Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
Wang Liang; Yin Baozuo; Yi Jianjun; Cui Chunming
(1. State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071; 2. Petrochemical Research Institute, PetroChina, Beijing 100195)
Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
Wang Liang1; Yin Baozuo2; Yi Jianjun2; Cui Chunming1
(1. State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071; 2. Petrochemical Research Institute, PetroChina, Beijing 100195)
Three sulfonyl aliphatic amines [(R2SO2)2NR1, viz.: compound 1, in which R1=Me, and R2=Ph; compound 2, in which R1=n-Bu, and R2=CF3; and compound 3, in which R1=C8H17, and R2=CF3], have been synthesized and employed as internal electron donors (IED) for the preparation of Ziegler-Natta catalysts for the polymerization of propylene. The contents of Ti, H and C in these catalysts have been determined by elemental analysis and UV-vis spectrophotometry. The effect of the structure and dosage of the electron donor, the Al/Ti ratio and the polymerization temperature on the catalyst performance has been studied. Under optimized conditions, the catalyst with a highest activity yielded polypropylene with high isotacticity in the absence of external electron donors.
heterogeneous catalyst; polypropylene; internal electron donor; polymerization
1 Introduction
Polypropylene, as a synthetic resin, has extensive applications in construction, home appliances, electronics, and packaging industry due to its outstanding performance[1]. At present, the polypropylene industry is at a critical stage of its rapid development. The total production of polypropylene reached 61.0 Mt in 2010[2]worldwide. The development of catalyst has facilitated the rapid growth of polypropylene industry[3]. High-activity MgCl2-supported Ziegler-Natta catalysts, which comprise TiCl4, MgCl2and electron donors, play a dominant role in polypropylene (PP) production[4]. Heterogeneous MgCl2-supported Ziegler-Natta catalysts are currently predominant catalysts used in industrial propylene polymerization processes to produce stereospecific polypropylene[5]. The internal electron donors (IED) play a vital role in controlling the catalyst stereoselectivity, and therefore, have great significance for the development of catalyst technology and polypropylene industry. Diesters and diethers are mostly used internal electron donors[6]. Phthalates and succinates have been developed as internal electron donors for polymerization catalysts. The aromatic diether such as 9,9-bis(methyl acetate)fluorene has been also studied as the internal electron donor[7]. Other new types of internal electron donors were reported, too. 1,3-Diol dibenzoate was first reported by the Beijing Research Institute of Chemical Industry[8]. Compared with conventional diesters, the catalysts employing 1,3-diol dibenzoate as internal electron donor possess excellent hydrogen pressure response and slow loss of the donor content[8]. Our group previously reported sulfonyl aromatic amines as internal electron donors for the preparation of Ziegler-Natta catalysts[9]. These catalysts exhibited excellent performance for the polymerization of propylene. For the continuation of this program, we brought up sulfonyl aliphatic amines as internal electron donor for propylene polymerization catalysts to make a comparison with previous results. Herein, we report on the synthesis and employment of these amines as internal electron donors for the preparation of Ziegler-Natta catalysts for the stereospecific polymerization of propylene. The results have demonstrated that these amines are promising internal electron donors for the development of practical catalysts for industry applications.
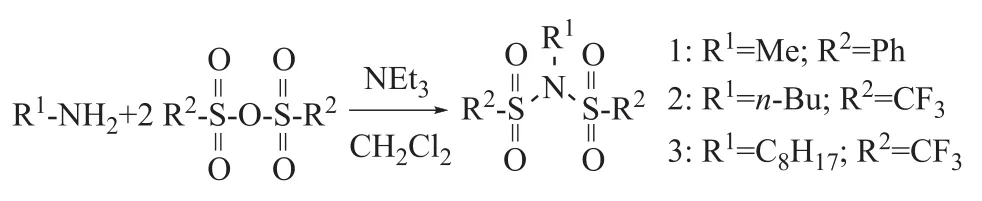
Figure 1 Synthesis of sulfonyl aliphatic amines
2 Experimental
Materials: All operations involving air-sensitive or moisture-sensitive compounds were carried out under an atmosphere of argon using standard Schlenk techniques or in a conventional argon-filled glove box. Ether, hexane and toluene were refluxed over sodium/benzophenone and distilled before use. Methylene chloride was dried over CaH2and freshly distilled prior to use. Propylene was obtained from the Dongxiang Special Gas Corp. in Tianjin, and passed through a column filled with pre-dried 4 Å molecular sieve before use. Et3Al (TEA) was purchased from the Crompton Corp.
