Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
Luo Hui; Fan Weiyu; Li Yang; Zhao Pinhui; Nan Guozhi
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao 266555)
Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
Luo Hui; Fan Weiyu; Li Yang; Zhao Pinhui; Nan Guozhi
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao 266555)
The continuous reduction in sulfur content of fuels would lead to diesel fuel with poor lubricity which could result in engine pump failure. In the present work, fatty acids were adopted as lubricity additives to low-sulfur diesel fuel. It was attempted to correlate the molecular structures of fatty acids, such as carbon chain length, degree of saturation and hydroxylation, to their lubricity enhancement, which was evaluated by the High-Frequency Reciprocating Rig (HFRR) method. The efficiency order was supported by the density functional theory (DFT) calculations and the molecular dynamics (MD) simulations. The lubricity enhancing properties of fatty acids are mainly determined by the cohesive energy of adsorbed films formed on iron surface. The greater the cohesive energy, the more efficiently the fatty acid would enhance the lubricity of low-sulfur diesel fuel.
lubricity; fatty acid; DFT; MD simulation; adsorption
1 Introduction
The environmental requirements demand the governments of numerous countries to impose strict regulations on the sulfur content of commercial petroleum fuels. However, the natural lubricating components of the fuels would be removed simultaneously during the desulfurization process, which would result in poor lubricity of the desulfurized fuel. The engine and fuel injection system would be damaged when using liquid fuel with poor lubricity[1-2]. This damage could be avoided by the addition of lubricity additives to restore the fuel lubricity. Various types of lubricity additives have been proposed, such as long chain fatty acids, fatty esters, fatty acid dimers, aliphatic amines and alkoxyamides, etc.[3-4]Among these compounds, long chain fatty acids have been found to be quite effective.
Boundary lubrication formed by the polar additives plays a key role in the lubrication of fuels[5-6]. As shown in Figure 1, the polar head of additives are adsorbed on the metal surface, either by chemisorption or physical adsorption, and the long chain tails are aligned directionally to form a film by the van der Waals interaction and dipole interaction between molecules. This film is considered to be most important for reducing the number of points at which true metal to metal contact occurs[7]. For a given polar group, it is shown that the lateral cohesive interactions between the hydrocarbon chains in the monolayer play a major role in reducing friction and that the sum of these interactions approximates the enthalpy of fusion[8].
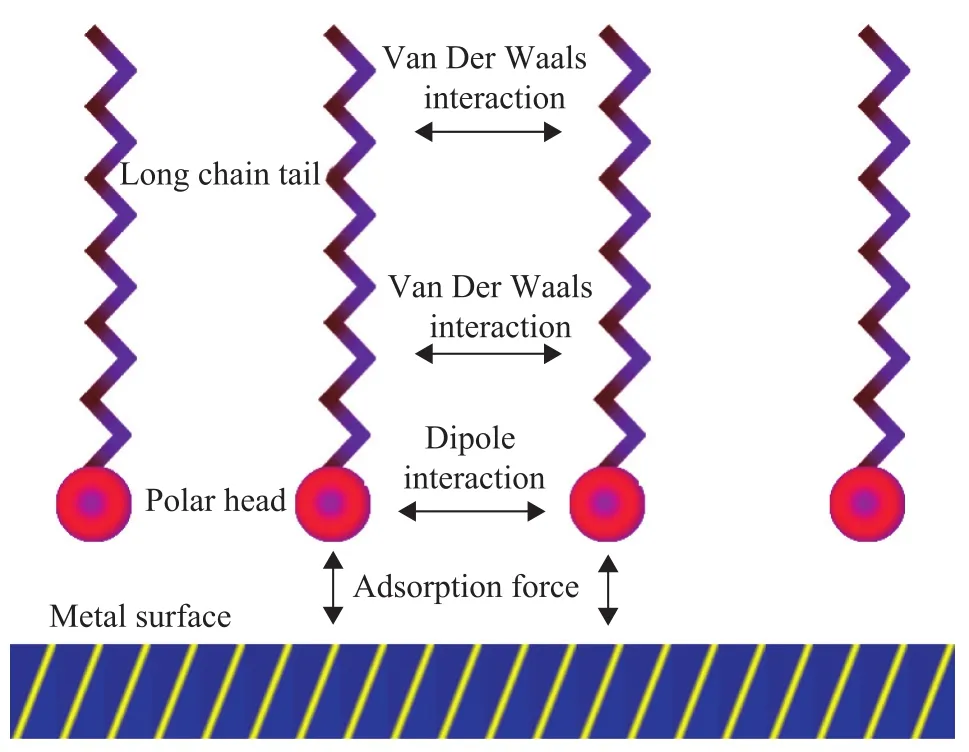
Figure 1 Schematic diagram of the polar additives adsorbed on the metal surface to form a film
The interactions between the additives and the metal surface, especially the interactions between the additives themselves, can hardly be measured experimentally. However, the density functional theory (DFT) calculations and molecular dynamics (MD) simulations would be the powerful methods to investigate the adsorption behavior at microscopic molecular level and provide useful information[9-12]. To assess an effective lubricity enhancing additive, the effect of the molecular structure of additives on their performance is the key issue. DFT calculations have been employed by many researchers to study the effect of the polar group of additives on their tribological performance[13-15]. However, interactions between the chain tails of additives and their effects on the performance of lubricity enhancement have rarely been studied.
