Arsenic Removal from Pinctada martensiiEnzymatic Hydrolysate by Using Zr(Ⅳ)-Loaded Chelating Resin
YANG Xiaoman1), 2), 3), DAI Wenjin2), 3), SUN Huili2), *, and PAN Jianyu2)
Arsenic Removal fromEnzymatic Hydrolysate by Using Zr(Ⅳ)-Loaded Chelating Resin
YANG Xiaoman, DAI Wenjin, SUN Huili, and PAN Jianyu
1),,264003,..2),,,510301,..3),100049,..
The present study investigated the removal of inorganic arsenic from Pinctada martensii enzymatic hydrolysate through unmodified resin (D296) and Zr(Ⅳ)-loaded chelating resin (Zr-D401). By loading Zr to macroporous chelating resin D401, the as exchange adsorption active sites are generated. This transforms D401 from a material that does not have the arsenic adsorption capacity into a material that has excellent arsenic exchange adsorption capacity. The static adsorption experiments were conducted to investigate the optimal removal condition for D296 and Zr-D401. The experimental results show that: the optimum condition for D296 is that T=25℃, pH=5, resin additive amount=1g(50mL), and contact time=10h, the corresponding arsenic removal rate being 65.7%, and protein loss being 2.33%; the optimum condition for Zr-D401 is that T=25℃, pH=8, resin additive amount=1g(50mL), and contact time=10h, the corresponding arsenic removal rate being 70.3%, and protein loss being 4.65%. These results show that both of the two resins are effective in arsenic removal for preserving useful substance. Our research provides scientific evidence and advances in the processing technology for heavy metal removal in shellfish.
; enzymolysis; arsenic removal; chelating resin
1 Introduction
The pearl oysteris a typical kind of shellfish in the South China Sea. It is widely distributed near the coast of Guangdong and Guangxi provinces. Following the development of the pearl industry,is now cultured at very large scales. Theoyster meat contains proteins, bioactive peptides, amino acids and taurine, and other minerals that are valuable sources for human nutrition (Zhang., 2000). However, due to the limitation of the processing technology, only a small part of oyster meat was utilized. The remainder of the meat has been discarded as aquatic product processing wastes, which is not only wasteful but also causes environmental pollution (Zheng., 2009; Zheng., 2012). As a processing tech- no- logy for shellfish, protein enzymatic hydrolysis converts shellfish’s under-utilized by-products into acceptable forms without losing nutritional value, which obviously has high economic profit (Clemente, 2000). In addition, enzymatic hydrolysis can improve the functional properties of protein. These functional proteins then display various biological activity, such as antioxidant (Barkia., 2010), antihypertensive (Je., 2009), ACE inhibitory (Dai., 2012), antimicrobial (Liu., 2008),.
However, along with the industrial development, tons of wastewater are dumped into the ocean. These hazardous substances such as heavy metals are not subject to degradation processes but tend to accumulate in sediments.are filter feeders straining particulate food from the surrounding water. This special feeding mode of shellfish causes the accumulation of pollutants in the shellfish, which consequently causes the percentages of heavy metals and other toxic substances to exceed the safety levels (Bourgoin, 1990; Li., 2003; Katano., 2003). There have been several reports related to the technology for removing hazardous substance from shellfish. The most common method is shellfish depuration technique (Qiao, 2007). However, such methods are mainly focused on the removal of bacteria (Martinez., 2009), shellfish poison (Xie., 2013) and microorganism (Ho and Tam, 2000) from shellfish, and they do not work well with heavy metal and arsenic removal. Moreover, there are other techniques, such as the use of chitosan (Liu., 2010; Sun., 2010) and cation exchange resin, which are employed for the removal of heavy metals in enzymatic hydrolysate. But these studies are mainly focused on Cd and Lead ions purification, and there have not been many useful techniques that deal with arsenic removal (Yang., 2012). Nevertheless some methods used in the arsenic removal from industrial wastewater can provide guidance for our removal experiment (Mohan and Pittman, 2007; Biswas., 2008; Pan., 2007; Ratna., 2004).
In our experiment, we transform D401 from a material that does not have the arsenic adsorption capacity into a material that has arsenic exchange adsorption capacity by loading Zr to macroporous chelating resin D401.The relevant research indicates that Zr-loaded resin showed strong selectivity and retainment of As (V) and As (III) (Suzuki,., 2000). In the present paper, we used unmodified resin (D296) and Zr (IV)-loaded chelating resin (Zr-D401) as purification material, and used Pinctada martensii enzymolysis hydrolyzate as samples to study the best technology and conditions to remove arsenic. Our research provides scientific evidence and advances in the processing technology for heavy metal removal in shellfish.