Synthesis of the sulfonyl aliphatic amine compounds was carried out according to the following procedure.
Amine (10 mmol), NEt3(22 mmol) and 30 mL of methylene chloride were introduced to a dry reaction flask previously purged with argon and equipped with a constantpressure funnel and a magnetic stirrer. The mixture was cooled down to -78 ℃. Then sulfonic anhydride (22 mmol) in 10 mL of methylene chloride was added slowly into the flask under stirring. After addition of sulfonic anhydride the reaction mixture was allowed for warm-up to room temperature under constant stirring for 2 h. Then the reaction mixture was washed twice with water. The organic phase was collected and dried over Mg2SO4. After the volatiles were removed, the final product was purified by vacuum distillation or recrystallization of the residue. The1H NMR spectra of compound 1, 2 and 3 are shown in Figures 2, 3, and 4, respectively. The1H NMR data are presented as follows:
Compound 1:1H NMR (400 MHz, CDCl3): δ 3.29 (s, 3H, N-CH3), 7.55 (t,J=7.6 Hz, 4H, Ph-H), 7.65 (t,J=7.6 Hz, 2H, Ph-H), 8.01 (d,J=8.4 Hz, 4H, Ph-H);
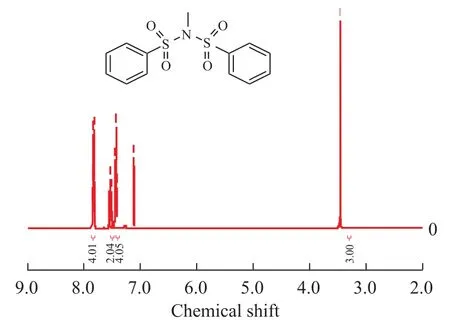
Figure 21H NMR spectrum of compound 1
Compound 2:1H NMR (400 MHz, CDCl3): δ 0.95 (t,J=7.6 Hz, 3H, CH3), 1.33 (q,J=7.6 Hz, 2H, CH2), 1.76-1.84 (m, 2H, CH2), 3.91 (t,J=8.4 Hz, 2H, N-CH2);
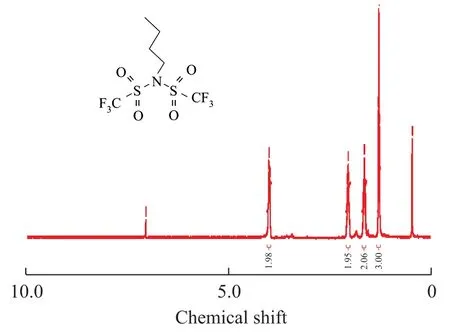
Figure 31H NMR spectrum of compound 2
Compound 3:1H NMR (400 MHz, CDCl3): δ 0.87 (t,J=6.8 Hz, 3H, CH3), 1.28-1.31 (m, 10H, CH2), 1.79-1.83 (m, 2H, CH2), 3.89 (t,J=8.4 Hz, 2H, N-CH2);

Figure 41H NMR spectrum of compound 3
Preparation of catalysts for propylene polymerization:
纵观现有的研究仍然存在着研究视角单一,对于组织边界、组织域的关注较少,缺乏对于组织机制及运作逻辑的结构性分析和建构过程的讨论。对于体育社会组织的制度约束、资源困境等关注过多,忽视了对于包括文化传统、道德习俗等非正式制度对于组织运作机制的形塑过程的分析,缺乏对于中国传统社会“合群立会”等历史视角的社会学想象力。从研究方法上看,个案研究过多,定量研究较少,从理论的解释力来看,理论与经验的不匹配、理论和方法背后的逻辑推演的不清晰,引进的西方理论的适用性等问题仍存在于目前的研究当中。
The catalysts, comprising TiCl4, MgCl2(C2H5OH)2.5, and sulfonyl amine serving as the internal electron donor,were prepared according to the reference except the carrier used[9].
