Hepatic abscess associated with Salmonella serotype B in a chronic alcoholic patient
Youngstown, USA
Hepatic abscess associated with Salmonella serotype B in a chronic alcoholic patient
Sidhartha S Tulachan, Charles E Wilkins, Anthony F Cutrona, David Gemmel and Thomas P Marnejon
Youngstown, USA
BACKGROUND:Hepatic abscess secondary toSalmonellais extremely rare in the United States and other Western countries.
METHODS:A 43-year-old Caucasian man, with a history of chronic alcohol abuse, was admitted to the hospital for intermittent abdominal pain, fever and diarrhea. Clinical, radiological and laboratory results were analyzed. Medical literature in PubMed pertaining to similar cases was reviewed.
RESULTS:Stool culture was positive forSalmonellaserotype B and a CT scan of the abdomen with contrast was consistent with a solitary hepatic abscess. Appropriate intravenous antibiotics followed by oral maintenance therapy for six weeks resulted in a complete clinical recovery and radiographic resolution.
CONCLUSIONS:The cause ofSalmonellahepatic abscess in our patient was most likely associated with decreased mucosal resistance to the bacteria, seeding of infection via transient portal bacteremia and loss of host immunity. Our case highlights the fact that appropriate antibiotic alone is sufficient in the management of a solitary hepatic abscess less than 3-5 cm.
(Hepatobiliary Pancreat Dis Int 2013;12:440-442)
hepatic abscess;Salmonella; alcoholic; hypochlorhydria; antibiotics
Introduction
Salmonellais ubiquitous and is a worldwide public health problem. The majority of persons who ingestSalmonellanever develop an illness.Salmonellainfection manifests as various clinical syndromes. The disease severity varies from mild selflimiting gastroenteritis to severe septicemia and focal abscess formation, the latter being a major cause of morbidity and mortality.[1]In this report, we discuss a case of a solitary right lobe hepatic abscess secondary toSalmonellaserotype B infection, managed by antibiotics alone.
Case report
A 43-year-old Caucasian man with a history of alcohol abuse, presented with sudden onset of severe abdominal pain and diarrhea. The abdominal pain was described as diffuse, crampy and intermittent in nature. The diarrhea occurred 8-10 times a day with watery, nonfoul smelling stool. No mucus or blood was noted. Other associated symptoms included hand tremor, nausea, low-grade fever and chills. There was no history of sick contacts or recent travel. Other significant past medical history included gastric ulcer disease treated with chronic proton pump inhibitor therapy.
On examination the patient was anxious, agitated, diaphoretic and mildly dehydrated. Temperature was 37.6 ℃, pulse 87 per minute, respiration 26 per minute, and blood pressure 158/96 mmHg. There was no pallor or icterus. Considerable guarding was present on abdominal examination with tenderness in the epigastric and right upper quadrant regions, without rebound tenderness. The liver was palpable 4 cm below the costal margin. The remainder of the examination was unremarkable.
Initial laboratory studies showed normal renal andhepatic function. The hematological parameters revealed a white cell count of 10.4×109/L with elevated absolute neutrophil count. Urine drug screening was positive for cocaine, benzodiazepines and opiates. Serum ethanol level was less than 10 mg/dL. The initial clinical impression was acute alcohol or opioid withdrawal and alcoholic gastritis. The patient was admitted and treated with intravenous fluids, thiamine, folic acid, clonidine and lorazepam. Stool work-up for ova and parasite,Clostridium difficle(C. diff) toxin and enteric pathogen, blood and urine cultures were performed. The stool was negative forC. diff, ova and parasites. Blood and urine cultures had no growth. An acute hepatitis panel and HIV-1/HIV-2 serology was also non reactive. Additional blood work revealed an elevated sedimentation rate of 47 mm/h and C-reactive protein of 21.2 mg/dL. CT scan of the abdomen with intravenous and oral contrast showed a mildly enlarged liver, a diffuse pattern of colitis involving the ascending and proximal transverse colon, and a 1.5×2.0 cm subtle low density lesion in the right lobe of the liver at the level of the gallbladder fossa consistent with a solitary hepatic abscess (Fig. A-C). Empiric intravenous cefepime and metronidazole were initiated.