The aim of this work is to correlate the molecular structures of some fatty acids used as lubricity additives with their lubricity properties. The lubricity enhancing properties of the fatty acids in low-sulfur diesel fuel were evaluated by a high frequency reciprocating rig (HFRR) apparatus. The HFRR results will be supported by the density functional theory (DFT) calculations and the molecular dynamics (MD) simulations.
2 Experimental and Computational Methods
2.1 Lubricity measurements
A hydrotreated diesel was used as the test fuel in this study, and its typical properties are listed in Table 1. The fatty acids, such as octanoic acid (C8:0), decanoic acid (C10:0), lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and ricinoleic acid (C18:1 OH), were used as lubricity additives. All the fatty acids were commercially available chemicals and their purity were nearly 99 m% without further purification.
The additives were added to the test fuel in a concentration range of between 50—500 μg/g. All lubricity measurements were performed by using the high-frequency reciprocating rig (HFRR) apparatus according to the ISO-12156 method. Specifically, 20 mL of test sample was placed in the test reservoir at 60 ℃, and the test was carried out at a frequency of 50 Hz under a load of 2 N for a duration of 90 min. The lubricity of the test sample was estimated by measuring the average wear scar diameter (WSD) of the spherical specimen with a photomicroscope. A smaller WSD value means better lubrication.
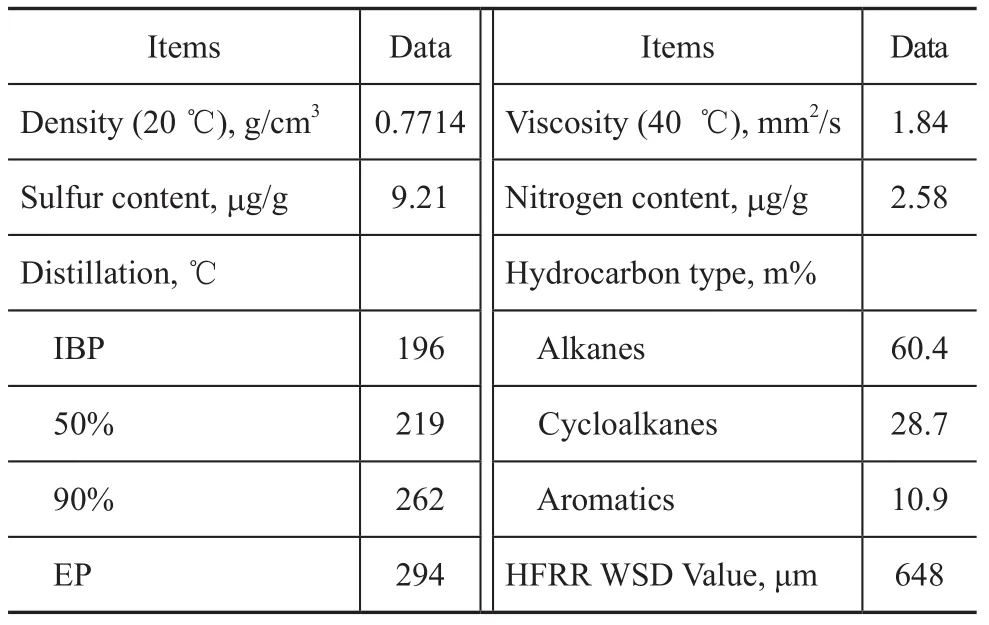
Table 1 Properties of hydrotreated diesel fuel
2.2 Quantum chemistry calculations
DFT calculations were performed by means of the DMol3program[16-17]in the Materials Studio 5.5 package developed by Accelrys Ltd. The molecules of the fatty acids were first simulated in Materials Visualizer. Then, the geometry optimizations were performed and the structural properties were obtained by means of the Generalized Gradient Approximation (GGA) with the Becke Lee Yang Parr (BLYP) correlation functions[18-19]. The doublenumeric quality base set with polarization functions (DNP) was applied in this work. The convergence criteria for these optimizations consisted of threshold values of 1.0×10-5Ha, 0.002 Ha/Å and 0.005 Å for energy, force and displacement convergence, respectively, and the selfconsistent field (SCF) density convergence threshold value was set at 1.0×10-6Ha.
The global reactivity of the molecules was analyzed through evaluating the energy of the highest occupied molecular orbital (EHOMO), the energy of the lowest unoccupied molecular orbital (ELUMO), the absolute electronegativity (χ), the hardness (η), and the electrophilicity index (ω).
