out using GPS explorer software (Applied Biosystems) equipped with MASCOT (Matrix Science) search engine, Peptide mass fingerprinting (PMF) data obtained from the MS mode (MALDI-TOF MS) and peptide sequencing data obtained from the MS/MS mode (MALDI-TOF/TOF) were employed. All searches allowed for two missed tryptic cleavage sites, carbamidomethylation as fixed modification and oxidation on methionine as variable modification. The MASCOT searching was performed using the default settings for the MALDI-TOF/TOF instrument as supplied by Matrix Science (peptide mass tolerance of 50and a fragment mass tolerance of 0.2Da).
2.6 Protein Determination and Clotting Assay
Total protein concentration was determined with Bradford method and bovine serum albumin as standard. The clotting activity of plasma and chromatographic fractions was investigated with the method described earlier (Perazzolo., 2005). Plasma (56mgmL), CP-de- pleted plasma and purified CP (2.8mgmL) were dialyzed overnight in Tris buffer (0.05molLTris-HCl, pH7.4, 0.4molLNaCl, 1mmolLEDTA) at 4℃. Then 0.4mL of each sample was mixed in plastic Eppendorf tubes with 0.05mL Tris-buffer, 35μL HLS (4.0mgmL) and 15μL 1molLCaCl. As a control, HLS was replaced by TGase (1UmL; Sigma). A second control was done by incubating the purified CP with 0.2molLEDTA rather than 1molLCaCl. After incubation at 20℃ for 5h, a rigid gel was formed when the tube was inverted, which could confirm the formation of a clot.