2 Materials and Methods
2.1 Material and Equipment
oyster meat was purchased from Nansha fish market, Guangzhou (China); macroporous strong-based anion exchange resin D296 and macro- porous chelating resin D401 were from the chemical factory of Nankai University (China); trypsin and flavourzy- me were from Guangxi Pang Bo Biological Technology, LLP (China). Zirconium reserving liquid (adding 48.34g ZrOCl·8HO and certain amount of buffer solution of per liter 0.1M HCl that leads to PH=2), NaOH, HCl, sodium acetate, 2-(Cyclohexylamino) ethanesulfonic acid,., were analytically pure. Experimental water was deionized water.
Equipment used included: PHS-3C, a high precision instrument (Shanghai Lei Ci Instrument Factory); Acculab, a high precision electronic balance (German Saiduoli, LLP); SK-2003AZ, AFS (Beijing Jin Suo Kun Technology Development Co., Ltd); KDN-2C, an azotometer (Shanghai Xian Jian Instrument Co., Ltd).
2.2 Experimental Method
2.2.1Preparation of enzymatic hydrolysate
Dispersions ofoyster meat (20% w/w) were prepared in deionized water. The hydrolysis process was carried out in a shaking water bath incubator. Trypsase (at 50℃ for 1h) and flavor enzyme (at 50℃ for 3h)were applied in turn to obtain the enzymatic hydrolysate; after the hydrolysis process, the mixtures were heated at 100℃ for 5min to inactivate the enzymes.
2.2.2 Resin pretreatment
After soaking in dehydrated alcohol for 24h, the resin was soaked in 3% HCl and 3% NaOH solution, respect- tively, with stirring every half hour for a total of 6h. Then resin was fully washed with deionized water until being neutral, followed by drying at 40℃ until constant weight.
2.2.3 Preparation of the Zr (IV)-loaded chelating resin D401 (Zr-D401)
Resin wasmodified according to the method proposed by Biswas (Biswas., 2008). In order to load zirconium, 40g dried macroporous chelating resin D401 was equilibrated with 1L zirconium reserving liquid at 30℃ for 24 h. The resin was then washed with deionized water until neutral pH, followed by drying at 40℃ until constant weight. The amount of Zr (IV) ion loaded onto the resin was calculated from the difference of the metal concentrations in the solution before and after loading.
2.2.4 Determination of total nitrogen loss rate
Micro-Kjeldahl method (Chang, 2010) was developed to determine total nitrogen (TN) content in enzymatic hydrolysate.
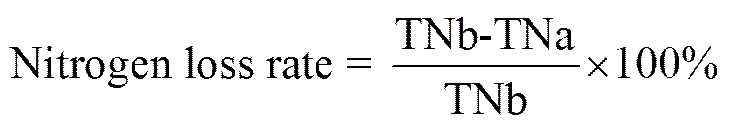
where TNb is the content of total nitrogen before removal, and TNa is that after removal.
2.2.5 Determination of inorganic arsenic
The inorganic arsenic content in enzymatic hydrolysate was detemined by hydride generation-atomic fluore- scence spectrometry (Feng and Fu, 1998). Given the inorganic arsenic content in enzymatic hydrolysate before and after removal, the arsenic removal rate is calculated as follows:
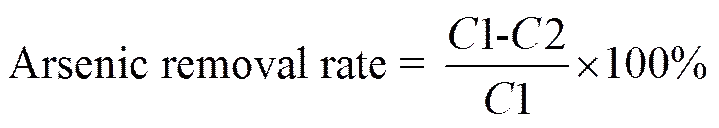
where1 and2 are the contents of inorganic arsenic in enzymatic hydrolysate before and after removal, respect- tively.
3 Results and Discussion
3.1 Effect of pH on the Removal of Arsenic by D296 and Zr-D401 Resin
The static adsorption experiments were conducted to investigate the removal behavior of arsenic by using macroporous strong-based anion exchange resin D296 and Zr (IV)-loaded chelating resin D401 (Zr-D401).
Environment changes, especially pH changes, affect not only the dissociation of Zr and the exchange- absorption behavior of inorganic arsenic on the anion exchange resin but also the speciation of arsenic, which is considered to be the most significant factor for the removal of arsenic.
With adding 1g Zr-D401 or D296 resin per 50mL hydrolyzate, keeping constant temperature 25℃and stirring for 12h, the arsenic adsorption efficiency under different pH conditions was observed as shown in Fig.1.
