Influence of Carbon Content on S Zorb Sorbent Activity
Xu Li
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Influence of Carbon Content on S Zorb Sorbent Activity
Xu Li
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
The reaction activity of S Zorb sorbents with different sulfur contents was investigated, and the structure and composition of carbon-containing sorbents were characterized by XRD, FT-IR and TG-MS in order to delve into the kind and morphology of carbon on the sorbent. Test results have revealed that coke could be deposited on the S Zorb sorbent during the operating process, and the coke content was an important factor influencing the reaction performance of the S Zorb sorbent. Retention of a definite amount of coke on the sorbent while securing the desulfurization activity of the S Zorb sorbent would be conducive to the reduction of octane loss of reaction product.
S Zorb sorbent; carbon content; desulfurization activity; octane loss
1 Introduction
By the end of 2012 the Sinopec Corp. has constructed ten commercial S Zorb units in an attempt to constantly and smoothly manufacture ultra-low sulfur (ULS) clean gasoline[1-4]. The S Zorb process achieves desulfurization of liquid fuel through selective adsorbing the sulfur atoms contained in sulfur compounds by the specific sorbent[4-7]. In the process of fuel desulfurization the retention of reasonable activity of the sorbent is very important. A lot of researches have shown[7-10]that during the desulfurization operation the active ingredients of S Zorb sorbent can give rise to the formation of zinc silicate and zinc aluminate that can affect the activity of the sorbent. However, during the regular operation of commercial units it has been disclosed that the sorbent activity is not only affected by the concentration of zinc silicate and zinc aluminate on the sorbent. Therefore, study on the factors that can influence the sorbent activity is of great significance for maintaining the activity of S Zorb sorbent and securing the long-cycle, smooth and optimized operation of the commercial S Zorb units.
This paper has investigated the rules governing the influence of carbon deposited on the S Zorb sorbent on the sorbent activity, and studied the kind and morphology of carbon deposited on the sorbent by means of XRD, FTIR, TG-MS and SEM to delve into the reasonable range of carbon concentration on the S Zorb sorbent suited to actual application.
2 Experimental
2.1 Sorbent samples
All sorbent samples were collected from a certain commercial S Zorb unit and comprised regenerated commercial sorbents labeled as samples A, B, C, and D. The gasoline sample used in the experiments was provided by a commercial FCC unit.
2.2 Evaluation of reaction performance of sorbent
A pressurized fixed fluidized bed reactor unit was adopted to evaluate the reaction performance of the S Zorb sorbent, with the simplified flow diagram of reaction unit presented in Figure 1. During the evaluation test a definite amount of sorbent was put in the reactor and was then heated and pressurized to the required temperature and pressure prior to being subjected to reduction by a hydrogen stream introduced under a specified flow rate. Under the required reaction conditions the feedstock after being preheated was routed at a specified space velocity in the reactor to take part in the adsorption reaction. The reaction product after being cooled down and separated was analyzed for determination of sulfur content and octane rating of gasoline product. The sorbent after reaction waspurged with nitrogen stream and then heated to the regeneration temperature prior to being regenerated under oxygen flow. The sorbent after termination of regeneration can be used again in the above-mentioned reaction cycle.