Propylene polymerization:
In a 250 mL reactor previously purged with argon, 100 mL of anhydrous heptane was added. The reactor was rapidly heated to the desired polymerization temperature under an atmosphere of propylene. Then a prescribed amount of catalyst (15—20 mg) and Et3Al were added to the reactor rapidly. Polymerization was carried out under a pressure in the range of 0.11—0.13 MPa. The reaction was continued for 30 min. The polymer was filtered off and dried at 50 ℃.
3 Results and Discussion
These amines can be conveniently prepared in high yield by a one-step reaction of commercially available amines with sulfonyl anhydride. The three catalysts with sulfonyl amine used as the internal electron donor (Cat-1 to Cat-3 in Table 1) have been prepared following the procedure described in the literature[9]. The elemental analysis data of C, H and Ti content of the catalysts are given in Table 2.
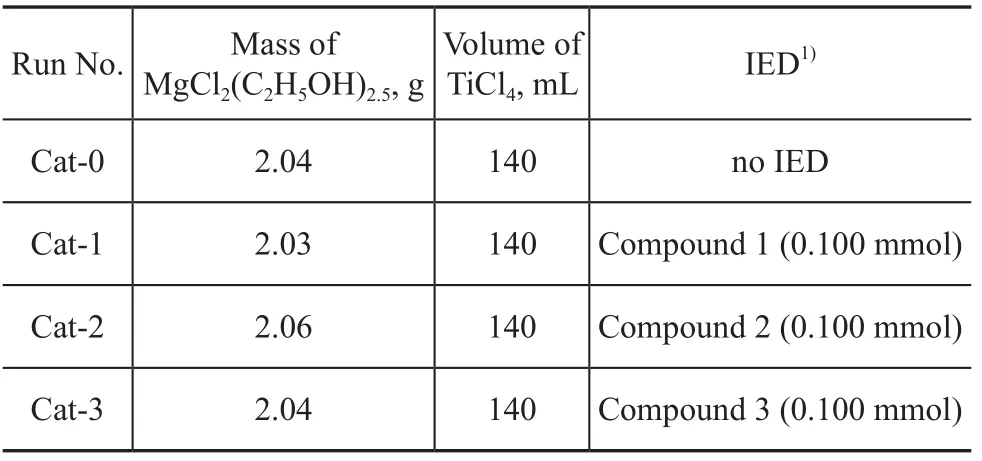
Table 1 Catalysts with different internal electron donors (IED)
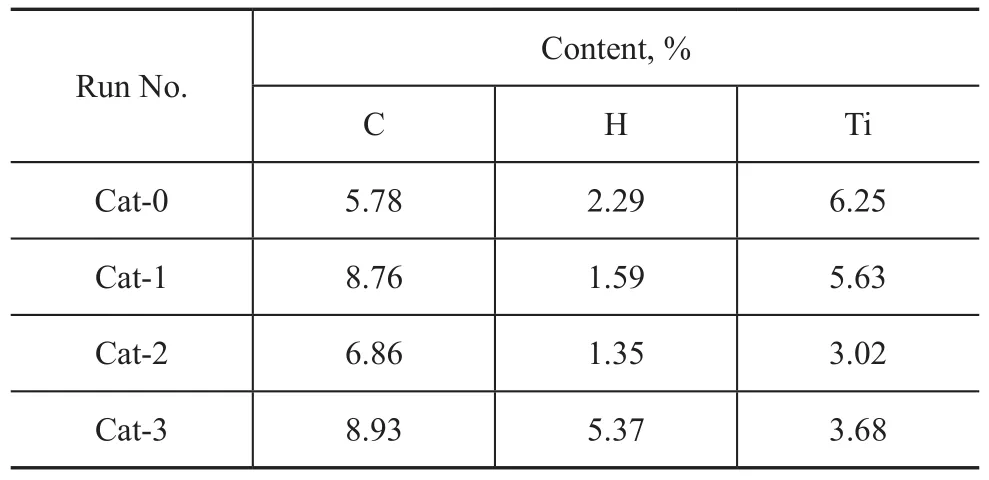
Table 2 Elemental analysis of catalysts with different IED
The propylene polymerization process was conducted according to the method described in the reference[9]. The data are listed in Table 3.