Though adding 1g Zr-D401 or D296 resin per 50mL hydrolysate, keeping constant temperature 25℃ for 12h, and adjusting pH to 2, 3, 4, 5, 6, 7, 8, 9, 10,11, the content of inorganic arsenic in enzymatic hydrolysate after removal was determined.
When other conditions remain unchanged, pH has great influence on arsenic removal effect. Durinfg the removal process, Macroporous strong-based anion exchange resin D296 has 2 maximum poifnts: at pH 5 with arsenic re- moval rate up to 63.4%, and at pH 8 with arsenic removal rate up to 52.1%; for Zr-D401 enzymatic hydrolyzate, the arsenic removal rate is up to 67.7% at pH 8, and at this pH the arsenic removal rate of Zr-D401 is higher than any other pH condition. Similar results have been reported: optimum pH range was found between 4-6 by An. (2011) and between 4.5-6.5 by Pakzadeh and Batista (2011) by using the method of ion-exchange. Biswas. (2008)reported that arsenate was strongly adsorbed in the pH range from 2 to 6, while arsenite was strongly adsorbed between pH 9 and 10 by Zr (IV)-loaded orange waste gel through the removal of As (V) and As (III).
According to Vaclavikova. (2008), the distribution of As (III) and As (V) in the species is shown in Figs.2 and 3. These figures show that at pH values of 5 to 8, the neutral HAsOspecies predominate in As (III), while at pH 8 the HAsOspecies predominate and at pH 5 the HAsOspecies predominate in As (V). Since the electrostatic forces between negatively charged As (V) and resin anion [exchange capacity sites] are stronger than neutral As species, it can be interpreted that As (V)is more easily to be removed than As (III). Similar results have been reported in previous studies (Manna., 1999). Furthermore, we speculate that enzymatic hydrolyzate of As (V) content was higher than As (III) content due to enzymatic oxidation process. In the aerobic environment of 50℃ under the constant temperature heating, according to the Eh-pH diagram for arsenic speciation in the neutral pH oxidizing environments, As (III) is likely to become As (V) due to oxidation (Smedley and Kinniburgh, 2002). Therefore, in enzymatic solution, the As (V) content was higher than the As (III) content. It also explains why inorganic arsenic was more easily to be removed at pH 5 to 8 in enzymatic solution.
3.2 Effect of Contact Time on the Arsenic Removal
Based on the study of the effect of pH on arsenic removal using D296 and Zr-D401, the optimal pH conditions were determined:pH 5 for D296, pH 8 for Zr-D401. At 25℃, by adding 1g Zr-D401 or D296 resin per 50mL hydrolyzate,the arsenic adsorption efficiency under different contact time conditions was observed as shown in Fig.4.
At 25℃, by adding 1g D296 or Zr-D401 resin per 50 mL hydrolyzate, keeping constant temperature at the optimum pH, and adjusting contact time to 0, 15, 30, 60, 120, 360, 600, 900 and 1440min, respectively, the content of inorganic arsenic in enzymatic hydrolysate after removal can then be determined.
Because of the particularity of the enzymatic hydro- lysate, when the contact time increases, microbial growth and organic matter degradation occur. In this case, or- ganic arsenic is transformed into inorganic arsenic and inorganic arsenic content increases. The transform- ed inorganic arsenic is toxic. Therefore, the long contact time does not provide any benefit on arsenic removal in enzymatic hydrolyzate. Moreover, it will result in loss of utility of the materials. The experimental results show that at 25℃and with resin additive amount =1g(50mL), pH 5 for D296, pH 8 for Zr-D401, the optimum contact time was 10h for both D296 and Zr-D401. The corresponding arsenic removal rates were 65.7% and 70.3%, respectively. Based on the comparison of the arsenic removal for the two resins, the removal rate of Zr-D401 increased rapidly within three hours. At contact time of 3h, the maximum removal rate of 90% was reached. In addition, Zr-D401 had shorter adsorption equilibrium time and higher adsorption capacity.
3.3 Effect of Resin Quantity on Arsenic Removal
The content of inorganic arsenic removed in enzymatic hydrolysate was determined after adding 0.1, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5 and 3g of Zr-D401 or D296 resin per 50mL hydrolysate, respectively, and adjusting pH and contact time to the optimum conditions, with temperature oscillating around 25℃. The efficiency of arsenic adsorption under different resin quantities is shown in Fig.5. When the amount of resin is increased from 0.1g to 1g, the D296 and Zr-D401 arsenic removal rate is increased markedly from 15.5% to 63.2% and from 18.6% to 66.1%, respectively; whereas when the amount of resin is increased from 1g to 3g, the arsenic removal rate is only increased by 7.7% and 13.7%, respectively. These results indicate that 1g of resin corresponds to a significant turning point. During the experiment, the limiting factors for arsenic removal changed from the amount of resin addedand contact area (when the resin is less than 1g) to the diffusion rate of ions and the existence of competitive ions (when the resin is increased to more than 1g). Based on economic considerations, the use of 1g of resin is considered as optimal.