3 Results
3.1 Purification of Shrimp Clotting Protein
Fc-CP was isolated from the plasma with anion-ex- change chromatography followed by hydrophobic-inter- action chromatography. The dialyzed protein was firstly applied to Q Sepharose FF column. With NaCl gradient elution, the bound protein was eluted and collected. The apparent molecular weight and purity of the eluted were analyzed with SDS-PAGE. PeaksⅠand Ⅱ were found to contain mainly hemocyanin, which was eluted from the column with 0.30molLand 0.45molLNaCl (Fig.1A; Fig.2, lane 1, 2). It appeared that fractions of peak Ⅲ eluted with 0.60molLNaCl contained clotting protein and some other hemocyanin (Fig.1A, Fig.2, lane 3). These fractions were then pooled, subjected to hydrophobic-interaction chromatography on a phenyl sepharose HP column which was eluted with a linear gradient of ammonium sulfate from 0.50molLto 0molL. Fc-CP interacted strongly to the medium eluting at the end of the (NH)SOgradient as a unique protein and its molecular weight was estimated to be 190kDa with SDS-PAGE (Fig.1B; Fig.2, lane 4).
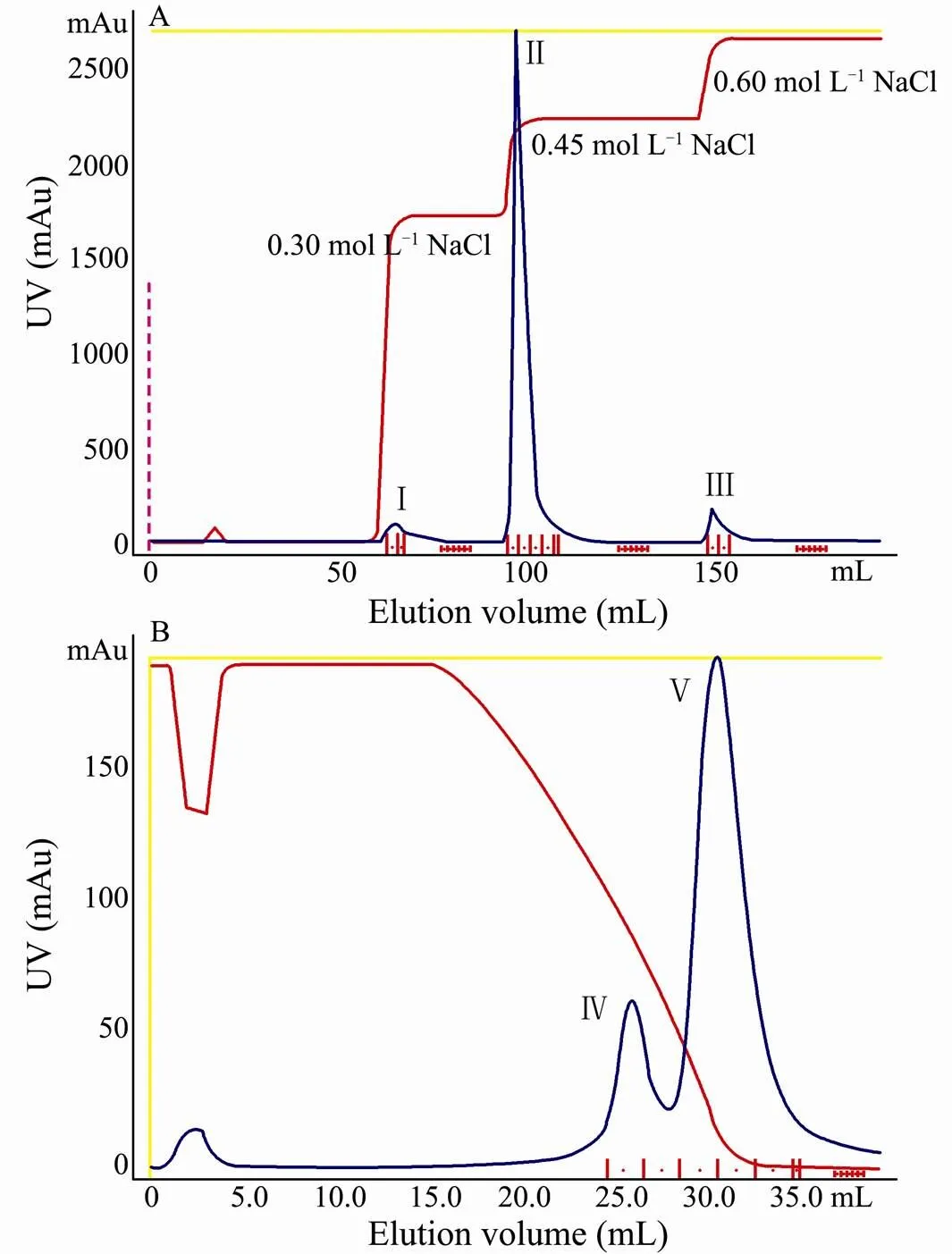
Fig.1 Purification chromatography of CP from F. chinensis on Q sepharose HP(A) and phenyl sepharose HP(B).