Absolute electronegativity (χ) and hardness (η) of the additives were calculated as described by Pearson[20]:
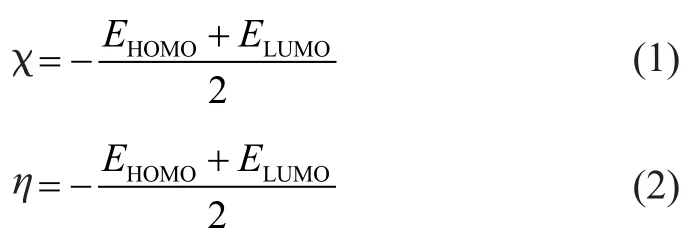
As introduced by Parr, et al.[21], the electrophilicity index was used to measure the electrophilic capacity of the molecules, which was defined as follows:

2.3 MD simulations
The adsorption behavior of the fatty acids onto Fe (110) plane was investigated by means of molecular dynamics (MD) simulations. The Forcite package contained in Accelrys Materials Studio 5.5 was adopted and the condensed-phase optimized molecular potentials for atomistic simulation studies (COMPASS)[22]forcefield was used for the MD simulations. The Fe (110) plane composed of eight Fe layers was first built and relaxed to minimize its energy by using the Smart Minimizer method. Next, the surface area was increased to 3.4753 nm×3.4753 nm and its periodicity was changed by constructing a super cell. Then, a vacuum slab with 6.0 nm of thickness was built on the Fe (110) plane, which was large enough to avoid the interaction between the adsorbate (fatty acids) and the periodic image of the bottom layer of atoms in the plane. Finally, 49 molecules of fatty acid were introduced into the simulation box, and the initial structure model is shown in Figure 2 (a), in which only stearic acid adsorbed on Fe surface was presented as the representative.
Prior to the dynamics run, all systems were optimized using the Smart Minimizer algorithm, and the convergence criterion adopted for the value of maximum force was 103kcal/(mol·Å) for the Steepest Descent method, 10 kcal/(mol·Å) for the Conjugate Gradient method, and 10-5kcal/(mol·Å) for the Newton-Raphson Gradient method. The minimized systems were subsequently relaxed by simulated annealing through preheating gradually from 300 K to 500 K during 20 ps using the velocity scale temperature control method in the constant particle volume temperature (NVT) ensemble. Equilibration and production simulations were conducted in the constant NVT ensemble, and the temperature was set at 298 K which was controlled by means of the Andersen method[23]. The summation methods for short-range interactions and nonbonds van der Waals and Coulomb interactions were the atom based, with a cutoff of 1.55 nm (0.10 nm for Spline width and 0.10 nm for Buffer width), and the intermolecular interactions beyond the cut distance were corrected with an approximation of the average density correction. The simulation time and step length were set at 100 ps and 1 fs, respectively, and a frame was exported on each 1000 fs for statistical analysis.
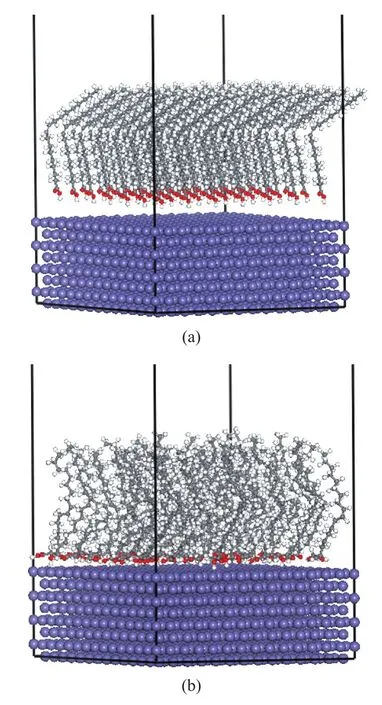
Figure 2 Structure model of oleic acid molecules adsorbed on the Fe (110) plane: (a) the initial structure, (b) the equilibrium structure after MD simulation
The equilibrium of the simulation systems was judged by the fluctuation of energy and temperature. Relative deviations of energy and temperature were less than 0.1 % and 10 %, respectively, and after about 20 ps it could be concluded that the simulation system had reached equilibrium after 20 ps. The equilibrium model is shown in Figure 2 (b).
The binding energy of fatty acids adsorbed on Fe (110) plane were calculated using Equation (5):
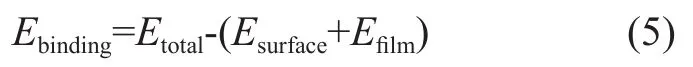
whereEtotalis the total energy of the surface and additives,Esurfaceis the energy of the surface without the additives, andEfilmis the energy of the adsorbed film of additives without the surface.
The cohesive energy of adsorbed film is defined as follows[24]:
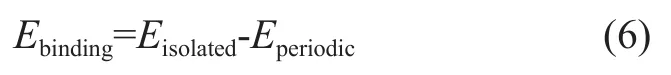
whereEisolatedis the energy of additive molecule in the isolated state, andEperiodicis the energy of additive molecule in the adsorbed film.
3 Results and Discussion
3.1 Lubrication properties
It is well known that the long chain fatty acids are surface active materials, which are used widely to enhance the lubricity of synthetic or mineral fuels. The fatty acids can be adsorbed on the rubbing surfaces and form a hydrodynamic film, resulting in a low shear stress layer at a majority of metal-to-metal contact points. Their long carbon chains could increase the thickness of the film and enhance the intermolecular forces in the film.