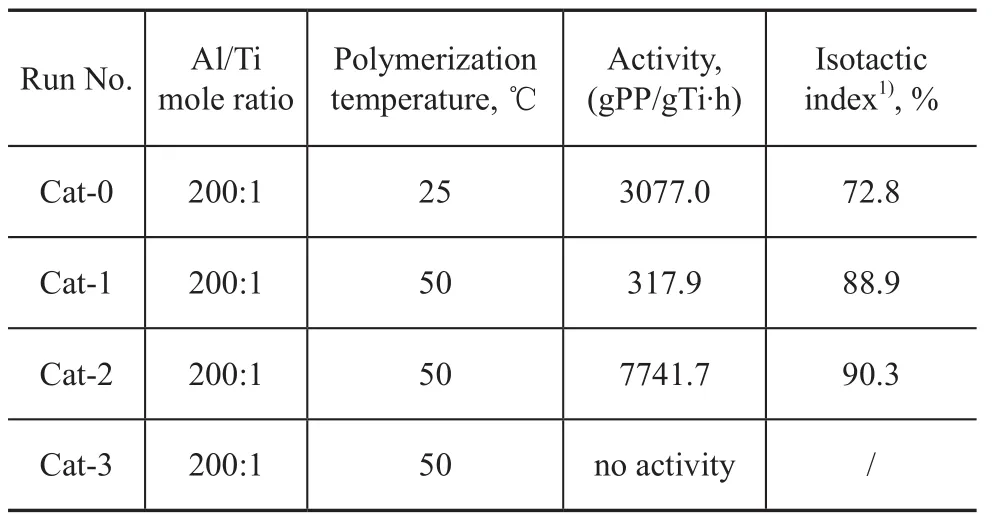
Table 3 Propylene polymerization results of catalysts with different IED
The results showed that the Cat-1 exhibited low activity and Cat-3 showed no activity at all. Only Cat-2 displayed acceptable activity. Therefore, we changed the dosage of compound 2 and prepared additional three catalysts (Cat-5 to Cat-7, composite dosages of which are listed in Table 4).
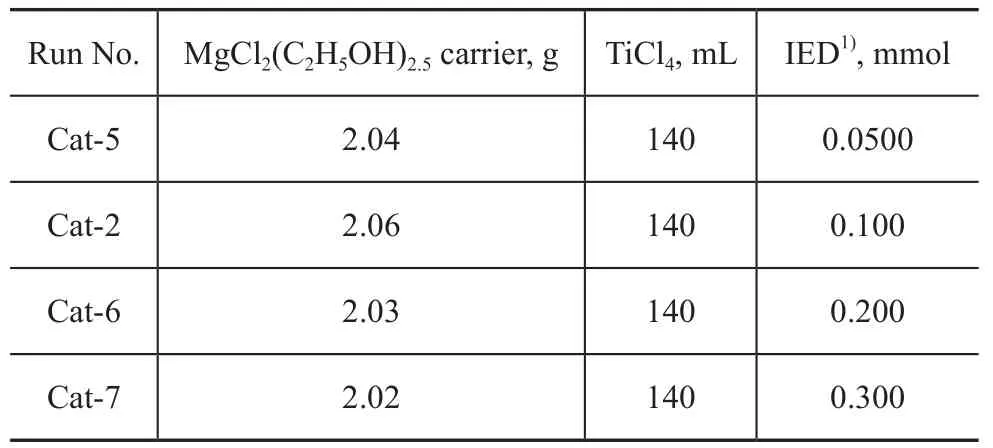
Table 4 Catalysts with compound 2 used as IED
The content of Ti, H and C in the catalysts is shown in Table 5.