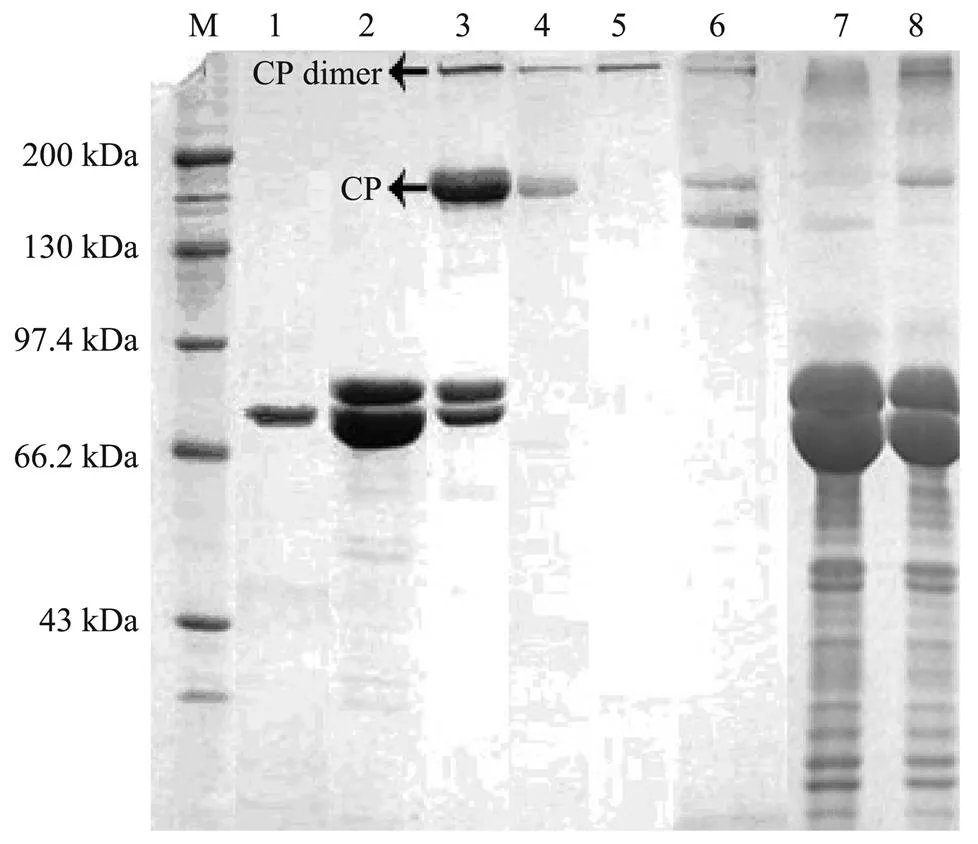
Fig.2 SDS-PAGE(7.5%)analysis of the clotting protein purified from plasma and other factions of F. chinensis. Lane 1, molecular weight markers; Lane 2, factions corresponding to the first peak on Q sepharose HP; Lane 2, fractions corresponding to the second peak on Q sepharose HP; Lane 3, fractions corresponding to the third peak on Q sepharose HP; Lane 4, fractions corresponding to the second peak on phenyl sepharose HP (puried CP); Lane 5, fractions corresponding to the second peak on phenyl sepharose HP under nonreducing conditions (puried CP dimmer); Lane 6, purified Fc-CP with a minor small band; Lane 7, F. chinensis serum; Lane 8, F. chinensis plasma.
3.2 Molecular Weight and Protein Composition
The native molecular mass of the purified CP fromwas estimated to be 380kDa with SDS-PAGE under non-reducing conditions (Fig.2, lane 5), and its coagulation capacity was confirmed with clotting assay. The subunit of Fc-CP was detected as a unique band of 190kDa under reducing conditions (Fig.2, lane 4). Thus, the protein was indicated as a homodimer linked by disulfide bonds. A minor protein band with lower molecular mass was sometimes observed. It was identified as Fc-CP by MALDI TOF/TOF MS analysis, and might be a fragment of CP. When shrimp serum (without anticoagulant) (Fig.2, lane 7) and plasma (anticoagulant used) (Fig.2, lane 8) obtained from hemolymph were analyzed by SDS-PAGE, the characteristic 190kDa band was not detected in the shrimp serum, indicating that CP was eliminated during clot formation.
3.3 Clotting Activity
The function of Fc-CP for the hemolymph coagulation was demonstrated in the presence of HLS containing endogenous TGase and Ca. Both crude shrimp plasma and purified Fc-CP can oligomerize to generate a stable clot if incubated with HLS and Ca(Table 1). On the other hand, when EDTA instead of Cawas added to the clotting assay, no gelation observed, confirming the Ca- dependence of the clotting reaction. The oligomerization was also observed when HLS was replaced by a commercial TGase.
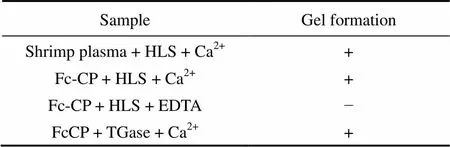
Table 1 In vitro coagulation assay using the F. chinensis crude plasma and Fc-CP
Note: The clot formation was recorded as follows: (+) stable clot formation, (−) no clot formation.