Using the same amount of resin, Zr-D401 has a higher arsenic removal rate than D296 in enzymatic hydrolysate. This can be explained by the two following points. First, the particle size of Zr-D401 is smaller than D296 and has a larger specific surface area than D296 for the same volume. Therefore, Zr-D401 has a larger effective area for adsorption and ion exchange. Second, the active group of Zr-D401 is hydrolysis Zr ion which can be exchanged with OHand HO ligands. It also has an exchange capacity with anionic form of As and neutral HAsO.
3.4 Protein Loss Rate Under Optimal Removal Condition
The protein loss rate during the inorganic arsenic removal process was calculated to evaluate the effective- ness for preserving useful substance.
Table 1 shows the optimum conditions for D296 and Zr-D401 resin to remove inorganic arsenic from enzy-matic hydrolysate and the corresponding protein loss rates. Under the optimal removal condition, the protein loss rates are 2.33% and 4.65% for D296 and Zr-D401 resin, respectively. These results indicate that both of the two resins are effective in arsenic removal for preserving useful substance.

Table 1 Protein loss rate under optimal removal condition
4 Conclusions
1) Our study shows that both D296 and Zr-D401 are effective materials for inorganic arsenic removal from enzymatic hydrolysate. By loading Zr to macroporous chelating resin D401, the As exchange adsorption active sites are generated. This transforms D401 from a material that does not have the arsenic adsorption capacity into a material that has excellent arsenic exchange adsorption capacity.
2) Within the scope of the experiment, the optimum conditions for D296 are 25℃, pH=5, resin additiveamount=1g(50mL), and contact time =10h, the corresponding arsenic removal rate being 65.7%, and protein loss being 2.33%; the optimum conditions for Zr-D401 are 25℃, pH=8, resin additiveamount=1g(50mL), and contact time=10h, the corresponding arsenic removal rate being 70.3%, and protein loss being 4.65%.
Acknowledgements
This work is supported by National Key Technologies R & D Program of China (2008 BAD94B08).
An, B., Liang, Q. and Zhao D., 2011. Removal of arsenic (V) from spent ion exchange brine using a new class of starch- bridged magnetite nanoparticles., 45: 1961- 1972.
Barkia, A., Bougatef, A., Ben Khaled, H., and Nasri, M., 2010. Antioxidant activities of sardinelle heads and/or viscera protein hydrolysates prepared by enzymatic treatment., 34: 303-320.
Biswas, B. K., Inoue, J. I., Inoue, K., Ghimire, K. N., Harada, H., Ohto, K., and Kawakita, H., 2008. Adsorptive removal of As (V) and As (III) from water by a Zr (IV)-loaded orange waste gel., 154: 1066-1074.
Bourgoin, B. P., 1990. Mytilus-edulis shell as a bioindicator of lead pollution-considerations on bioavailability and variability., 61: 253-262.
Chang, S. K., 2010. Protein analysis. In:. 4 th ed. Springer, New York, 133-146.
Clemente, A., 2000. Enzymatic protein hydrolysates in human nutrition., 11: 254- 262.
Dai, Z. Y., Zhang, Y. P., Zhang, H., and Lu, Y. B., 2012. Preparation and characterization of mussel (mytilus edulis) protein hydrolysates with angiotensin-i-converting enzyme (ace) inhibitory activity by enzymatic hydrolysis., 36: 66-74.
Feng, X., and Fu, B., 1998. Determination of arsenic, antimony, selenium, tellurium and bismuth in nickel metal by hydride generation atomic fluorescence spectrometry., 371: 109-113.
Ho, B. S. W., and Tam, T. Y., 2000. Natural depuration of shellfish for human consumption: A note of caution., 34: 1401-1406.
Je, J. Y., Lee, K. H., Lee, M. H., and Ahn, C. B., 2009. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis., 42: 1266-1272.
Katano, S., Matsuo, Y., and Hanaoka, K., 2003. Arsenic compounds accumulated in pearl oyster Pinctada fucata., 53: 245-251.
Li, W. H., Wei, C., Zhang, C., Van Hulle, M., Cornelis, R., and Zhang, X. R., 2003. A survey of arsenic species in chinese seafood., 41: 1103-1110.
