Urinary trypsinogen-2 for diagnosing acute pancreatitis: a meta-analysis
Chengdu, China
Urinary trypsinogen-2 for diagnosing acute pancreatitis: a meta-analysis
Tao Jin, Wei Huang, Kun Jiang, Jun-Jie Xiong, Ping Xue, Muhammad A Javed, Xiao-Nan Yang and Qing Xia
Chengdu, China
BACKGROUND:Currently, serum amylase and lipase are the most popular laboratory markers for early diagnosis of acute pancreatitis with reasonable sensitivity and specificity. Urinary trypsinogen-2 (UT-2) has been increasingly used but its clinical value for the diagnosis of acute pancreatitis and post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis has not yet been systematically assessed.
DATA SOURCES:A comprehensive search was carried out using PubMed (MEDLINE), Embase, and Web of Science for clinical trials, which studied the usefulness of UT-2 as a diagnostic marker for acute pancreatitis. Sensitivity, specificity and the diagnostic odds ratios (DORs) with 95% confidence interval (CI) were calculated for each study and were compared with serum amylase and lipase. Summary receiver-operating curves were conducted and the area under the curve (AUC) was evaluated.
RESULTS:A total of 18 studies were included. The pooled sensitivity and specificity of UT-2 for the diagnosis of acute pancreatitis were 80% and 92%, respectively (AUC=0.96, DOR=65.63, 95% CI: 31.65-139.09). The diagnostic value of UT-2 was comparable to serum amylase but was weaker than serum lipase. The pooled sensitivity and specificity for the diagnosis of post-ERCP pancreatitis were 86% and 94%, respectively (AUC=0.92, DOR=77.68, 95% CI: 24.99-241.48).
CONCLUSIONS:UT-2 as a rapid test could be potentially used for the diagnosis of post-ERCP pancreatitis and to an extent, acute pancreatitis. Further studies are warranted to confirm these results.
(Hepatobiliary Pancreat Dis Int 2013;12:355-362)
urinary trypsinogen-2; acute pancreatitis; endoscopic retrograde cholangiopancreatography; diagnostic odds ratios; meta-analysis
Introduction
Acute pancreatitis (AP) is an inflammatory disease, and its clinical manifestations vary from mild self-limiting form to severe form, characterized by pancreatic necrosis, multi-organ failure and death.[1]Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis occurs in 1%-15% of patients who undergo this procedure.[2]Early diagnosis and treatment of AP/post-ERCP pancreatitis are critical in reducing morbidity and mortality of the patients. However, sometimes, it can be hard to differentiate AP from other non-pancreatic acute abdominal diseases (AADs), such as intestinal obstruction, perforated gastric or duodenal ulcer and mesenteric ischemia.[3]Unfortunately, to date there is no "gold standard biomarker" for the diagnosis of AP.
In human pancreatic juice, there are three trypsinogen (TPS) isoenzymes, namely, cationic (TPS-1) and anionic TPS (TPS-2), and a minor isoenzyme (TPS-3).[4]Physiologically, the trypsinogens are stored in inactive forms in the cytoplasmic zymogen granules of pancreatic acinar cells. These are secreted into the adjacent duct lumen and are subsequently delivered to the small intestine where they become activated by enterokinase.[5,6]In AP, premature activation of trypsinogen to trypsin occurs, leading to the activationof other zymogens and the autodigestion of the pancreas.[7,8]As a result, a large amount of TPS-2 is secreted from the injured pancreas into urine and blood within few hours after onset of the disease in patients with AP.[9,10]In 1990, Itkonen et al[9]developed an immunofluorometric technique to detect serum TPS-1 and TPS-2, and subsequently commercial urinary TPS-2 (UT-2) dipstick (Actim Pancreatitis®) based on the same technique was introduced.[11]Although the use of UT-2 dipstick was frequently reported in diagnosing AP and post-ERCP pancreatitis, its diagnostic accuracy has not been systematically assessed.
The aim of this meta-analysis is to assess the value of UT-2 in differentiating AP from extrapancreatic AADs and in predicting post-ERCP pancreatitis. The diagnostic values of UT-2 in predicting AP were also compared with those of serum amylase and lipase.
Methods
Literature search
Case controlled or cohort studies published in English from January 1990 to April 2012 that evaluated the value of UT-2 in screening AP and post-ERCP pancreatitis were searched in PubMed (MEDLINE), Embase and Web of Science. The search was based on the following combinations of key words: "acute pancreatitis", "pancreatitis" or "endoscopic retrograde cholangiopancreatography", and "trypsinogen", "trypsinogen-2" or "urinary trypsinogen-2". More publications were identified through manually searching from major gastrointestinal journals in the library. Only clinical trials with details and full-text were included. Final inclusion of articles was determined by consensus.