3.4 Determination of N-Terminal Amino Acid Sequence
The N-terminal amino acid sequence of the 190-kDa Fc-CP subunit which was separated with SDS-PAGE and electrotransferred to Immobilion P was determined with Edman method and further compared with homologous proteins described earlier in other crustaceans (Fig.3). Fifteen identified residues were LQPGLEYQYRYSARV and they were identical to the N-terminals of the CPs of pink shrimp(15 amino acids) (Perazzolo., 2005), tiger shrimp(15 amino acids) (Yeh., 1998) and white shrimp(10 amino acids) (Montano-Perez., 1999). The sequence was also found to be similar to other crustacean CP N-termi- nals, for example, 73% to crayfish(Kopacek., 1993), 66% to lobster(Doolittle and Riley, 1990) and 87% to sand crayfish(Komatsu and Ando, 1998).

Fig.3 Alignment of the N-terminals of clotting protein of F. chinensis with CPs of other crustaceans. L.v., Litopenaeus vannamei; F. p., Farfantepenaeus paulensisi; P. m., Penaeus monodoni; M. j., Marsupenaeus japonicusi; P. l., Pacifastacus leniusculusi; P. i., Panulirus interruptusi; I. c., Ibacus ciliatusi.
3.5 Identification of the Targeted Proteins with Proteomic Method
The bands of purified protein (190kDa and the minor with lower molecular mass) were excised, digested in-gel with trypsin, and analyzed with mass spectrometry MALDI TOF/TOF, generating the spectra of peptides. PMF (peptide mass fingerprinting) of the tryptic peptides of the purified protein is shown in Fig.4A. The 22 intense peaks were further analyzed with tandem mass spectrometry. The peptide masses obtained from the MALDI-MS combined with MS/MS were used to search the entire NCBInr database using the MASCOT search engine (Matrix Science). As a result, Fc-CP matched with a known hemolymph clottable protein (accession no.gi|46396031) from the tiger shrimpwith an intensity matched of 28.407; a total ion score of 135 and a total ion C.I. of 100%. The protein was estimated to be 188338.7Da in molecular weight with a PI of 5.27. In total 22 peptides were counted (Table 2).