Over the next two days, the clinical condition of the patient deteriorated. He remained febrile with a temperature of 39.1 ℃, persistent hepatic tenderness and diarrhea. Stool culture grewSalmonellaserotype B. The antibiotics were changed to intravenous ciprofloxacin. The condition of the patient was improved, and he was discharged on the tenth day of admission, after a good clinical recovery. Oral ciprofloxacin was continued for a total of six weeks and the patient was scheduled to follow up in the outpatient clinic. A repeat abdominal CT after completion of antibiotic therapy showed a complete resolution of the previously described solitary hepatic abscess (Fig. D-F).
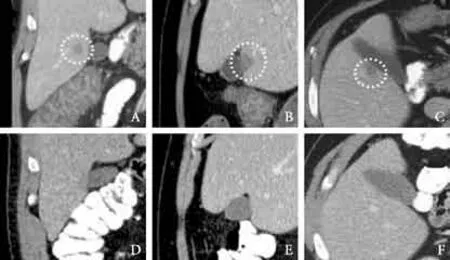
Fig.CT scan showing a low-density lesion (encircled) in the right hepatic lobe next to the gallbladder fossa.A-Cis before andD-Fis after 6 weeks of antibiotic treatment.
Discussion
The three major forms of hepatic abscess, classified by etiology, are pyogenic, amebic, and fungal. The most common pathogens of the pyogenic hepatic abscesses areEscherichia coli,Klebsiella pneumoniae,Bacteroides,Enterococci,Streptococci, andStaphylococci. The overall incidence of pyogenic hepatic abscess in the United States is 3.6 per 100 000 according to a populationbased study.[2]The most common infections areStreptococcusspecies (29.5%),Escherichia coli(18.1%) and polymicrobial (16.3%). However, the incidence of pyogenic hepatic abscess secondary toSalmonellais not known. A literature review utilizing a PubMed search yielded only 18 case reports in Western countries since 1952.[1,3-6]
Although the blood culture was negative in our patient, the diagnosis ofSalmonellahepatic abscess was based on clinical symptoms, radiographic findings, positive stool culture and effective response to appropriate antimicrobial therapy. Previous studies have shown that concomitant bacteremia is found in only 33%-50% of patients with pyogenic hepatic abscess.[7,8]Moreover,Salmonellaebacteria are less frequently isolated from blood cultures or from focal extraintestinal sites.[5,9,10]It is generally accepted that hepatic abscess is caused bySalmonellawhen CT image is compatible with hepatic abscess andSalmonellais isolated in stool culture. The most common site of hepatic abscess is the right lobe, and underlying pathology is frequently found in the region of portal drainage. The likely source of the hepatic abscess in our patient could therefore possibly be secondary to the seeding of infection from the large bowel via transient portal bacteremia.[11]
Salmonellainfection can involve any part of the gastrointestinal tract, but extraintestinal disease usually involves the hepatobiliary system and spleen. More than 2000Salmonellaserotypes have been described, but only a few serotypes are invasive and can cause extraintestinal diseases in humans.[12]The virulence factor ofSalmonellais based on genes that encode the polysaccharide portion of lipopolysaccharide (LPS) and flagellin. Based on the Kauffmann-White classification, serotypes A, B and D share a common trisaccharide backbone in LPS and are considered more virulent than others.[12,13]Similarly, a number of comorbid conditions are associated with a significantly higher risk of developing pyogenic hepatic abscess such as malignancy, sickle cell trait, liver cirrhosis, impaired bowel motility, and immunocompromized status namely HIV infection and diabetes mellitus.[5,14,15]In our patient, the likely risk factors included hypochlorhydria due to chronicuse of proton pump inhibitors, which can decrease the mucosal barrier against this pathogen[16,17]and chronic alcohol abuse, which can impair host immunity.[18]
Different strategies have been proposed for the treatment of pyogenic hepatic abscesses depending on their initial size and location. Some authors[19]suggest that abscesses less than 3 cm may be successfully treated with antibiotics alone, whereas others[20]recommend a cutoff of less than 5 cm. Larger uniloculated abscessess should be treated with intravenous antibiotics and percutaneous drainage in the form of ultrasound guided needle aspiration or percutaneous catheter drainage. Surgical or laparoscopic drainage is recommended for large multi-loculated collections.[19,20]All reportedSalmonellahepatic abscess cases were treated with antibiotics and either surgical or percutenous catheter drainage. Our case is the first reported case ofSalmonellahepatic abscess, which was treated to complete resolution by antibiotics alone.
In summary, we described a case of a solitary hepatic abscess associated withSalmonellaserotype B and illustrated that appropriate antibiotic therapy alone is effective for complete resolution of an abscess less than 3-5 cm.