Inclusion and exclusion criteria
Three authors identified and screened potential eligible clinical trials. Inclusion criteria for this metaanalysis were as follows: (1) English language articles published in peer-reviewed journals; (2) AP or severe AP defined by the Atlanta criteria;[12](3) human trials testing the value of UT-2 in differentiating AP from nonpancreatic AADs or in predicting post-ERCP pancreatitis; and (4) studies in which 2×2 table could be extracted. Exclusion criteria were: (1) abstracts, letters, editorials, expert opinions, reviews and case reports; (2) studies without extrapancreatic AADs as control cases; and (3) UT-2 for predicting postoperative pancreatitis.
Data extraction and quality assessment
Data were extracted by three independent observers using standardized forms. The recorded data included population characteristics, residence, date of patient admission and urinary sampling, and etiology. Diagnostic parameters including true positivity (TP), false positivity (FP), false negativity (FN) and true negativity (TN) were extracted directly or by calculating the sensitivity and specificity of UT-2 for the diagnosis of either AP or post-ERCP pancreatitis. TP, FP, FN and TN were also extracted for serum amylase and lipase if these were reported in the included studies. The quality of the included studies was assessed independently by two reviewers using the Standards for Reporting of Diagnostic Accuracy (STARD) initiative guidelines.[13]
Statistical analysis
Meta-analysis was made with Meta-DiSc 1.4 software (Hospital Ramóny Cajal, Madrid, Spain). Pooled sensitivity, specificity, and diagnostic odds ratio (DOR) with diagnostic valueQwere calculated. The mentioned parameters were pooled respectively with a corresponding 95% confidence interval (95% CI). Receiver-operating characteristics were also generated and expressed by area under curve (AUC). The AUC represents the accuracy of diagnosis (>0.97 excellence, 0.93-0.96 very good, 0.75-0.92 good, 0.50-0.75 bad). Similarly, DOR indicates its diagnostic capability for differentiating disease group from negative group.[14]Heterogeneity was evaluated using Cochran'sQtest and aPvalue of 0.1 was considered significantly different.I2statistics was used to measure the percentage of total variation across the studies because of heterogeneity (I2of 50% or more indicating the presence of heterogeneity).[15]Meta-analysis was performed using a fixed-effect model if there was no homogeneity among the studies, otherwise the random effects were analyzed.[16]The sensitivity was analyzed by excluding each study for the analysis of the effect on the overall results. Subgroup analyses were dependent on the following items: STARD score ≥16, sample size≥50 in each study, admission at ≤72 hours after onset of symptoms, or prediction of severe AP.
Results
Study identification
Twenty-eight clinical studies were identified. Ten studies were excluded due to lack of required data (5 studies),[17-21]serum TPS-2 (2)[22,23]and postoperative AP (3).[24-26]At last, 18 studies were included for analysis with 15 studies[10,11,26-38]for AP and 3 studies[39-41]for post-ERCP pancreatitis, respectively. Of the 15studies[10,11,26-38]focused on prediction of AP, two[11,30]used patients recruited in the same period and, therefore only 14 studies[10,11,26-29,31-38]were used for the analysis of the overall diagnostic value of UT-2 in AP; Nine[26,29-34,37,38]of these studies compared the value of UT-2 to lipase while ten[26-29,31-34,37,38]also compared UT-2 with serum amylase. The search flowchart is shown in Fig. 1.
Study characteristics and quality assessment
Of the 15 included studies for assessing predicting accuracy in AP, five studies[10,11,27,28,30]were conducted by a same group in Finland. The predominant etiology of patients recruited in these studies was alcohol abuse, whereas, biliary pathology was the main causative agent in the others. Except two studies[10,27]which were retrospectively designed, all studies were prospective in nature. Studies included in predicting post-ERCP pancreatitis were all prospectively designed. All studies used immunofluorometric technique to test UT-2 and defined a cutoff value at 50 µg/L for UT-2 to predict AP/post-ERCP pancreatitis; only one study[27]used 55 µg/L as their cutoff value. Table 1 describes study characteristics in detail. Table 2 shows diagnostic parameters and STARD score for each study.

Fig. 1.Flow diagram illustrating the process of identification of relevant studies. AP: acute pancreatitis, post-ERCP: postendoscopic retrograde cholangiopancreatography.