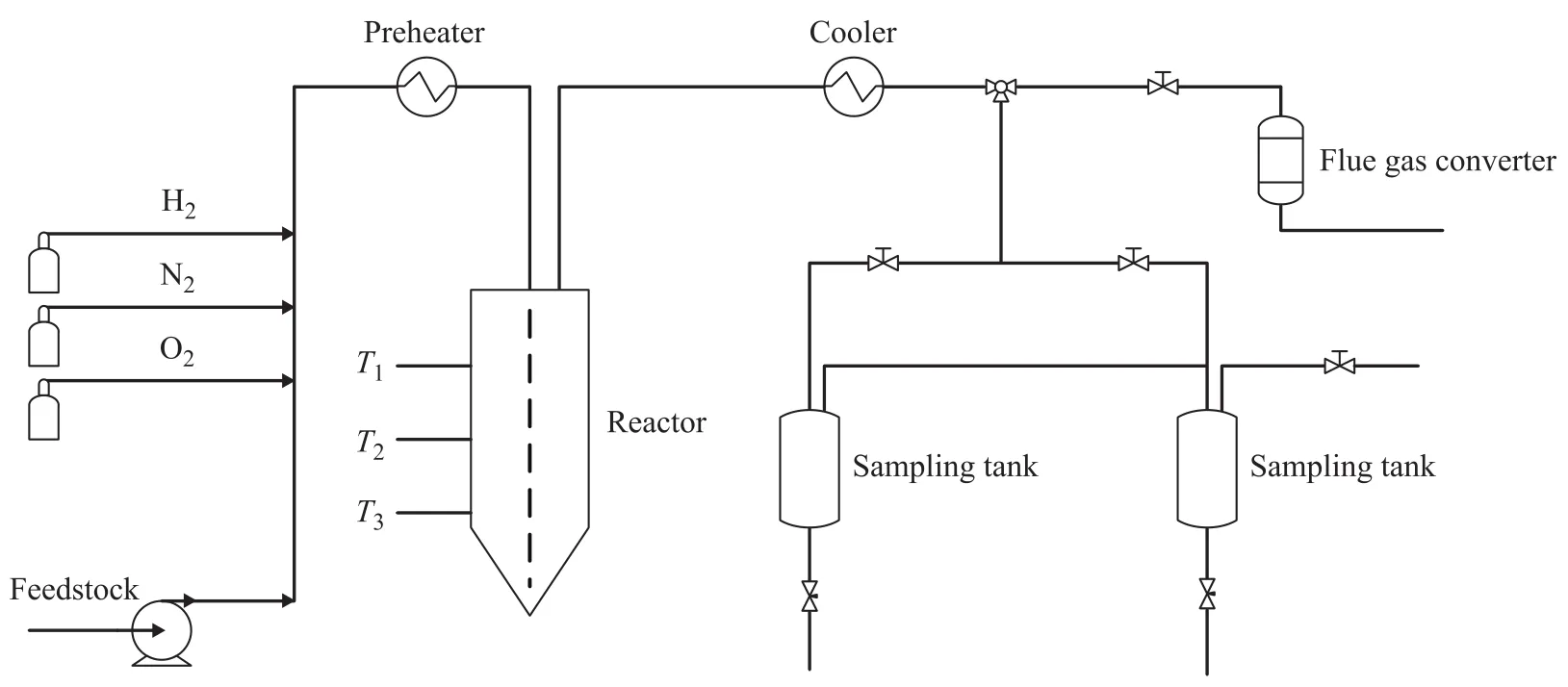
Figure 1 Sketch of reaction unit for evaluation of reaction performance of the S Zorb sorbent
2.3 Characterization of physico-chemical performance of sorbent
The structure of the sorbent was analyzed by a Rigaku TTR-III X type X-ray diffractometer, and the morphology of sorbent was analyzed by a Hitachi S4800 scanning electron microscope.
A Thermo Fisher’s Nicolet 6700 infra-red spectrophotometer was adopted to obtain the spectrograms of the feed oil and products. The carbon and sulfur content of sorbent was determined through high-temperature combustion in combination with infra-red spectrometry coupled with measurement by a LECO Corporation’s 600 series carbon and sulfur analyzer. A Netzsch GmbH’s mass spectrometer (type STA409PC-QMS403) in conjunction with thermal analysis was applied to perform TGA-MS analyses of samples.
3 Results and Discussion
3.1 Reaction performance of S Zorb sorbents with different carbon contents
The composition and sulfur and carbon contents of four sorbent samples are presented in Table 1. The reaction performance of four sorbent samples was studied in a bench-scale fixed fluidized reactor unit, with the reaction being conducted at a sorbent dosage of 240 g, a reaction temperature of 425 ℃, a reaction pressure of 2.4 MPa, a hydrogen/hydrocarbons molar ratio of 0.3, and a reaction space velocity of 4 h-1. The feedstock used in the S Zorb sulfur removal process was a FCC gasoline obtained from a certain FCC unit, with the S content equating to 486 µg/g and olefin volume fraction equating to 39.66% in the gasoline feedstock. The results for evaluating the reaction performance of sorbent samples are presented in Table 2.