Table 2 A description of MALDI-TOF/TOF MS peptide spetctrum of Fc-CP
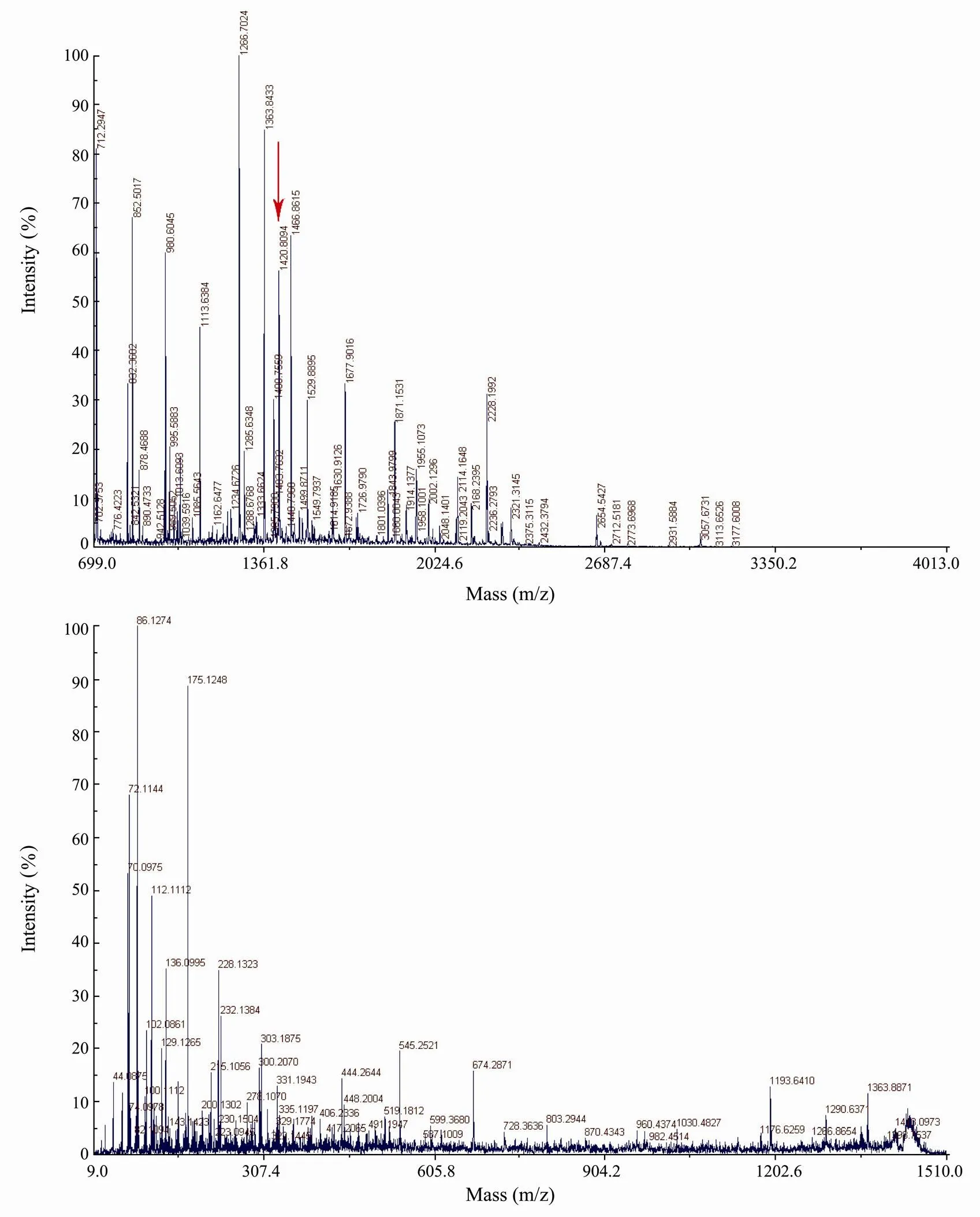
Fig.4 MS analysis of the clotting protein of F. chinensis with a 4700 Proteomics Analyzer. A, The MALDI-TOF MS map in which matched peptide peaks with mass value; B, the MALDI-TOF/TOF MS analysis of the peptide with the biggest ion score (m/z 1420.8049).
4 Discussion
As one of the key differences between vertebrates and arthropods, the body fluid of vertebrates is mostly confined to blood and lymphatic vessels whereas that of arthropods is in an open circulatory system (Bidla., 2005; Furusawa., 2008; Kwok and Tobe, 2006). Hemolymph clotting is thus an integral part of innate immunity and is of vital importance to invertebrates (Jiravanichpaisal., 2006). The invertebrate model of plasma coagulation is highly diverse but less studied. Investigation on them can help to understand the general principle of hemostasis in animals. Three types of hemolymph coagulation in crustaceans have been described. Type A is characterized by rapid agglutination of hemocytes without clotting of the plasma; type B involves cell aggregation coupled with limited clotting of the plasma; and type C shows limited cell lysis leading to plasma clotting and little cell aggregation (Yeh., 1999). These three types are likely the variations of basic mechanism involving both hemocytes and hemolymph. Shrimp and other decapods undergo type C coagulation. The clotting reaction of shrimp hemolymph is based on a combination of clotting protein and cell-derived transglutaminase. At wound sites, CPs oligomerize to prevent hemolymph loss through breaking exoskeleton and dissemination of bacteria.