Meta-analysis results
UT-2 for diagnosing AP among AADs
Of the 15 studies on predicting AP, 14[10,11,26-29,31-38]aimed to differentiate AP (n=852) from non-pancreatic AADs (n=1807). As shown in Fig. 2 and Table 3, the pooled sensitivity, specificity, AUC and DOR of UT-2 in diagnosing AP from other AADs were 80% (95% CI: 0.77-0.82), 92% (95% CI: 0.91-0.94), 0.96 and 65.63 (95%CI: 31.65-139.09), respectively.

Fig. 2.Forest plots of sensitivity and specificity, and summary ROC curve for urinary trypsinogen-2 test in AP. The pooled sensitivity (A), specificity (B) and summary ROC curve (C). ROC: receiveroperating characteristics curve.

Table 1.Characteristics of included studies for diagnosing AP and post-ERCP pancreatitis
UT-2 vs serum amylase or lipase for diagnosing AP
Pooled results from 10 studies[26-29,31-34,37,38]showed that UT-2 had similar sensitivity, specificity and AUC value (80% vs 78%, 92% vs 93%, 0.96 vs 0.94) as serum amylase. Ho wever, it seemed to have slightly better DOR than serum amylase (56.41 vs 44.22) (Fig. 3). Whereas, data from nine studies[26,29-34,37,38]indicated that UT-2 has weaker diagnostic values than serum lipase, the pooled sensitivity, specificity, AUC value and DOR being 77% vs 81%, 91% vs 96%, 0.95 vs 0.96 and 43.54 vs 84.13, respectively (Fig. 4). These data suggest that UT-2 is weaker than serum lipase in diagnosingAP but may have some edge over serum amylase, as is reflected by higher DOR.

Table 2.Diagnostic parameters of included studies and quality assessment
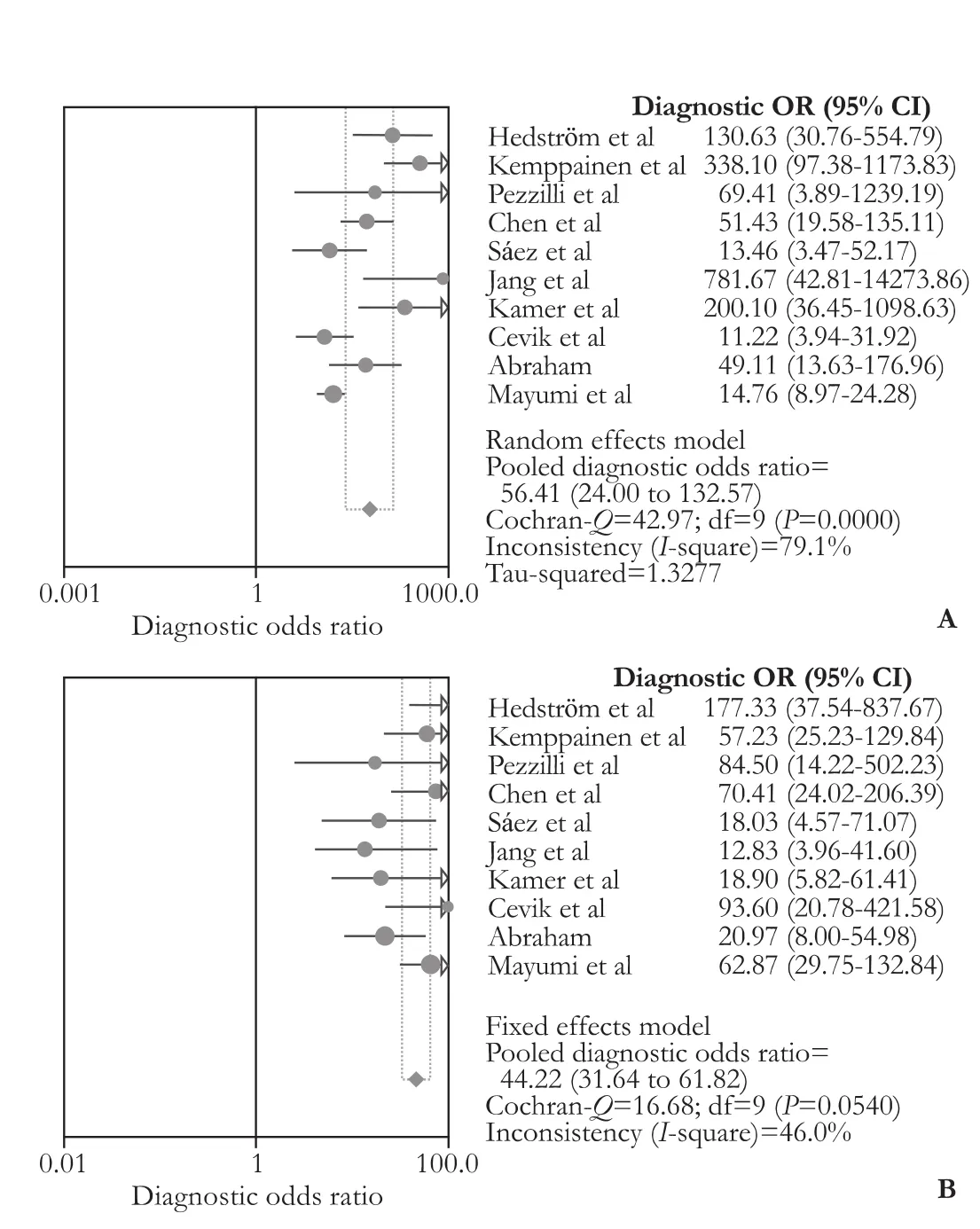
Fig. 3.Forest plots of DOR for comparison of urinary trypsinogen-2 and serum amylase. The pooled DOR of urinary trypsinogen-2 (A) and serum amylase (B). DOR: diagonstic odds ratio.