Liu, B. J., Wang, D. F., Dong, F., Sun, J. P., He, S. Q., Xu, W., and Xu, Y., 2010. Removal of cadmium from Chlamys ferrari skirt border enzymatic hydrolysate by cross-linked chitosan resins., 37: 11-14 (in Chinese with English abstract).
Liu, Z. Y., Dong, S. Y., Xu, J., Zeng, M. Y., Song, H. X., and Zhao, Y. H., 2008. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin., 19: 231-235.
Manna, B., Bhat, S., Dasgupta, M., and Ghosh, U., 1999. Stud-ies on removal of arsenic from water using hydrated zircon- ium oxide., 8: 51-56.
Martinez, O., Rodriguez-Calleja, J. M., Santos, J. A., Otero, A., and Garcia-Lopez, M. L., 2009. Foodborne and indicator bacteria in farmed molluscan shellfish before and after depuration., 72: 1443-1449.
Mohan, D., and Pittman, C. U., 2007. Arsenic removal from water/wastewater using adsorbents — a critical review., 142: 1-53.
Pakzadeh, B., and Batista, J. R., 2011. Surface complexation modeling of the removal of arsenic from ion-exchange waste brines with ferric chloride., 188: 399-407.
Qiao, Q. L., 2007. A study on classification of shellfish farming area and purification technique., 22: 11-14 (in Chinese with English abstract).
Pan, B., Zhang, Q., Du, W., Zhang, W., Pan, B., Zhang, Q., Xu, Z., and Zhang, Q., 2007. Selective heavy metals removal from waters by amorphous zirconium phosphate: Behavior and mechanism., 41: 3103-3111.
Ratna, K. P., Chaudhari, S., Khilar, K. C., and Mahajan, S., 2004. Removal of arsenic from water by electrocoagulation., 55: 1245-1252.
Smedley, P., and Kinniburgh, D., 2002. A review of the source, behaviour and distribution of arsenic in natural waters., 17: 517-568.
Sun, J. P., Wang, F., Li, G. Y., Zhang, F. L., Liu, B. J., and Xu, W., 2010. The removal of cadmium from chlamys ferrari by chitosan oligosaccharide complexes with Ca and Mg., 37: 10-13 (in Chinese with English abstract).
Suzuki, T. M., Bomani, J. O., Matsunaga, H., and Yokoyama, T., 2000. Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic., 43: 165-172.
Vaclavikova, M., Gallios, G. P., Hredzak, S., and Jakabsky, S., 2008. Removal of arsenic from water streams: An overview of available techniques., 10: 89-95.
Xie, W. C., Liu, X. L., Yang, X. H., Zhang, C. H., and Bian, Z. Y., 2013. Accumulation and depuration of paralytic shellfish poisoning toxins in the oyster Ostrea rivularis Gould-Chitosan facilitates the toxin depuration., 30: 446- 452.
Yang, X. M., Dai, W. J., and Sun, H. L., 2012, Heavy metal control and removal technology analysis in marine shellfish enzymatic hydrolysates., 36: 116-119 (in Chinese with English abstract).
Zhang, C. H., Wu, H. M., Hong, P. Z., Deng, S. G., and Lei, X. L., 2000. Nutrients and composition of free amino acid in edible part of Pinctada martensii., 24: 135-139 (in Chinese with English abstract).
Zheng, H. N., Zhang, C. H., Cao, W. H., Liu, S. C., and Ji, H. W., 2009. Preparation and characterisation of the pearl oyster (Pinctada martensii) meat protein hydrolysates with a high Fischer ratio., 44: 1183-1191.
Zheng, H. N., Zhang, C. H., Qin, X. M., Gao, J. L., and Li, T. Y., 2012. Study on the protein fractions extracted from the mu- scle tissue of Pinctada martensii and their hydrolysis by pancreatin., 47: 2228-2234.
(Edited by Ji Dechun)
10.1007/s11802-013-2341-3
ISSN 1672-5182, 2013 12 (3): 392-396
. E-mail: shl@scsio.ac.cn
(March 23, 2013; revised April 18, 2013; accepted April 24, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- Shallow Water Body Data Processing Based on the Seismic Oceanography
- Prediction of China’s Submerged Coastal Areas by Sea Level Rise due to Climate Change
- A Homogeneous Linear Estimation Method for System Error in Data Assimilation
- The Suspended Sediment Concentration Distribution in the Bohai Sea, Yellow Sea and East China Sea
- Role of Ekman Transport Versus Ekman Pumping in Driving Summer Upwelling in the South China Sea