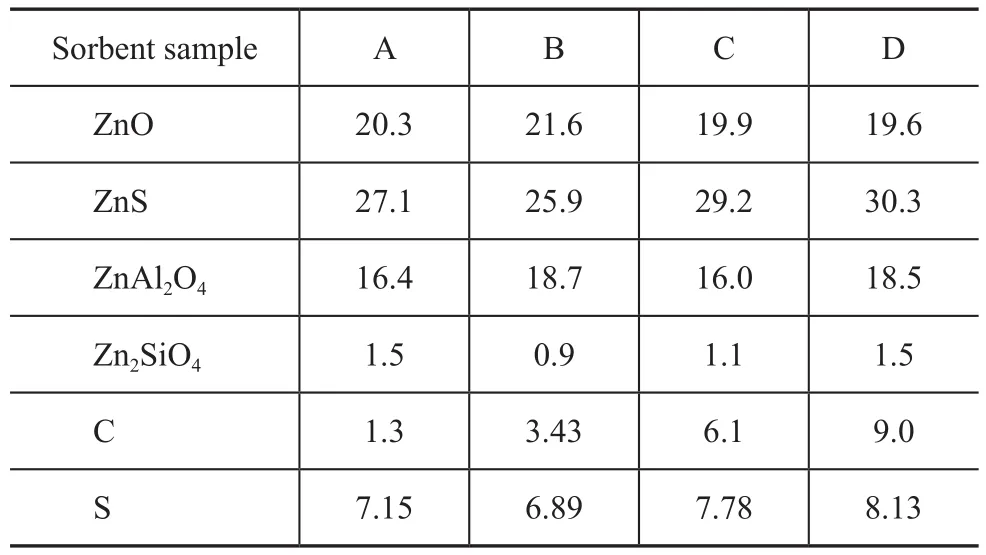
Table 1 Compounds and carbon and sulfur mass fractions in the sorbent %
The previous study[7]has revealed that the concentration of zinc-containing compounds in the sorbent can affect the reaction performance of the sorbent. The higher the ZnO content in the sorbent, the higher its sulfur removal activity would be. ZnS in the sorbent can be transformed to ZnO via normal regeneration reaction. The formation of non-active ingredients such as ZnAl2O4and Zn2SiO4can result in reduction of sorbent activity. It can be learned upon comparing the data listed in Table 1 that the content of non-active ingredients-zinc aluminate and zinc silicate in four sorbent samples was nearly equal at around 18% and 1%, respectively. Among the active ingredients the zinc oxide content in sorbent sample A, B,C and D was 20.3%, 21.6%, 19.9% and 19.6%, respectively, whereas the ZnS content in four sorbent samples was 27.1%, 25.9%, 29.2% and 30.3%, respectively. The content of sulfur adsorbed on the sorbent sample A, B, C and D was 7.15%, 6.89%, 7.78% and 8.13%, respectively. The major difference between the sorbent sample A, B, C and D was associated with the carbon content, which was equal to 1.3%, 3.43%, 6.1% and 9.0%, respectively.
It can be seen from the reaction performance of sorbents depicted in Table 2 that the four sorbent samples led to differing outcome of sulfur removal reaction. The carbon content on the sorbent was an important factor that could influence the reaction performance of the sorbent. When the carbon content on the sorbent increased from 1% to 9%, the sulfur removal activity of the sorbent dropped from 99.7% to 90.5%, but the gasoline octane loss decreased, with the antiknock index loss reduced from 0.55 to 0.2.
Since during the operation of commercial S Zorb unit it is desirable to achieve the set target of desulfurization while reducing the octane loss of products as far as possible, it is necessary to retain the carbon content of sorbent in a reasonable extent in the course of sorbent application. However, the coke formation rate on the sorbent varies significantly due to the difference in the properties of S Zorb feedstocks, while the gasoline desulfurization units also bring forth different requirements on the gasoline quality, which can result in diversifying demand for the sorbent activity. Therefore, in the course of actual application of the S Zorb sorbent it is necessary to map out an optimal range of carbon content on the sorbent based on the specific features of the S Zorb unit.