Contributors:TSS wrote the first draft of this report. All authors contributed to the intellectual context and approved the final version. TSS is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Rovito V, Bonanno CA.Salmonellahepatic abscess: an unusual complication of theSalmonellacarrier state? Am J Gastroenterol 1982;77:338-339.
2 Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol 2010;105:117-124.
3 Poon MC, Sanders MG. Hepatic abscess caused bySalmonellaparatyphi B. Can Med Assoc J 1972;107:529-531.
4 Sheikh I, Sievers C, Mullen K.Salmonellaenteritidis liver abscess. Ann Hepatol 2011;10:370-371.
5 Vidal JE, da Silva PR, Schiavon Nogueira R, Bonasser Filho F, Hernandez AV. Liver abscess due toSalmonellaenteritidis in a returned traveler with HIV infection: case report and review of the literature. Rev Inst Med Trop Sao Paulo 2003; 45:115-117.
6 Hirschowitz BI. Pyogenic liver abscess: a review with a case report of a solitary abscess caused bySalmonellaenteritidis. Gastroenterology 1952;21:291-299.
7 Dan LL. Liver abscess. In: Dan LL, ed. Harrison's principle of internal medicine. 18th ed. New York: McGraw-Hill Companies, Inc; 2012:1081-1082.
8 Bosanko NC, Chauhan A, Brookes M, Moss M, Wilson PG. Presentations of pyogenic liver abscess in one UK centre over a 15-year period. J R Coll Physicians Edinb 2011;41:13-17.
9 Ramos JM, García-Corbeira P, Aguado JM, Alés JM, Soriano F. Classifying extraintestinal non-typhoidSalmonellainfections. QJM 1996;89:123-126.
10 Clarke R, Tomlinson P.SalmonellaBrandenburg: changing patterns of disease in Southland Province, New Zealand. N Z Med J 2004;117:U1144.
11 Struthers JK. Infections of blood: Bacteraemia. In: Struthers JK, ed. Clinical bacteriology, 1st ed. London: Manson Publishing Ltd; 2003:66-67.
12 Fierer J.Salmonellaoutcomes. J Infect Dis 2008;198:1724.
13 Fierer J, Guiney DG. Diverse virulence traits underlying different clinical outcomes ofSalmonellainfection. J Clin Invest 2001;107:775-780.
14 Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004;2:1032-1038.
15 Sarma PS, Narula J. MultipleSalmonellatyphi subcutaneous abscesses in a sickle cell anaemia patient. J Assoc Physicians India 1996;44:563-564.
16 Holt P. SevereSalmonellainfection in patients with reduced gastric acidity. Practitioner 1985;229:1027-1030.
17 Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009;122:896-903.
18 Chen SC, Lee YT, Lai KC, Cheng KS, Jeng LB, Wu WY, et al. Risk factors for developing metastatic infection from pyogenic liver abscesses. Swiss Med Wkly 2006;136:119-126.
19 Baek SY, Lee MG, Cho KS, Lee SC, Sung KB, Auh YH. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. AJR Am J Roentgenol 1993;160: 799-802.
20 Khan R, Hamid S, Abid S, Jafri W, Abbas Z, Islam M, et al. Predictive factors for early aspiration in liver abscess. World J Gastroenterol 2008;14:2089-2093.
April 11, 2012
Accepted after revision September 5, 2012
Author Affiliations: Department of Internal Medicine (Tulachan SS, Wilkins CE and Marnejon TP), Department of Infectious Disease (Cutrona AF), and Department of Research (Gemmel D), St Elizabeth Health Center, 1044 Belmont Avenue, Youngstown, OH 44501, USA
Sidhartha S Tulachan, MD, PhD, Department of Internal Medicine, St Elizabeth Health Center, 1044 Belmont Avenue, Youngstown, OH 44501, USA (Tel: 1-330-480-7643; Fax: 1-330-480-3777; Email: sstulachan@gmail.com)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60069-7
 Hepatobiliary & Pancreatic Diseases International2013年4期
Hepatobiliary & Pancreatic Diseases International2013年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Biliary-colonic fistula caused by cholecystectomy bile duct injury
- A new veno-venous bypass type for ex-vivo liver resection in dogs
- Effect of L-cysteine on remote organ injury in rats with severe acute pancreatitis induced by bile-pancreatic duct obstruction
- Endobiliary radiofrequency ablation for malignant biliary obstruction
- The diagnostic value of high-frequency ultrasonography in biliary atresia
- Impact of periampullary diverticula on the outcome and fluoroscopy time in endoscopic retrograde cholangiopancreatography