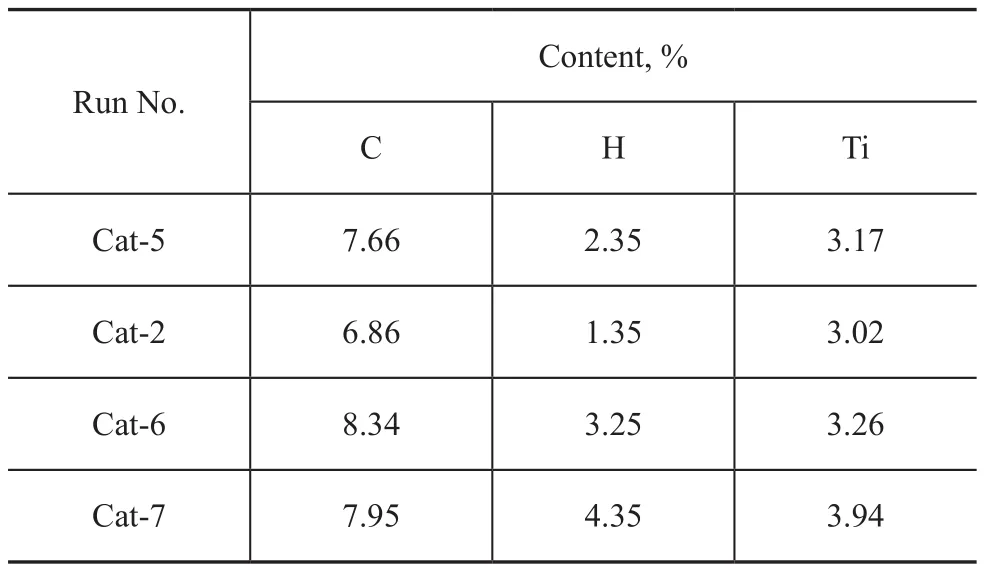
Table 5 Elemental analysis of catalysts with compound 2 used as IED
The results of propylene polymerization are shown in Table 6.
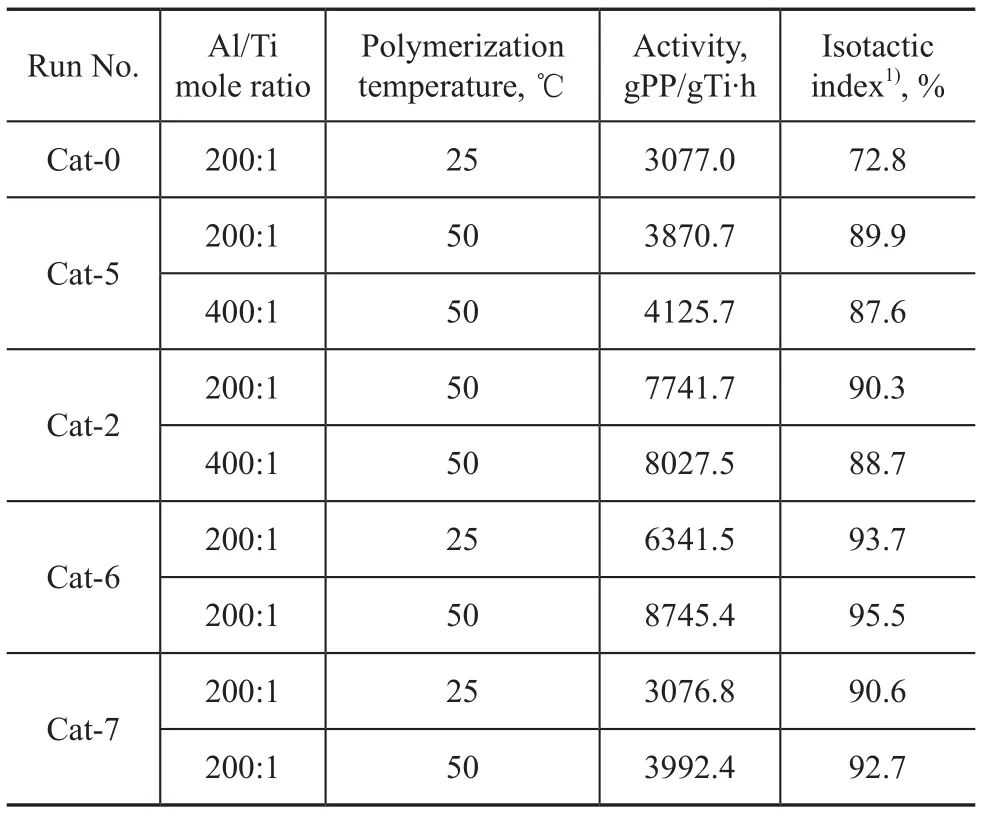
Table 6 Propylene polymerization results of catalysts with compound 2 used as IED
The polymerization results showed that the dosage of internal electron donor, the Al/Ti molar ratio and the polymerization temperature have remarkable effect on the catalytic activity and isotacticity. In the absence of the internal electron donors, the catalyst activity (Cat-0) and isotacticity of the polymer product are very low. At an internal electron donor/Mg molar ratio of 1:20 (Cat-5), the activity of the catalyst was slightly increased with very low isotacticity of the polymer product. When the molar ratio of the internal electron donor to Mg changed to 2:10 (Cat-6), the activity of the catalyst and the stereoregularity of the polymer were significantly improved. This result is consistent with those reported in the previous paper[10], demonstrating that the electron donors play an important role relating to the catalyst activity and stereoregularity of the resulted polymers. However, when the molar ratio of the internal electron donor to Mg was increased to 3:10 (Cat-7), the activity of the catalyst was suppressed. It indicated that surplus electron donor can reduce the catalytic activity, which is consistent with that mentioned in the previous paper[11-12]. These results indicated that the dosage of the internal electron donor significantly