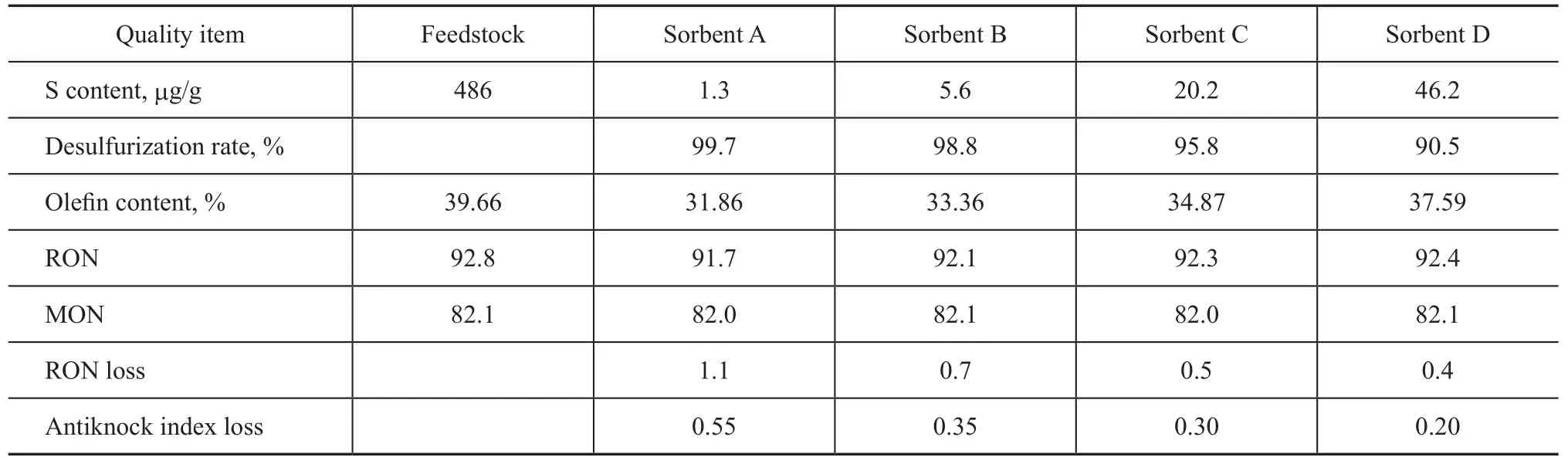
Table 2 Reaction performance of sorbents with different carbon contents
3.2 SEM, XRD and FT-IR analyses of S Zorb sorbents with different carbon contents
Figure 2 depicts the SEM images of sorbent samples B and D, with the XRD patterns of sorbent samples B and D presented in Figure 3. It can be seen from Figure 2 and Figure 3 that there is no notable difference between the SEM images and XRD patterns of the two sorbent samples despite different carbon levels they contain. The sorbent samples were spherical in shape with grain size ranging from 40 µm to 120 µm and they had similar
structure because the XRD patterns did not identify peaks of other carbon-containing compounds. It is evident that differing carbon content did not change the structure of sorbent, and difference in activity of sorbent with different carbon contents might be ascribed to the covering of sorbent active sites by some coke deposits.
The SEM images of the S Zorb sorbent samples are presented in Figure 4. It can be seen from Figure 4 that an absorbance peak of O—H stretching vibrations was identified at 3 423 cm-1, while at 1 062 cm-1a peak of anti-symmetrical stretching vibrations of Si—O—Si in silicon dioxide was seen. However, in FT-IR spectrograms no peaks of alkanes and aromatics were detected, suggesting that the carbon deposited on the sorbent might be composed of highly symmetrical substance, such as graphite carbon.
3.3 Thermal decomposition behaviour of S Zorb sorbent with different carbon contents
The TG-MS spectrograms of the sorbent samples B and D are presented in Figure 5, which shows that the thermal decomposition peak temperatures varied with differing carbon contents in the sorbent sample, with the comparison of respective peak temperatures shown in Table 3. Inorder to illustrate the difference in the nature of carbon thermal decomposition during the S Zorb sorbent regeneration process, Figure 6 depicts the corresponding TGMS spectra of industrial spent sorbent B. It is evident that no CO2evolution peaks related with sorbent samples B and D were identified at 419 ℃, confirming that no combustion-prone coke was found on the outside surface of sorbent samples B and D. The thermal decomposition temperature of the sorbent sample D with much higher carbon content was apparently higher than that of the sorbent sample B. The peak temperature of CO2evolution from the sorbent B was located at 513 ℃ and 585 ℃, respectively, whereas the sorbent D showed only a CO2evolution peak temperature of 584 ℃. It can be learned by comparing the SO2evolution peak temperatures that higher carbon content in the sorbent could affect the conversion of sulfur compounds, and the SO2evolution peak temperature of the sorbent D was apparently higher than the sorbent B. Hence it is evident that the higher the carbon content in the sorbent, the more stringent the requirement for the sorbent regeneration.