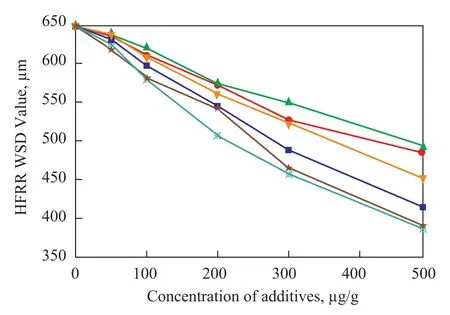
Figure 3 HFRR lubricity evaluation for saturated fatty acids of increasing chain length in low-sulfur diesel fuel
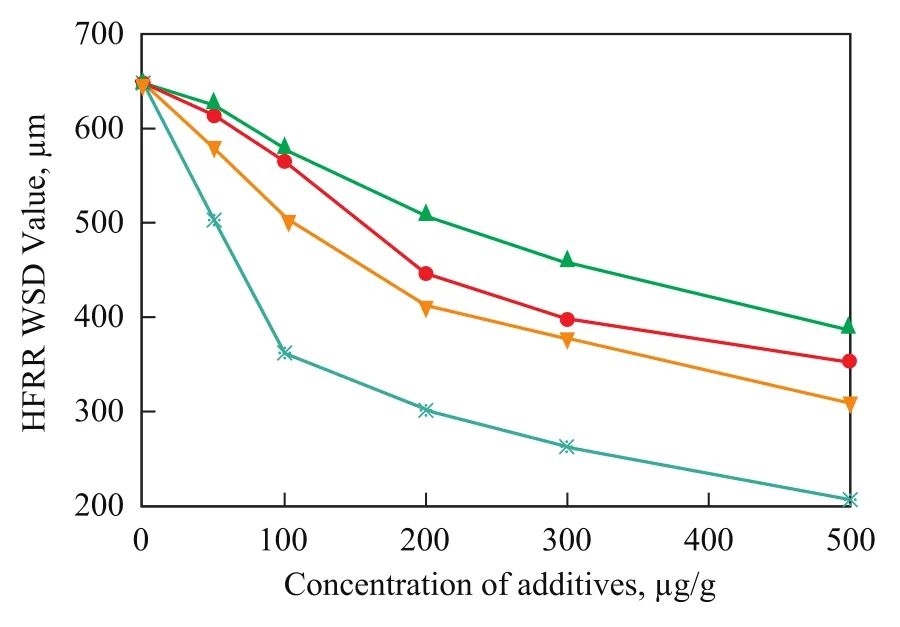
Figure 4 HFRR lubricity evaluation for 18-carbon-chain fatty acids in low-sulfur diesel fuel
Figure 3 presents HFRR lubricity versus carbon chain length of saturated fatty acids. It can be seen that the lubricity of the diesel fuel blend improves regularly with an increasing concentration of fatty acids in diesel fuel and there is a specific influence of the carbon chain length of these fatty acids on the fuel lubricity. The saturated fatty acid with longer carbon chain shows a higher efficiency to improve the lubricity of the fuel.
The results of HFRR analysis for 18-carbon-chain fatty acids with different chain structures are shown in Figure 4, and this series of fatty acids exhibited a clear trend associating the carbon bond unsaturation degree with fuel lubricity enhancement. It can be confirmed that the fuel lubricity enhancement increased with an increase in the degree of carbon bond unsaturation. Especially, the hydroxylated fatty acid, ricinoleic acid, which was used in this study displayed more effective lubricity enhancement than the non-hydroxylated one, oleic acid, and was ultimately better than all the nonhydroxylated fatty acids studied.
3.2 Quantum-chemical parameters of the fatty acids
Geometric and electronic structures of the fatty acids were calculated by means of DFT, with the optimized structures shown in Figure 5, in which only stearic acid and linoleic acid are presented as the representatives. The quantum chemical parameters are presented in Table 2. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are very important quantum chemical indicators.EHOMOis directly related to the electron-donating ability of the molecule, andELUMOis associated with the ability of the molecule to accept electrons. The higher the value ofEHOMOis, more probably the molecule would donate electrons to appropriate acceptor molecules with low energy. Conversely, the lower the value ofELUMOis, more possibly the molecule would accept electrons[10].
It can be seen from Table 2 that the total energy (ETotal) of fatty acids decreases with an increasing carbon chain length, and the value ofETotaldecreases approximately by 2.0×105kJ/mol for each additional ethyl chain. The chain length has no significant effect on other parameters of saturated fatty acids, such asEHOMO, ΔE,χ,ηandω. So the carbon chain length has no effect on the ability offatty acid to accept electrons or donate electrons. Double bonds introduced in the carbon chain of the fatty acid could lead to significant increase inEHOMO, or decrease in ΔE,χ,ηandω. And these parameters would change obviously with an increase in the number of double bonds. This means that the ability of fatty acid to donate electrons would be improved by an addition of double bonds. If the carbon chain of the fatty acid contains double bond and hydroxyl group at the same time, such as ricinoleic acid,ETotaldecreases approximately by 2.0×105kJ/mol, but the changes inEHOMO, ΔE,χ,ηandωare less obvious compared with the effect of double bonds alone.