CP has been isolated and characterized from the hemolymph of several crustaceans. Diverse methods,., repeatedly sequential anion-exchange chromatography (Yeh., 1998), have been adopted for its purification. We tried the method at beginning in purifying Fc-CP; unfortunately, it failed to satisfy us. We developed a rapid two-step purification process which included anion-ex- change chromatography and hydrophobic-interaction chromatography. The plasma was dialyzed in order to equilibrate it with the initial buffer for ion exchange chromatography. Dialyzed and centrifugation-clarified plasma was loaded onto the Q-sepharose HP column. The Fc-CP was eluted from the matrix with a high concentration of salt. The Fc-CP-rich fractions were furtherusing a phenyl sepharose HP column. The Fc-CP was eluted in low salt as a purified protein because of the hydrophobicity of this lipoprotein, while other proteins, mainly hemocyanin, were eliminated during this step. The composition, structure, location and physiological function of CP have been studied in many crustaceans (Kopacek., 1993; Montano-Perez., 1999; Yeh., 1998). CP is a large homodimeric glycolipoprotein in crustacean hemolymph, which has previously been biochemically and functionally characterized in crayfish., and was first demonstrated corresponding to VHDL (very high density lipoprotein) (Hall., 1995) and further confirmed in other crustaceans. This glycolipoprotein has dual physiological function, being involved in crustacean immunity and lipid transport. The CP of crustacean hemolymph consists of two subunits with a molecular weight of about 200kDa. Each subunit has both lysine and glutamine sidechains which are recognized and covalently linked to each other by TGases (Yeh., 1999). The molecular mass of Fc-CP was estimated to be 380kDa with SDS-PAGE under non-re- ducing condition and 190kDa under reducing condition, which represents an unusual low molecular weight compared with other crustaceans such as in shrimps.(420kDa) (Montano-Perez., 1999) and crayfish(420kDa) (Kopacek., 1993). A minor 180kDa degradation form of Fc-CP, identified as the native CP by MALDI TOF/TOF MS, was detected in purified Fc-CP. A similar degraded form was also found during purification of crayfish CP (Kopacek., 1993), tiger shrimp CP (Yeh., 1998) and kuruma prawn (Cheng., 2008). CP was found to exist in most shrimp tissues. CP gene expresses in a similar pattern in tiger shrimp and Kuruma prawn (Cheng., 2008; Yeh., 2007). The study demonstrated that epidermis, gill, and central nervous system of shrimp were important expression tissues of CP gene. CP is one of high-abun- dant proteins in shrimp hemolymph, and its concentration in normal intermolt shrimps was around 3.0mgmL. After successive bleeding for a week, CP concentration increased from 3.0mgmLto more than 12mgmLin shrimp hemolymph (Yeh., 2007). Other researchers have found that the concentration of CP in penaeid shrimp was higher in summer than in winter, and varied by molting cycle; its plasma level increased by 2-folds before molting and decreased after molting to the normal (Yeh., 1998). The results indicated that biosynthesis of CP was stimulated to compensate its loss or reserve its possible loss. This is consistent with a wound-healing role of the protein.
Proteomics plays an ever-increasing and pivotal role in biological research. MS, hyphenated with a range of electrophoretic and multidimensional chromatographic separation techniques, has emerged as a key platform technology in proteomics for the rapid and high-throughput identification, characterization, and quantitation of proteins (Samyn., 2006). Protein identification is a significant bottleneck in researches of some species with unknown genomes or uncompleted protein database at present (Vertommen., 2011). The identification of proteins derived from organisms with unsequenced genomes mainly depends on homology searching (D’Amato., 2010). Recently, the development of a new tandem mass spectrometry (MS/MS) generation apparatus with a MALDI source allows proteins to be directly identified using either the PMF and/or high quality tandem MS/MS data (Pan., 2010; Shevchenko., 2001). MS/MS to obtain protein sequence information has further enhanced the applications of this new proteomics technology in marine invertebrates (Franco., 2011). In this study, the purified Fc-CP was successfully identified by MS/MS and Mascot searching. The strategy has been proved feasible and reliable for protein identification of shrimp. The identity of the purified protein was also confirmed by N-terminal sequencing. N-terminal sequencing of the purified protein identified the first 15 amino-acid sequence as LQPGLEYQYRYSARV which was found to be identical to those of penaeids(Perazzolo., 2005),.(Yeh., 1998) and.(Montano-Perez., 1999). It was interesting that the molecular weight of Fc-CP is also closer to that of.(380 kDa) (Yeh., 1998). It also had a high similarity to N-terminal sequences of other crustacean CPs. The results showed that the CP is a highly conserved protein in crustaceans.