affected the activity of the catalyst. These results, combined with our previous study on this type of electron donors, indicated that sulfonyl amines are promising internal electron donors for the industry applications. The outstanding performance of these sulfonyl amines used as internal electron donors may be attributed to the synergy of the sufonyl group with nitrogen atom. Sulfonyl aromatic amines show better performance than sulfonyl aliphatic amines functioning as IED. Nevertheless, due to more convenient preparation and environmentally friendly behavior compared to sulfonyl aromatic amines, sulfonyl aliphatic amines show remarkably potential for the industry applications.
4 Conclusions
Three new sulfonyl aliphatic amines have been synthesized and used as internal electron donors for the preparation of Ziegler-Natta catalysts. The experimental results demonstrated that the internal electron donors have substantial effect on the catalytic activity and isotacticity of the resulted polymers. The highly active catalysts can be obtained when the molar ratio of the internal electron donor to Mg reaches 2:10. Under the optimized conditions, the activity of the catalysts for propylene polymerization reactions reached 6.3 kgPP/gTi·h with the isotactic index equating to 93.7% in the absence of external electron donors. These results indicated that sulfonyl aliphatic amines are promising internal electron donors for the industry applications.
Acknowledgments:We are grateful to the PetroChina for the financial support (Contract Number: 08-07-01-20).