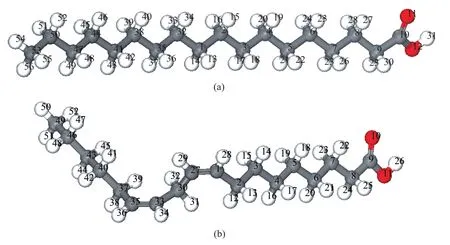
Figure 5 Optimized geometries of the fatty acids obtained by DFT calculations: (a) stearic acid; (b) linoleic acid
Judging from HFRR values of these fatty acids and the results of DFT calculations, it can be found out that an increase ofETotalor a decrease of ΔE,χ,ηandωcan lead to a more lubricity enhancing efficiency.
3.3 Results of MD simulations
In the current study, the fatty acids have been simulated as adsorbate on Fe (110) substrate to investigate the preferential adsorption phenomenon. Figure 6 shows the relative concentration profile of fatty acids in the adsorbed films on Fe (110) substrate. These concentration profiles suggest that the films have a higher density close to Fe (110) substrate indicating that the fatty acids can stick on the iron surface because of adsorption. With the increase of carbon chain length, the thickness of the adsorbed film increases obviously. However, the double bonds or hydroxyl group contained in carbon chain has no significant effect on the concentration profile of fatty acids.
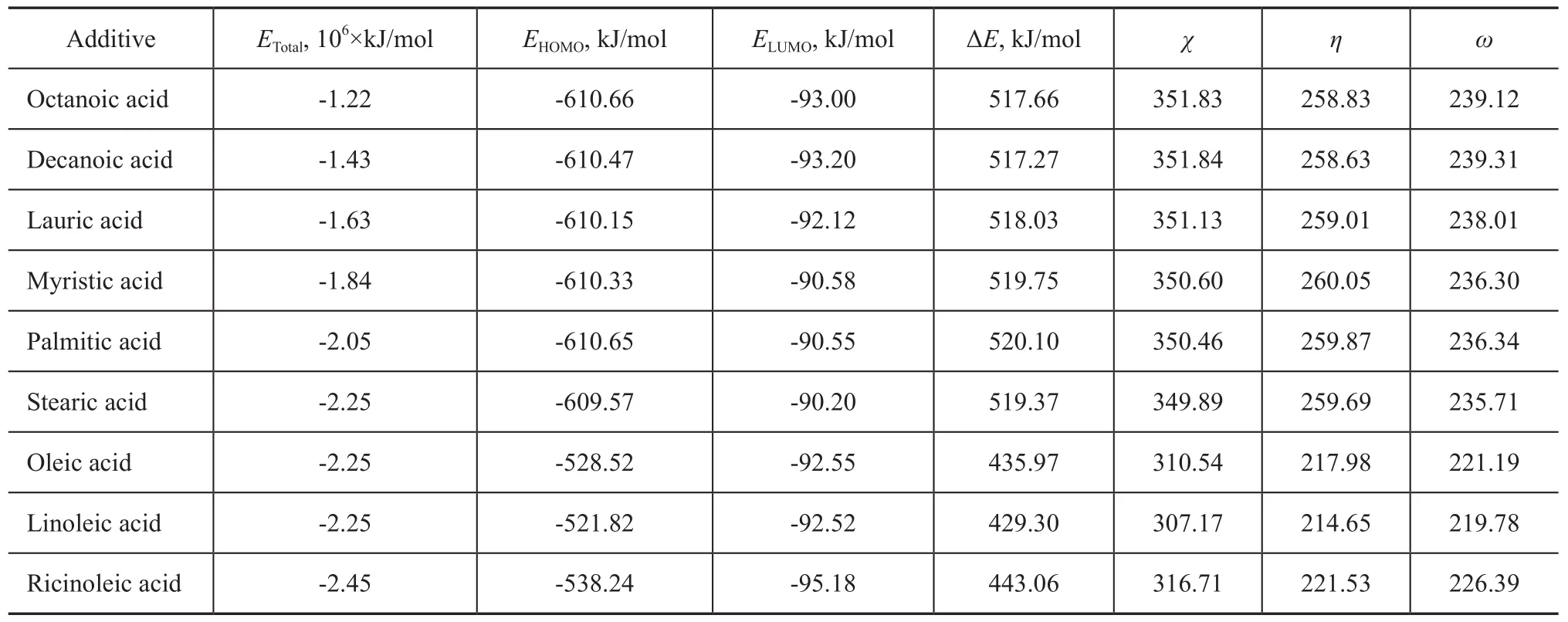
Table 2 Quantum chemical parameters derived for the fatty acids calculated with DFT method
Besides the adsorption interactions between additives and Fe surface, the interactions of additive molecules themselves in the adsorbed film could not be ignored as it has been demonstrated in Figure 1. The binding energy of fatty acids adsorbed on Fe (110) plane and the cohesive energy of the adsorbed film are shown in Table 3.
It can be seen from Table 3 that the fatty acids adsorbed on Fe (110) plane have the same or nearly the same binding energy, which demonstrates that for the fatty acids, the interaction between additives and Fe surface is not the most important factor affecting the lubricity enhancing properties. With the increase in carbon chain length of saturated fatty acids, the cohesive energy of the film increases; and the double bonds or hydroxyl group introduced in the carbon chain can enhance the cohesive energy. Ricinoleic acid could form an adsorbed film with a maximum cohesive energy during the simulation process.