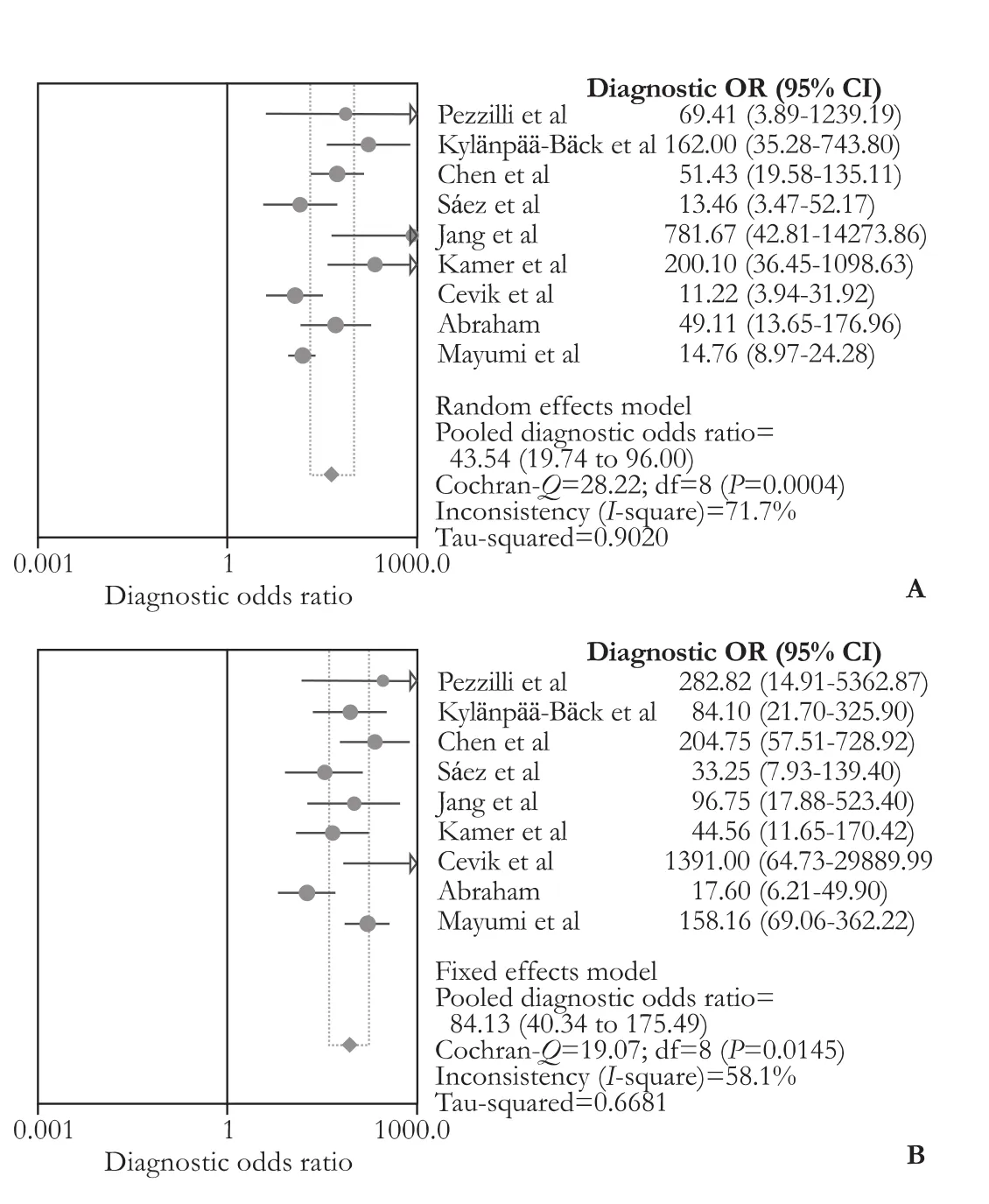
Fig. 4.Forest plots of DOR for comparison of urinary trypsinogen-2 and serum lipase. The pooled DOR of urinary trypsinogen-2 (A) and serum lipase (B). DOR: diagonstic odds ratio.