In conclusion, CP fromhemolymph can be purified through co-application of anion-exchange chromatography and hydrophobic-interaction chromatography. The protein was identified by Edman N-terminal sequencing and MALDI TOF/TOF MS analysis. Cloning of the Fc-CP gene and its expression analysis will be the next step to elaborate the mechanism of the clotting reaction in more detail and understand the immune responses in crustaceans better.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30600458).
Bidla, G., Lindgren, M., Theopold, U., and Dushay, M. S., 2005. Hemolymph coagulation and phenoloxidase in Drosophila larvae., 29 (8): 669-679.
Cheng, W., Tsai, I. H., Huang, C. J., Chiang, P. C., Cheng, C. H., and Yeh, M. S., 2008. Cloning and characterization of hemolymph clottable proteins of kuruma prawn () and white shrimp ()., 32 (3): 265- 274.
D’Amato, A., Cereda, A., Bachi, A., Pierce, J. C., and Righetti, P. G., 2010. In depth exploration of the hemolymph ofvia combinatorial peptide ligand libraries., 9 (6): 3260-3269.
Doolittle, R. F., and Riley, M., 1990. The amino-terminal sequence of lobster fibrinogen reveals common ancestry with vitellogenins., 167 (1): 16-19.
Fagutao, F. E., Maningas, M. B. B., Kondo, H., Aoki, T., and Hirono, I., 2012. Transglutaminase regulates immune-related genes in shrimp., 32 (5): 711- 715.
Franco, C. F., Santos, R., and Coelho, A. V., 2011. Proteome characterization of sea star coelomocytes–The innate immune effector cells of echinoderms., 11 (17): 3587- 3592.
Furusawa, T., Rakwal, R., Nam, H. W., Hirano, M., Shibato, J., Kim, Y. S., Ogawa, Y., Yoshida, Y., Kramer, K. J., Kouzuma, Y., Agrawal, G. K., and Yonekura, M., 2008. Systematic investigation of the hemolymph proteome ofat the fifth instar larvae stage using one- and two-dimensional proteomics platforms., 7 (3): 938-959.
Hall, M., Vanheusden, M. C., and Soderhall, K., 1995. Iden- tification of the major lipoproteins in crayfish hemolymph as proteins involved in immune recognition and clotting., 216 (3): 939-946.
Jiravanichpaisal, P., Lee, B. L., and Soderhall, K., 2006. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization., 211 (4): 213-236.
Komatsu, M., and Ando, S., 1998. A very-high-density lipoprotein with clotting ability from hemolymph of sand crayfish,., 62 (3): 459-463.
Kopacek, P., Hall, M., and Soderhall, K., 1993. Characterization of a clotting protein, isolated from plasma of the fresh-water crayfish., 213 (1): 591-597.
Kwok, R., and Tobe, S. S., 2006. Hemolymph clotting in crustaceans: Implications for neuropeptide extraction from invertebrate hemolymph., 27 (3): 590-596.
Montano-Perez, K., Yepiz-Plascencia, G., Higuera-Ciapara, I., and Vargas-Albores, F., 1999. Purification and characteri- zation of the clotting protein from the white shrimp., 122 (4): 381-387.