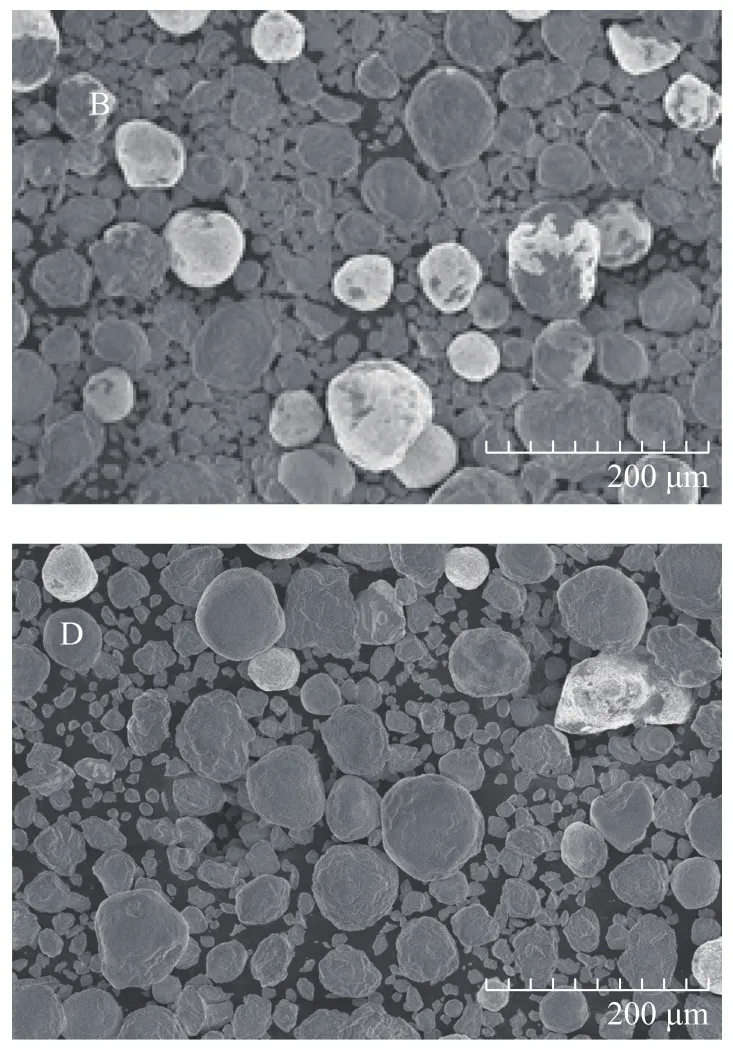
Figure 2 SEM images of S Zorb sorbent samples
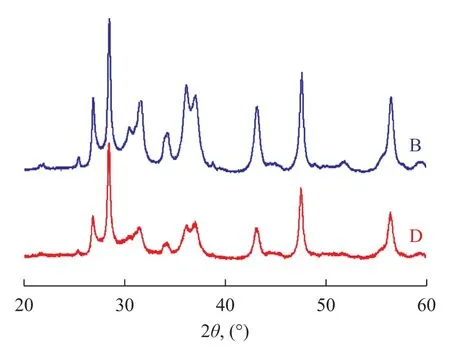
Figure 3 XRD patterns of S Zorb sorbent samples
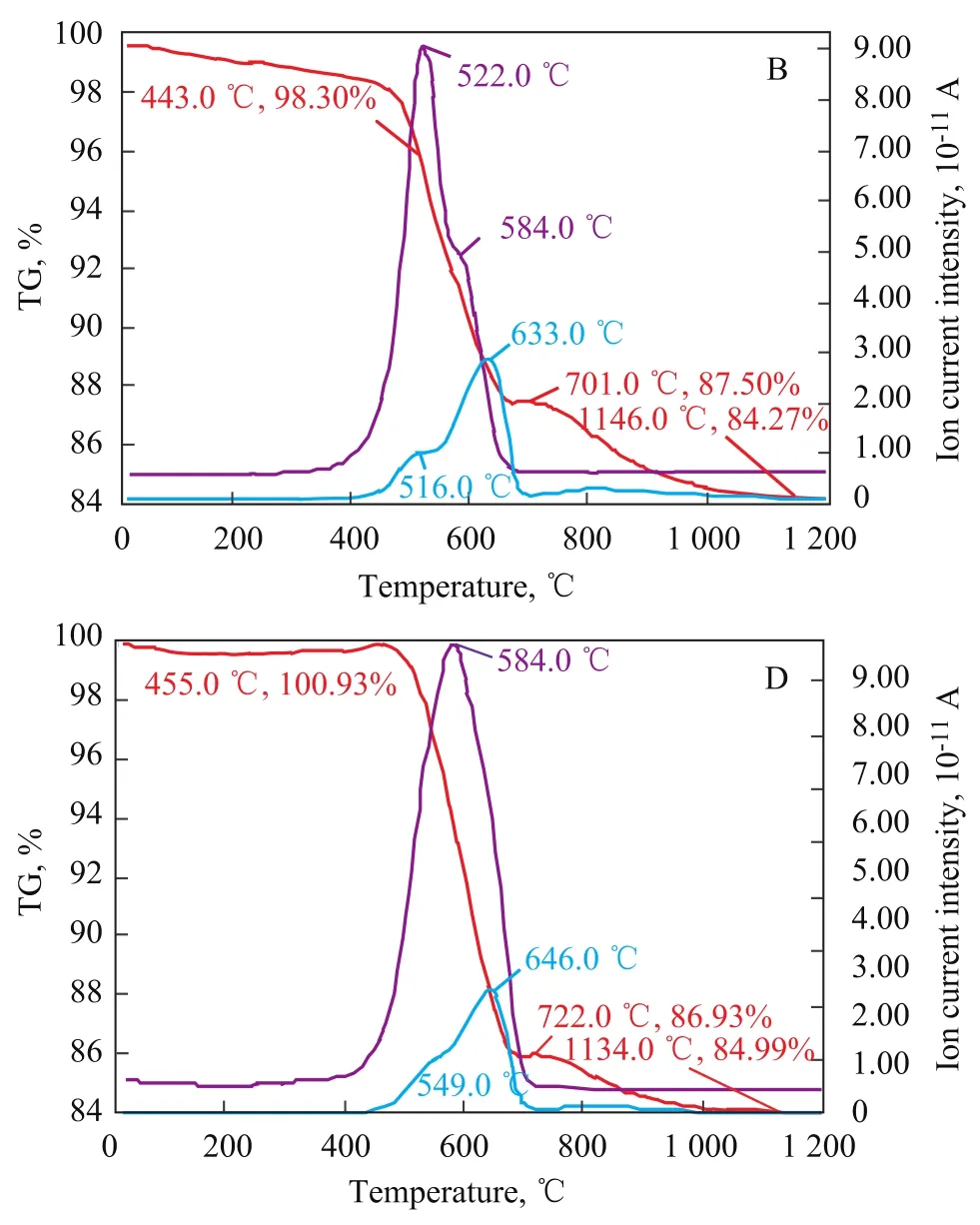
Figure 5 TG-MS spectra of S Zorb sorbent samples
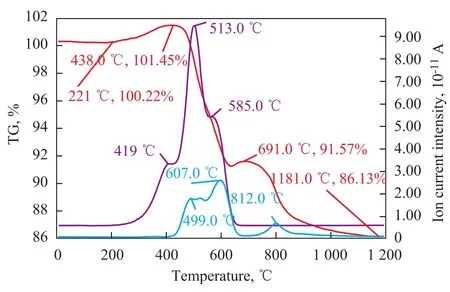
Figure 6 TG-MS spectra of sorbent B-DS
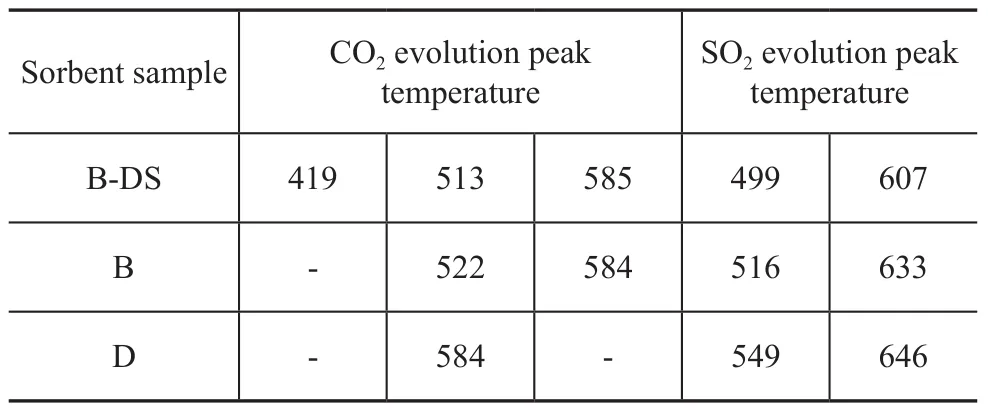
Table 3 Signal peak temperatures in S Zorb sorbents’mass spectra ℃
4 Conclusions
The coke content on the S Zorb sorbent was an important factor influencing the reaction performance of the S Zorb sorbent. The higher the carbon content in the sorbent, the lower the sulfur removal activity of the S Zorb sorbent would be along with less octane loss. During the application of the S Zorb sorbent a definite amount of carbon retained on the sorbent would be conducive to the reduction of gasoline octane loss if the required desulfurization activity of the sorbent is guaranteed. Varying feedstock properties and different demand for product slate would require different ranges of carbon content on the sorbent. TEM, XRD, FT-IR and TG-MS analyses of the S Zorb sorbents have revealed that the carbon deposited on the sorbent might be highly symmetrical