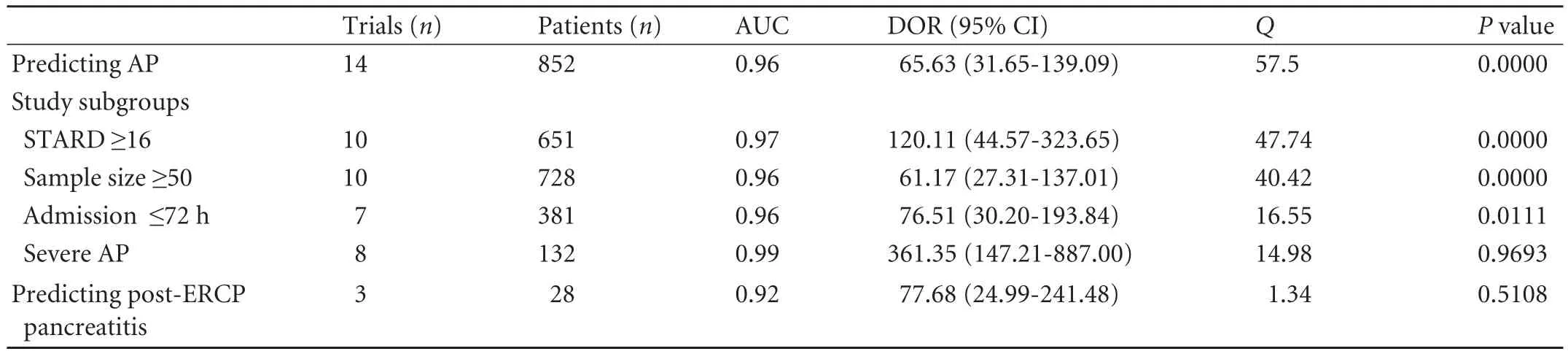
Table 3.Meta-analysis outcomes of included studies for predicting AP and post-ERCP pancreatitis
Sensitivity and subgroup analysis
Outcomes for sensitivity and subgroup analysis are shown in Table 3.
UT-2 for diagnosing post-ERCP pancreatitis
In total, 285 patients underwent ERCP. We did not find any significant inter study heterogeneity (Q=1.34,P=0.5108,I2=0.0%). The pooled sensitivity, specificity, AUC and DOR were 86% (95% CI: 0.67-0.96), 94% (95% CI: 0.91-0.97), 0.92 and 77.68 (95% CI: 24.99-241.48), respectively (Table 3).
Discussion
Currently, laboratory pancreatic enzyme tests such as serum amylase and lipase are the most commonly used biomarkers for early diagnosis of AP due to their cost-effectiveness, and reasonable sensitivity and specificity.[42]Pancreatic enzymes such as serum phospholipase A2[43]and serum pancreatic elastase[44]have been shown to confer no benefit as diagnostic tests. TPS-2 has also been assessed for its diagnostic potential in this setting. Two retrospective studies[10,27]carried out by a Finnish group showed that UT-2 had better sensitivity and specificity in predicting AP than amylase and lipase. A later larger prospective cohort study[28]by the same group enrolling 53 patients with AP and 447 patients with AAD (non-pancreatic) showed that UT-2 has a sensitivity of 94% and a specificity of 95%, better than serum amylase (85% and 91%) and urinary amylase (83% and 88%) in predicting AP. UT-2 estimation doesn't require laboratory facilities and is undertaken almost instantaneously (within 5 minutes) as opposed to serum amylase and lipase, results for which may require an hour to get back to the physician. Therefore it is a potential suitable marker to diagnose AP. However, existing literature doesn't justify generalized application of UT-2 in clinical practices. Therefore, we designed this meta-analysis to provide up-to-date evidence on the issue.
Pooled results from our meta-analysis indicated that UT-2 performs well in differentiating AP from other non-pancreatic AADs (AUC=0.96, DOR=65.63, 95% CI: 31.65-139.09). The diagnostic value of UT-2 was similar to that of serum amylase (Fig. 3) but was found to be inferior to serum lipase (Fig. 4). Since serum amylase and/or lipase were used as reference tests to establish the diagnosis of AP, their values might be overestimated,[45]which is not the case with UT-2.
The high sensitivity of UT-2 might be down to its urinary and serum concentrations which remains elevated for 4 to 30 days.[28]Mero et al[46]also reported that the total TPS immunoreactivity in serum lasts for more than 9 days. High specificity of UT-2 could potentially be useful in its application in emergency department to improve accuracy of diagnosing AP. However, positive UT-2 tests could also point towards the presence of biliary diseases,[47]gastrointestinal and ovarian cancers,[48]and other AADs. This might limit its suitability as a standalone diagnostic biomarker for AP but it could be potentially useful as an alternative of or in combination with serum amylase and/or lipase. In countries where laboratory facilities may be scanty in far flung hospitals, UT-2 might be a very useful tool for the early diagnosis of AP.
To investigate the source of heterogeneity, sensitivity and subgroup analysis were performed based on the study quality, sample size, admission time and disease severity. Cutoff value and test technique were not assessed as they were the same among all the studies (except one study used 55 µg/L for cutoff value). Data suggested that there was no significant heterogeneity among studies for predicting severe AP. These data suggested that the proportion of severe AP patients is avery important factor for homogeneity. In fact, the DOR of UT-2 to predict severe AP was more than 5 times higher than to predict AP (361.35 vs 65.63). This also explained that in high quality studies pooled DOR was also higher than the overall DOR (120.11 vs 65.6) as 9 out of 10 studies have enrolled severe AP patients, which represented the real clinical situation.[49]
Although the definition of AP remains the same for post-ERCP induced disease as well, it is more difficult to identify because of the presence of pain and hyperamylasemia is usually associated with the procedure.[50]Imaging remains the most important criterion in confirming the diagnosis of post-ERCP pancreatitis,[2]and is expensive for routine use. Only four studies[17,39-41]investigated the role of UT-2 in predicting post-ERCP pancreatitis. Pooled results from these studies suggest that UT-2 might be a good diagnostic marker for post-ERCP pancreatitis (within 3-6 hours), but this needs to be validated in larger studies.
In summary, results from our meta-analysis point towards a potential benefit of UT-2 in diagnosing post-ERCP pancreatitis in clinical practice. It has also been found to be of similar value in diagnosing AP to that of serum amylase. However, this study has several limitations: variability in study design of included studies and small sample size being the major ones. Future studies with stringently defined inclusion criteria and reasonable proportion of severe patients (about 20%) will be needed to establish the efficiency of UT-2 in the diagnosis of AP and post-ERCP pancreatitis.
Contributors:JT and HW contributed equally to this work. HW and XQ designed the research, corrected and approved the manuscript. JT, HW, JK and XJJ developed the literature search and carried out statistical analysis of the studies. JT, XP, JMA and YXN performed data extraction. JT, HW and XQ wrote the manuscript. All authors read and approved the final manuscript. XQ is the guarantor.
Funding:This study was supported by grants from the National Natural Science Foundation of China (2009SZ0201) and National Institute of Health Research UK.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007;132:1127-1151.
2 Woods KE, Willingham FF. Endoscopic retrograde cholangiopancreatography associated pancreatitis: A 15-year review. World J Gastrointest Endosc 2010;2:165-178.
3 Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101: 2379-2400.
4 Paju A, Stenman UH. Biochemistry and clinical role of trypsinogens and pancreatic secretory trypsin inhibitor. Crit Rev Clin Lab Sci 2006;43:103-142.
5 Sutton R, Petersen OH, Pandol SJ. Pancreatitis and calcium signalling: report of an international workshop. Pancreas 2008;36:e1-14.
6 Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 2008;135:632-641.
7 Mukherjee R, Criddle DN, Gukovskaya A, Pandol S, Petersen OH, Sutton R. Mitochondrial injury in pancreatitis. Cell Calcium 2008;44:14-23.
8 Criddle DN, McLaughlin E, Murphy JA, Petersen OH, Sutton R. The pancreas misled: signals to pancreatitis. Pancreatology 2007;7:436-446.
9 Itkonen O, Koivunen E, Hurme M, Alfthan H, Schröder T, Stenman UH. Time-resolved immunofluorometric assays for trypsinogen-1 and 2 in serum reveal preferential elevation of trypsinogen-2 in pancreatitis. J Lab Clin Med 1990;115:712-718.
10 Hedström J, Korvuo A, Kenkimäki P, Tikanoja S, Haapiainen R, Kivilaakso E, et al. Urinary trypsinogen-2 test strip for acute pancreatitis. Lancet 1996;347:729-730.
11 Kylänpää-Bäck M, Kemppainen E, Puolakkainen P, Hedström J, Haapiainen R, Perhoniemi V, et al. Reliable screening for acute pancreatitis with rapid urine trypsinogen-2 test strip. Br J Surg 2000;87:49-52.
12 Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586-590.
13 Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-44.
14 Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129-1135.
15 Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.
16 DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
17 Petersson U, Borgström A, Ohlsson K, Fork FT, Toth E. Enzyme leakage, trypsinogen activation, and inflammatory response in endoscopic retrograde cholangiopancreatographyinduced pancreatitis. Pancreas 2002;24:321-328.
18 Segal I, Chaloner C, Douglas J, John KD, Zaidi A, Cotter L, et al. Acute pancreatitis in Soweto, South Africa: relationship between trypsinogen load, trypsinogen activation, and fibrinolysis. Am J Gastroenterol 2002;97:883-892.
19 Huang QL, Qian ZX, Li H. A comparative study of the urinary trypsinogen-2, trypsinogen activation peptide, and the computed tomography severity index as early predictors of the severity of acute pancreatitis. Hepatogastroenterology 2010;57:1295-1299.
20 Lempinen M, Stenman UH, Finne P, Puolakkainen P, Haapiainen R, Kemppainen E. Trypsinogen-2 andtrypsinogen activation peptide (TAP) in urine of patients with acute pancreatitis. J Surg Res 2003;111:267-273.
21 Lempinen M, Stenman UH, Puolakkainen P, Hietaranta A, Haapiainen R, Kemppainen E. Sequential changes in pancreatic markers in acute pancreatitis. Scand J Gastroenterol 2003;38:666-675.
22 Kobayashi K, Sasaki T, Serikawa M, Inoue M, Itsuki H, Chayama K. Assessment of trypsinogen-2 levels as an early diagnostic for post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas 2011;40:1206-1210.
23 Sainio V, Puolakkainen P, Kemppainen E, Hedström J, Haapiainen R, Kivisaari L, et al. Serum trypsinogen-2 in the prediction of outcome in acute necrotizing pancreatitis. Scand J Gastroenterol 1996;31:818-824.
24 Räty S, Sand J, Nordback I. Detection of postoperative pancreatitis after pancreatic surgery by urine trypsinogen strip test. Br J Surg 2007;94:64-69.
25 Uemura K, Murakami Y, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, et al. Randomized clinical trial to assess the efficacy of ulinastatin for postoperative pancreatitis following pancreaticoduodenectomy. J Surg Oncol 2008;98:309-313.
26 Mayumi T, Inui K, Maetani I, Yokoe M, Sakamoto T, Yoshida M, et al. Validity of the urinary trypsinogen-2 test in the diagnosis of acute pancreatitis. Pancreas 2012;41:869-875.
27 Hedström J, Sainio V, Kemppainen E, Puolakkainen P, Haapiainen R, Kivilaakso E, et al. Urine trypsinogen-2 as marker of acute pancreatitis. Clin Chem 1996;42:685-690.
28 Kemppainen EA, Hedström JI, Puolakkainen PA, Sainio VS, Haapiainen RK, Perhoniemi V, et al. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. N Engl J Med 1997;336:1788-1793.
29 Pezzilli R, Morselli-Labate AM, d'Alessandro A, Barakat B. Time-course and clinical value of the urine trypsinogen-2 dipstick test in acute pancreatitis. Eur J Gastroenterol Hepatol 2001;13:269-274.
30 Kylänpää-Bäck ML, Kemppainen E, Puolakkainen P, Hedström J, Haapiainen R, Korvuo A, et al. Comparison of urine trypsinogen-2 test strip with serum lipase in the diagnosis of acute pancreatitis. Hepatogastroenterology 2002; 49:1130-1134.
31 Chen YT, Chen CC, Wang SS, Chang FY, Lee SD. Rapid urinary trypsinogen-2 test strip in the diagnosis of acute pancreatitis. Pancreas 2005;30:243-247.
32 Sáez J, Martínez J, Trigo C, Sánchez-Payá J, Compañy L, Laveda R, et al. Clinical value of rapid urine trypsinogen-2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis. World J Gastroenterol 2005;11:7261-7265.
33 Jang T, Uzbielo A, Sineff S, Naunheim R, Scott MG, Lewis LM. Point-of-care urine trypsinogen testing for the diagnosis of pancreatitis. Acad Emerg Med 2007;14:29-34.
34 Kamer E, Unalp HR, Derici H, Tansug T, Onal MA. Early diagnosis and prediction of severity in acute pancreatitis using the urine trypsinogen-2 dipstick test: a prospective study. World J Gastroenterol 2007;13:6208-6212.
35 Aysan E, Sevinc M, Basak E, Tardu A, Erturk T. Effectivity of qualitative urinary trypsinogen-2 measurement in the diagnosis of acute pancreatitis: a randomized, clinical study. Acta Chir Belg 2008;108:696-698.
36 Andersen AM, Novovic S, Ersbøll AK, Jorgensen LN, Hansen MB. Urinary trypsinogen-2 dipstick in acute pancreatitis. Pancreas 2010;39:26-30.
37 Cevik Y, Kavalci C, Ozer M, Daş M, Kiyak G, Ozdoğan M. The role of urine trypsinogen-2 test in the differential diagnosis of acute pancreatitis in the Emergency Department. Ulus Travma Acil Cerrahi Derg 2010;16:125-129.
38 Abraham P. Point-of-care urine trypsinogen-2 test for diagnosis of acute pancreatitis. J Assoc Physicians India 2011; 59:231-232.
39 Kemppainen E, Hedström J, Puolakkainen P, Halttunen J, Sainio V, Haapiainen R, et al. Urinary trypsinogen-2 test strip in detecting ERCP-induced pancreatitis. Endoscopy 1997;29:247-251.
40 Sankaralingam S, Wesen C, Barawi M, Galera R, Lloyd L. Use of the urinary trypsinogen-2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreat ography. Surg Endosc 2007;21:1312-1315.
41 Tseng CW, Chen CC, Lin SZ, Chang FY, Lin HC, Lee SD. Rapid urinary trypsinogen-2 test strip in the diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatog raphy. Pancreas 2011;40:1211-1214.
42 Al-Bahrani AZ, Ammori BJ. Clinical laboratory assessment of acute pancreatitis. Clin Chim Acta 2005;362:26-48.
43 Sternby B, O'Brien JF, Zinsmeister AR, DiMagno EP. What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study. Mayo Clin Proc 1996;71:1138-1144.
44 Millson CE, Charles K, Poon P, Macfie J, Mitchell CJ. A prospective study of serum pancreatic elastase-1 in the diagnosis and assessment of acute pancreatitis. Scand J Gastroenterol 1998;33:664-668.
45 Smotkin J, Tenner S. Laboratory diagnostic tests in acute pancreatitis. J Clin Gastroenterol 2002;34:459-462.
46 Mero M, Schröder T, Tenhunen R, Lempinen M. Serum phospholipase A2, immunoreactive trypsin, and trypsin inhibitors during human acute pancreatitis. Scand J Gastroenterol 1982;17:413-416.
47 Terada T, Nakanuma Y. Immunohistochemical demonstration of pancreatic alpha-amylase and trypsin in intrahepatic bile ducts and peribiliary glands. Hepatology 1991;14:1129-1135.
48 Hedström J, Haglund C, Haapiainen R, Stenman UH. Serum trypsinogen-2 and trypsin-2-alpha(1)-antitrypsin complex in malignant and benign digestive-tract diseases. Preferential elevation in patients with cholangiocarcinomas. Int J Cancer 1996;66:326-331.
49 Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008;371:143-152.
50 Badalov N, Tenner S, Baillie J. The Prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009;10:88-97.
March 29, 2012
Accepted after revision March 24, 2013
Author Affiliations: Sichuan Provincial Pancreatitis Center, Department of Integrated Traditional Chinese and Western Medicine (Jin T, Jiang K, Xue P, Yang XN and Xia Q) and Department of Hepato-Biliary-Pancreatic Surgery (Xiong JJ), West China Hospital, Sichuan University, Chengdu 610041, China; NIHR Liverpool Pancreas Biomedical Research Unit, Royal Liverpool University Hospital, University of Liverpool, Liverpool L69 3BX, UK (Huang W and Javed MA)
Qing Xia, MD, PhD, Sichuan Provincial Pancreatitis Center, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital, Sichuan University, Chengdu 610041, China (Tel: 86-28-85423373; Fax: 86-28-85423373; Email: xiaqing@medmail.com.cn)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60056-9
 Hepatobiliary & Pancreatic Diseases International2013年4期
Hepatobiliary & Pancreatic Diseases International2013年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Effect of L-cysteine on remote organ injury in rats with severe acute pancreatitis induced by bile-pancreatic duct obstruction
- Impact of periampullary diverticula on the outcome and fluoroscopy time in endoscopic retrograde cholangiopancreatography
- The diagnostic value of high-frequency ultrasonography in biliary atresia
- Endobiliary radiofrequency ablation for malignant biliary obstruction
- Hepatic abscess associated with Salmonella serotype B in a chronic alcoholic patient
- Biliary-colonic fistula caused by cholecystectomy bile duct injury