Pan, C., Park, B. H., McDonald, W. H., Carey, P. A., Banfield, J. F., VerBerkmoes, N. C., Hettich, R. L., and Samatova, N. F., 2010. A high-throughputsequencing approach for shotgun proteomics using high-resolution tandem mass spec- trometry., 11: 118.
Perazzolo, L. M., Lorenzini, D. M., Daffre, S., and Barracco, M. A., 2005. Purification and partial characterization of the plasma clotting protein from the pink shrimp., 142 (3): 302- 307.
Samyn, B., Sergeant, K., Memmi, S., Debyser, G., Devreese, B., and Van Beeumen, J., 2006. MALDI-TOF/TOF de novo sequence analysis of 2-D PAGE-separated proteins from, a bacterium with unsequenced genome., 27 (13): 2702-2711.
Shevchenko, A., Sunyaev, S., Loboda, A., Shevehenko, A., Bork, P., Ens, W., and Standing, K. G., 2001. Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time of flight mass spectrometry and BLAST homology searching., 73 (9): 1917-1926.
Theopold, U., Schmidt, O., Soderhall, K., and Dushay, M. S., 2004. Coagulation in arthropods: defence, wound closure and healing., 25 (6): 289-294.
Vazquez, L., Alpuche, J., Maldonado, G., Agundis, C., Pereyra- Morales, A., and Zenteno, E., 2009. Immunity mechanisms in crustaceans., 15 (3): 179-188.
Vertommen, A., Moller, A. L. B., Cordewener, J. H. G., Swennen, R., Panis, B., Finnie, C., America, A. H. P., and Carpentier, S. C., 2011. A workflow for peptide-based proteomics in a poorly sequenced plant: A case study on the plasma membrane proteome of banana., 74 (8): 1218-1229.
Wang, R., Liang, Z., Hall, M., and Soderhall, K., 2001. A transglutaminase involved in the coagulation system of the freshwater crayfish,. Tissue locali- sation and cDNA cloning., 11 (7): 623-637.
Wojdyla, K., Rogowska-Wrzesinska, A., Wrzesinski, K., and Roepstorff, P., 2011. Mass spectrometry based approach for identification and characterisation of fluorescent proteins from marine organisms., 75 (1): 44-55.
Yeh, M. S., Chen, Y. L., and Tsai, I. H., 1998. The hemolymph clottable proteins of tiger shrimp,, and related species., 121 (2): 169-176.
Yeh, M. S., Huang, C. J., Cheng, J. H., and Tsai, I. H., 2007. Tissue-specific expression and regulation of the haemolymph clottable protein of tiger shrimp ()., 23 (2): 272-279.
Yeh, M. S., Huang, C. J., Leu, J. H., Lee, Y. C., and Tsai, I. H., 1999. Molecular cloning and characterization of a hemo- lymph clottable protein from tiger shrimp ()., 266 (2): 624- 633.
Yepiz-Plascencia, G., Jimenez-Vega, F., Romo-Figueroa, M. G., Sotelo-Mundo, R. R., and Vargas-Albores, F., 2002. Mole- cular characterization of the bifunctional VHDL-CP from the hemolymph of white shrimp., 132 (3): 585-592.
(Edited by Qiu Yantao)
10.1007/s11802-013-2026-y
ISSN 1672-5182, 2013 12 (3): 477-483
. Tel: 0086-532-82898572 E-mail: wanglei@qdio.ac.cn
(May 8, 2012; revised May 29, 2012; accepted November 9, 2012)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- Shallow Water Body Data Processing Based on the Seismic Oceanography
- Prediction of China’s Submerged Coastal Areas by Sea Level Rise due to Climate Change
- A Homogeneous Linear Estimation Method for System Error in Data Assimilation
- The Suspended Sediment Concentration Distribution in the Bohai Sea, Yellow Sea and East China Sea
- Role of Ekman Transport Versus Ekman Pumping in Driving Summer Upwelling in the South China Sea