[1] (a) Guo R, Li L, Li Y L, et al. Study on property and structure of PP resin prepared with CMMS and DCPMS as external donor[J]. Qilu Petrochemical Technology, 2006, 34(4): 392-394 (in Chinese); (b) Xu W Q, Li X J, Wang X S, et al. Synthesis and catalytic activity of Ziegler-Natta type catalyst-2,2-diisobutyl-1,3-propylene glycol dichlorobenzoate[J]. Chinese Journal of Synthetic Chemistry, 2010, 18(2):176-179 (in Chinese).
[2] He Y. Supply and demand of polypropylene and its development worldwide[J]. Chemical Techno-Economics, 2005, 23(3): 17-20 (in Chinese)
[3] Soga K, Shiono T. Ziegler-Natta catalysts for olefin polymerization[J]. Progress in Polymer Science, 1997, 22(7): 1503-1546
[4] Zhong C F, Gao M Z, Mao B Q. Influence of “TMA-depleted” MAO and alkylaluminiums on propylene polym-erization at high temperature with TiCl4/MgCl2catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2006, 243: 198-203
[5] Stukalov D V, Zakharov V A, Potapov A G, et al. Supported Ziegler–Natta catalysts for propylene polymerization. Study of surface species formed at interaction of electron donors and TiCl4with activated MgCl2[J]. Journal of Catalysis, 2009, 266: 39-49
[6] (a) Cavallo L, Piero D S, Melchior A, et al. Key interactions in heterogeneous Ziegler-Natta catalytic systems: Structure and energetics of TiCl4-Lewis base complexes[J]. Journal of Physical Chemistry, 2007, 111(11): 4412-4419; (b) Xu D M, Ma Z, Mi X, et al. Studies on catalyst containing novel asymmetric diether for propene polymerization[J]. Chemical Research in Chinese Universities, 2002, 23(5): 982-984 (in Chinese)
[7] Li Z H, Li H Y, Hu Y L. Progress of Ziegler-Natta catalysts containing diether internal donors and their use in propylene polymerization[J]. Polymer Bulletin, 2009, 5: 30-35
[8] Liu H T, Ma J, Ding C M, et al. Propylene polymerization catalyst with 1,3-diol dibenzoate as internal donor[J]. Petrochemical Technology, 2006, 35(2): 127-131 (in Chinese)
[9] Li H S, Yi J J, Cui C M. Bis(trifluoromethylsulfonyl) phenylamines as internal donors for Ziegler-Natta polymerization catalysts[J]. China Petroleum Processing and Petrochemical Technology, 2008(3): 51-54
[10] (a) Liu B P, Nitta T, Nakatani H, et al. Stereospecific nature of active sites on TiCl4/MgCl2Ziegler–Natta catalyst in the presence of an internal electron donor[J]. Macromolecular Chemistry and Physics, 2003, 204(3): 395-402; (b) Correa A, Piemontesi F, Morini G, et al. Key elements in the structure and function relationship of the MgCl2/TiCl4/ Lewis base Ziegler-Natta catalytic system[J]. Macromolecules, 2007, 40(25): 9181-9189
[11] Sacchi M C, Forlini F, Tritto I, et al. Activation effect of alkoxysilanes as external donors in MgCl2and supported Ziegler-Natta catalysts[J]. Macromolecules, 1992, 25(22): 5914-5918
[12] Yin B Z, Wang L, Yi J J, Cui C M. Study on Property of Polypropylene Catalyst with New Internal Donor[J]. China Petroleum Processing and Petrochemical Technology, 2011, 13(4): 70-73
Recieved date: 2013-02-28; Accepted date: 2013-04-18.
Professor Cui Chunming, Telephone: +86-22-23506975; E-mail: cmcui@nankai.edu.cn.
- 中国炼油与石油化工的其它文章
- Influence of Carbon Content on S Zorb Sorbent Activity
- Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
- Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
- Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil