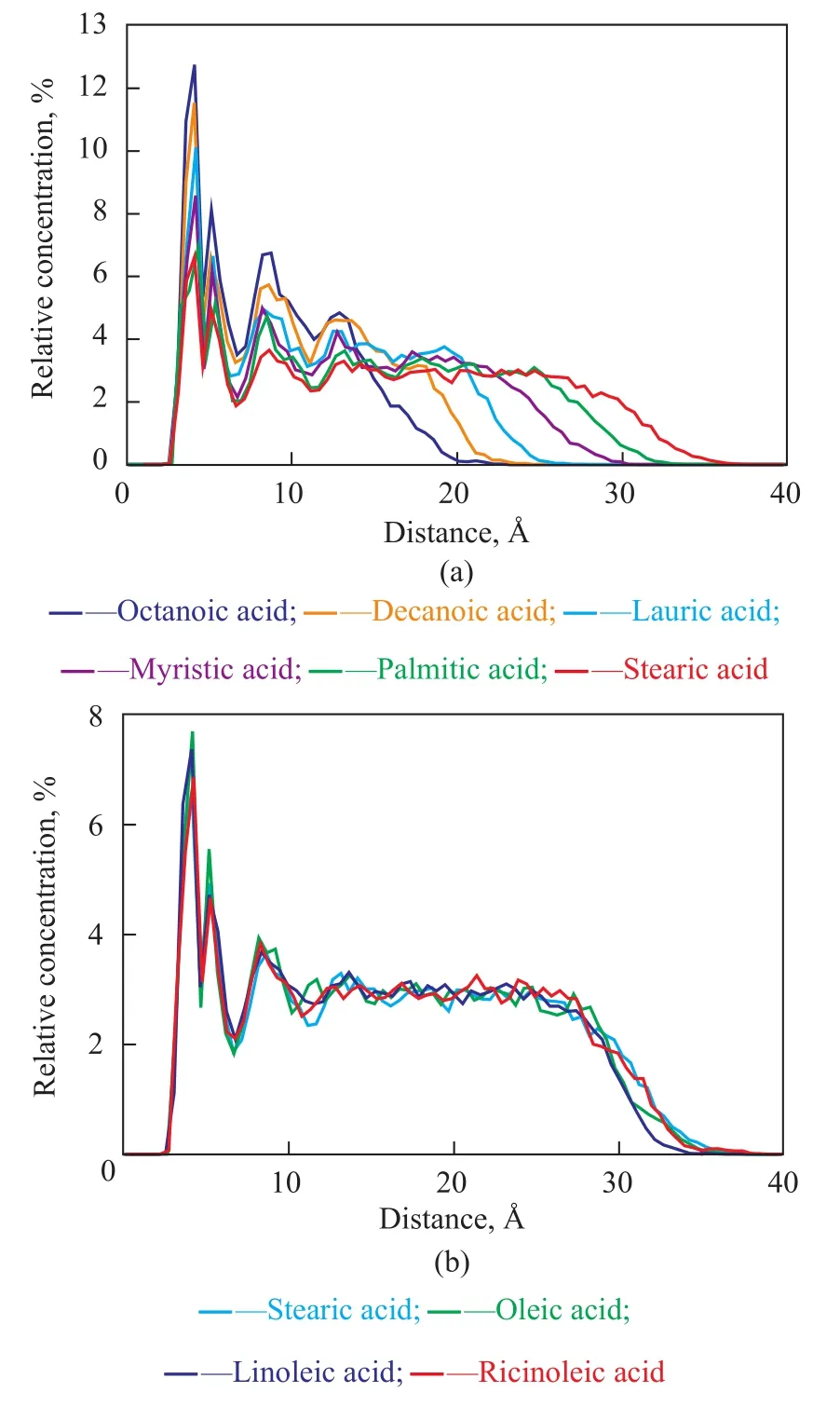
Figure 6 Concentration profile of fatty acids in the adsorbed films on Fe (110) surface: (a) saturated fatty acids with different carbon chain length; (b) 18-carbon-chain fatty acids
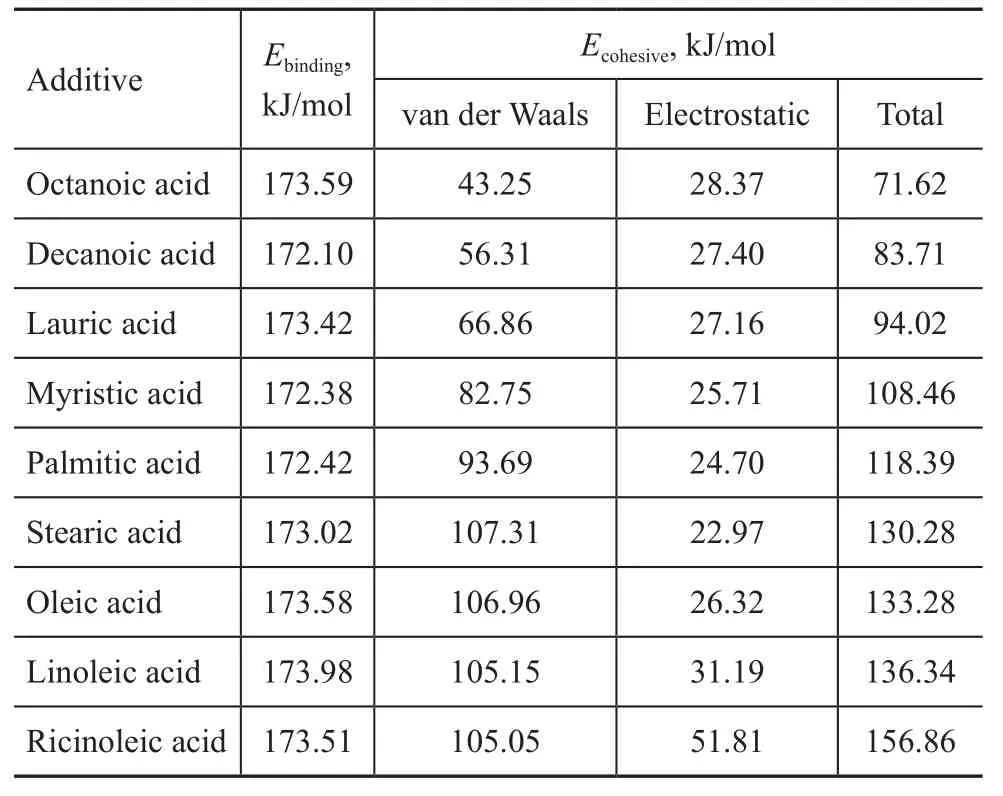
Table 3 Results calculated by means of MD simulation for adsorption of fatty acids on Fe (110) plane
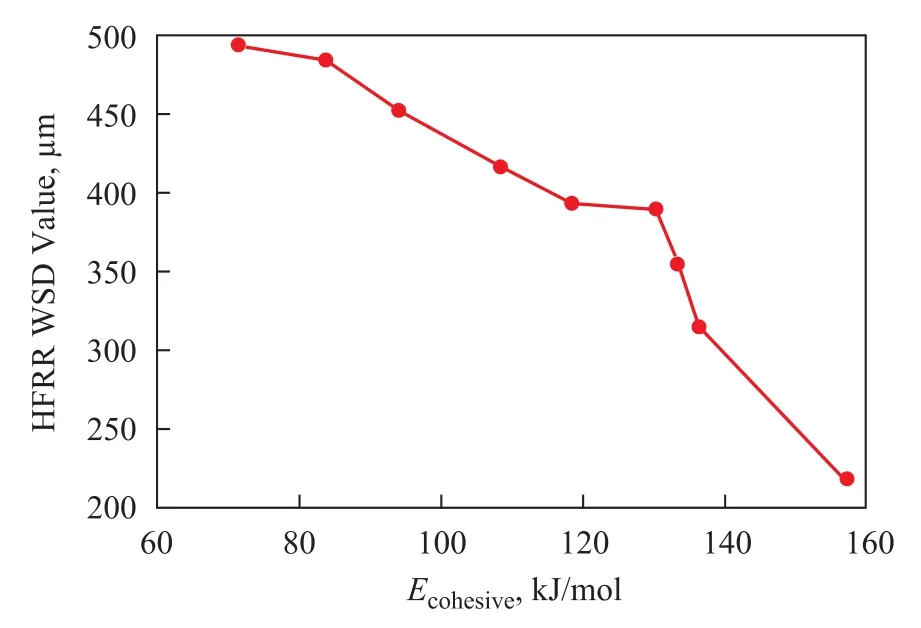
Figure 7 Relationship between the cohesive energy and HFRR lubricity evaluation of the fatty acids at a concentration of 500μg/g in diesel fuel
Figure 7 presents HFRR lubricity of the fatty acids at a concentration of 500 µg/g versus the cohesive energy of the adsorbed films, and it can be seen that the increase in cohesive energy leads to a regular increase of the lubrication effectiveness. Highest value of cohesive energy indicates that ricinoleic acid is the most efficient lubricity enhancer among the fatty acids tested thereby.
The cohesive energy of the adsorbed film consists of two parts: the van der Waals part and the electrostatic part. With the increase of carbon chain length, the van der Waals part of the cohesive energy increases, but the electrostatic part decreases slightly. The double bonds or hydroxyl group contained in carbon chain have no obvious effect on the van der Waals interaction, but can enhance the electrostatic interaction, especially with respect to hydroxyl group.
4 Conclusions
HFRR measurements of fatty acids in low-sulfur diesel fuel suggest that an increase in the carbon chain length leads to a higher lubrication efficiency. For the C18 fatty acids series, lubricity enhancement increased with an increasing unsaturation degree of fatty acids, and ricinoleic acid was better than all the nonhydroxylated fatty acids. The lubricity enhancement efficiencies were explained in terms of molecular parameters obtained from DFT calculations and the adsorption behavior described through MD simulation. The total energy of molecules could be lowered by an increase of the carbon chain length or an addition of hydroxyl group. Other electronic properties could change with the introduction of double bonds in the carbon chain of fatty acids. The cohesive energy of adsorbed films formed on the iron surface could be improved by an increase of the carbon chain length, which is mainly attributed to the increase of the van der Waals interaction. An increase in the unsaturation degree of fatty acid or an addition of hydroxyl group could also lead to higher cohesive energy, which is mainly attributed to the increase of electrostatic interaction. The lubricity enhancing properties of fatty acids are mainly determined by the cohesive energy, and an increase of cohesive energy leads to an increase in fuel lubricity.
Acknowledgment:This work was supported by the Fundamental Research Funds for the Central Universities of China (11CX06036A).
[1] Wei Danping. Thirty years of research on fuel lubricity [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2000, 16 (1): 31-39 (in Chinese)
[2] Margaroni D. Fuel lubricity [J]. Industrial Lubrication and Tribology, 1998, 50 (3): 108-118
[3] Anastopoulos G, Lois E, Serdari A, et al. Lubrication properties of low-sulfur diesel fuels in the presence of specific types of fatty acid derivatives [J]. Energy Fuels, 2001, 15 (1): 106-112
[4] Anastopoulos G, Lois E, Karonis D, et al. Impact of oxygen and nitrogen compounds on the lubrication properties of low sulfur diesel fuels [J]. Energy, 2005, 30 (2-4): 415-426
[5] Jahanmir S, Beltzer M. An adsorption model for friction in boundary lubrication [J]. ASLE Transactions, 1986, 29 (3): 423
[6] Wei D P, Spikes H A. Fuel lubricity-fundamentals and review [J]. Fuels International, 2000, 1 (1): 45-65
[7] Spikes H A, Cameron A. A comparison of adsorption and boundary lubrication failure [J]. Proceedings of the Royal Society Lond A, 1974, 336 (1607): 407-419
[8] Belter M, Jahanmir S. Role of dispersion interactions between hydrocarbon chains in boundary lubrication [J]. ASLE Transactions, 1987, 30 (1): 47-54
[9] Prathab B, Subramanian V, Aminabhavi T M. Molecular dynamics simulations to investigate polymer-polymer and polymer-metal oxide interactions [J]. Polymer, 2007, 48 (1): 409-416
[10] Khaled K F. Experimental, density function theory calculations and molecular dynamics simulations to investigate the adsorption of some thiourea derivatives on iron surface in nitric acid solutions [J]. Applied Surface Science, 2010, 256 (22): 6753-6763
[11] Wang L L, Chen W K. Density functional theory for adsorption of HCHO on the FeO (100) surface [J]. Journal of Natural Gas Chemistry, 2010, 19 (1): 21-24
[12] Argyris D, Ho T, Cole D R, at al. Molecular dynamics studies of interfacial water at the alumina surface [J]. J Phys Chem C, 2011, 115 (5): 2038-2046
[13] Zhang J Y, Liu W M, Xue Q J. The effect of molecular structure of heterocyclic compounds containing N, O and S on their tribological performance [J]. Wear, 1999, 231 (1): 65-70
[14] Tan Y Q, Huang W J, Wang X Y. Molecular orbital indexes criteria for friction modifiers in boundary lubrication [J]. Tribol Int, 2002, 35 (6): 381-384
[15] Huang W J, Tan Y Q, Chen B S, et al. The binding of antiwear additives to iron surfaces: quantum chemical calculations and tribological tests [J]. Tribol Int, 2003, 36 (3): 163-168
[16] Delley B. From molecules to solids with the DMol3approach [J]. J Chem Phys, 2000, 113 (18): 7756-7764
[17] Delley B. DMol3DFT studies: from molecules and molecular environments to surfaces and solids [J]. Compu Mat Sci, 2000, 17 (2): 122-126
[18] Becke A D. A multicenter numerical integration scheme for polyatomic molecules [J]. J Chem Phys, 1988, 88 (4):2547-2553
[19] Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density [J]. Phys Rev B, 1988, 37 (2): 785-789
[20] Pearson R G. Absolute electronegativity and hardness correlated with molecular orbital theory [J]. Proc Nati Acad Sci, 1986, 83 (22): 8440-8441
[21] Pearson R G. Recent advances in the concept of hard and soft acids and bases [J]. J Chem Educ, 1987, 64 (7): 561-567
[22] Sun H. COMPASS: Anab initioforce-field optimized for condensed-phase applications-overview with details on alkane and benzene compounds [J]. J Phys Chem B, 1998, 102 (38): 7338-7364
[23] Andersen, H C. Molecular dynamics simulations at constant pressure and/or temperature [J]. J Chem Phys, 1980, 72 (4): 2384-2393
[24] Fermeglia M, Pricl S. Multiscale modeling for polymer systems of industrial interest [J]. Progress in Organic Coatings, 2007, 58 (2-3): 187-199
Recieved date: 2013-01-21; Accepted date: 2013-03-28.
Professor Fan Weiyu, E-mail: fanwyu@ upc.edu.cn.
- 中国炼油与石油化工的其它文章
- Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
- A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil
- Influence of Carbon Content on S Zorb Sorbent Activity
- Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method