substance, such as graphite carbon. The carbon deposits did not affect the crystalline structure of the sorbent, and reduction of sorbent desulfurization activity might be attributed to covering of some active sites on the sorbent by carbon deposits. The more the carbon content on the sorbent, the higher the carbon thermal decomposition temperature would be.
[1] Zhang Jingcheng, Liu Yunqi, An Gaojun, et al. The adsorptive desulfurization technologies for ultra-clean oil production[J]. Progress in Chemistry, 2008, 20(11): 1834-1845 (in Chinese)
[2] Zhu Yunxia, Xu Hui. Improvement and development of S-Zorb process[J]. Petroleum Refinery Engineering, 2009, 39(8): 7-12 (in Chinese)
[3] Song Yichang. Production route of gasoline meeting national emission standard Ⅴ for motor vehicles[J]. Petroleum Processing and Petrochemicals, 2012, 43(12): 50-54 (in Chinese)
[4] Wang Wenshou, Mao Anguo, Liu Xianlong, et al. Study on the adsorptive removal of sulfur containing compounds in FCC gasoline [J]. Petroleum Processing and Petrochemicals, 2012, 43(6): 6-10 (in Chinese)
[5] Ryzhikov A, Bezverkhyy I, Bellat J P. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step[J]. Applied Catalysis B: Environmental, 2008, 84(3/4): 766-772
[6] Bezverkhyy I, Ryzhikov A, Gadacz G, et al. Kinetics of thiophene reactive adsorption on Ni/SiO2and Ni/ZnO[J]. Catalysis Today, 2008, 130(1): 199-205
[7] Xu Guangtong, Diao Yuxia, Zou Kang, et al. Cause analysis of sorbent deactivation in S Zorb unit for gasoline desulfurization[J]. Petroleum Processing and Petrochemicals, 2011, 42(12): 1-6 (in Chinese)
[8] Zhang Xin, Xu Guangtong, Zou Kang, et al. Formation mechanism of gahnite in S Zorb sorbents [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(2): 242-247 (in Chinese)
[9] Zou Kang, Huang Nangui, Xu Guangtong. Study of Rietveld quantitative phase analysis of S Zorb sorbent[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(4): 38-44 (in Chinese)
[10] Lin Wei, Wang Lei, Tian Huiping. An analysis of the formation rate of zinc silicate in S Zorb sorbents [J]. Petroleum Processing and Petrochemicals, 2011, 42(11): 1-4 (in Chinese)
Recieved date: 2013-02-28; Accepted date: 2013-04-18.
Dr. Xu Li, Telephone: +86-10-82369233; E-mail: xuli.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
- Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
- Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
- Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil

