Antiulcer activity of aqueous extract of leaves of Madhuca indica J. F. Gmel against naproxen induced gastric mucosal injury in rats
Smeeta M Mohod, Subhash L Bodhankar
Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Paud Road, Erandwane, Pune 411038, Maharashtra, India
Antiulcer activity of aqueous extract of leaves of Madhuca indica J. F. Gmel against naproxen induced gastric mucosal injury in rats
Smeeta M Mohod, Subhash L Bodhankar*
Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Paud Road, Erandwane, Pune 411038, Maharashtra, India
GSH
Histamine
Madhuca indica
MDA
MPO
Naproxen induced ulcer
NO
SOD
Objective: To evaluate antiulcer potential of aqueous extract of Madhuca indica (M. indica) J. F. Gmel leaves in rats. Methods: Aqueous extract of M. indica J.F. Gmel leaves was tested at the dose of 100, 200 and 400 mg/kg, p.o. against naproxen (30 mg/kg, p.o) induced gastric ulcer. Omeprazole (30 mg kg, p.o.) was used as a positive standard. Ulcerated area was measured by Image J software. Various antioxidant parameter like SOD, GSH, MDA, MPO, NO and histamine were also determined. Results: After 4 week treatment period, desired aim was achieved using aqueous extract of plant of M. indica at the dose of 200 and 400 mg/kg, p.o. (P<0.01, P<0.001) showed significant reduction in ulcerated area and ulcer index as compared to control group. Omeprazole (30 mg/kg, p.o.) was more effective in reducing ulcerated area after 30 days treatment period. In addition, SOD, GSH, NO significantly increased; MDA, MPO content significantly lowered when compared with control group. Histamine content didn’t show any significant change at all the three doses. Conclusions: Our finding suggests that aqueous extract of M. indica J.F. Gmel leaves is effective in gastric ulcer protection.
1. Introduction
Peptic ulcer is a conglomerate of heterogeneous disorders, which manifests itself as a break in the lining of the gastrointestinal mucosa bathed by acid and/or pepsin[1,2]. NSAIDs represent one of the most widely used classes of drugs to alleviate the symptoms (e.g. pain and swelling) of osteoarthritis, rheumatoid arthritis and other inflammatory disorders[3]; however, the use of NSAIDs is limited by their ability to induce the formation of erosions and ulcers in the gastrointestinal (GI) tract[4,5].
Approximately 50% of individuals who use NSAIDs develop gastric erosions, while an estimated 2% to 4% of these individuals develop clinically significant GI ulcers and bleeding, sometimes leading to death[6,7].
Various classes synthetic antiulcer drugs like antacids,proton pump inhibitors, anticholinergics, H2-receptor antagonists and cytoprotective agents are being used in clinical practices, but these entire drugs have been associated with undesirable side effects and drug-drug interaction. Therefore, search for an ideal antiulcer drug continues and has also been extended to herbal drugs for their easy availability, better protection, low cost and lesser toxicity[8,9].
Reactive oxygen species which include superoxide anion and hydroxyl radicals have been implicated in several degenerative diseases including digestive system disorders such as hypersecretion and gastric mucosal damage[10].
Antioxidants are considered as possible protective agent reducing oxidative damage to the human body[11-14]. In recent years, there has been growing interest in use of natural antioxidant, especially those derived from edible material such as fruits, spices, herbs and vegetables[15,16]. Therefore, the development and use of more effective antioxidant of natural origin is desired[17].
Madhuca indica J. F. Gmel (M. indica) is a plant belonging to the family Sapotaceae. It is a large, shady deciduous tree both wild and cultivated, found in different parts ofBangladesh. It is also distributed more or less throughout India especially in the states of Jharkhand, Uttar Pradesh, Bihar, Maharashtra, Madhya Pradesh, Kerala, Gujarat and Orissa[18,19].
The plant of M. indica is mentioned in literature as an effective remedy for peptic ulcer. It has been traditionally used for treatment of ulcer, rheumatism, itches, bleeding, spongy gum, tonsillitis and diabetes mellitus[20]. The leaves contained myricetin, quercetin, myricitrin, triterpenoid and quercitrin[21]. β-carotene and xanthophylls, erythrodiol, n-hexacosanol, n-octacosanol; palmitic acid, myricetin and its myricetin and 3-O-arabinoside and 3-O-L-rhamnoside, quercetin and its 3-galactoside; 3β-caproxy and 3βpalmitoxy-olean-12-en-28-ol, oleanolic acid, β-sitosterol and its 3-O-β-D-glucoside and stigmasterol were also isolated from leaves[22]. A new isoflavone, 3,4-dihdroxy-5,2-dimethoxy-6,7-methylendioxy, has also been reported from the plant[23].
No documented reports are available so far on the evaluation of the leaves of this plant for possible antioxidant and gastro protective activities. Therefore, to justify the traditional claim, the objective of the study to assess the gastro protective and antioxidant effect of aqueous extract of leaves of M. indica in laboratory animals.
2. Matearials and methods
2.1. Plant material
M. indica (Sapotaceae) leaves were collected from areas adjoining the district of Amravati, Maharashtra, India and was authenticated at Agarkar Research Institute, Pune, India and the voucher specimen was deposited at Institute (Voucher specimen sample no-L-054).
2.2. Preparation of extract
Weighed quantity (500 g) of air dried powder (Mesh size-16) of the leaves of M. indica (J. F. Gmel) was macerated with distilled water (MI-AQE) at room temperature for 7 days and filtered. The filtrate was dried on a tray dryer maintained at 60 °C. Semisolid aqueous extract was dissolved in distilled water in order to prepare the appropriate concentration of stock suspension.
2.3. Animals
Healthy male and female wistar rats (150-200 g) and male swiss albino mice (18-22 g) were obtained from National Toxicology Centre, Pune, India and housed in animal house in groups of six animals in polypropylene cages. The animals were maintained at (25±2) °C, relative humidity of 45% to 55% and under standard environmental conditions (12 h light 12 h dark cycle). All the animals were acclimatized for 10 days to the animal house conditions prior to the start of experimental protocol. The animals had free access to food (Amrut laboratory animal feed, Sangali, MS, India) and water ad libitum. The research protocol was approved by Institutional Animal Ethical Committee (IAEC) constituted as per the directions of the CPCSEA. All experiments were carried out between 12:00-16:00 hours.
2.4. Acute toxicity test
Acute toxicity study was performed in healthy adult male albino mice (18-22 g) as per guideline no AOT 425 of the Organization for Economical Co-operation and Development (OECD). Aqueous extract of leaves of M. indica was administered at various doses in mice were observed continuously for 2 h for behavioral and autonomic profiles and for any other sign of toxicity or mortality up to a period of 7 days.
2.5. Anti-ulcer activity Naproxen-induced ulcers[24-25]
Rats were divided into three sets as A, B and C with six groups in each set. Further, each group consisted of six rats. The animals were fasted for 24 h for naproxen induced ulcer. Group 1: Normal control; Group 2: Vehicle distilled water + Naproxen (30 mg/kg, p.o.); Group 3: Omeprazole (standard drug)–30 mg/kg, p.o.; Group 4: MI-AQE 100 mg/kg, p.o.; Group 5: MI-AQE 200 mg/kg, p.o.; Group 6: MI-AQE 400 mg/ kg, p.o.
Rats in sets A, B and C were treated with standard drug (group III) and MI-AQE (group IV to VI) for 10, 20 and 30 days respectively. Groups of II to VI of each set of rats was administered naproxen at the dose of 30 mg/kg, p.o. for 3 consecutive days staring from 7th day for 10 days treatment period in set A, 17th day for 20 days treatment period in set B and on 27th day for 30 days treatment period in set C. All the animals were fasted for 24 h before administration of first dose of naproxen. The animals had free access to feed following the first dose of naproxen. Animals of set A, B and C were scarified on completion of 10th day, 20th day and 30th day respectively. The stomach of each rat was removed, inspected internally and ulcerated area was calculated by image processing software Image J (National Institute of Health, U.S.A.). Antioxidant parameters such as MDA, GSH, SOD, MPO, NO and histamine were determined by standard reported method.
2.6. Biochemical estimation
Five hundred milligrams of tissue from the glandular portion of stomach was excised, washed chopped and homogenized at 3 000 r/min in chilled Tris buffer (10 mmol/L, pH 7.4) at a concentration of 10% (w/v). The homogenate was centrifuged at 10 000 g at 0 °C for 20 min, to obtain supernatant volume of 5.5 mL. It was divided into aliquot to determinesuperoxide dismutase (SOD) (0.2 mL) was estimated using the method developed by Mishra and Fridovich[26]. Reduced glutathione (GSH) (0.2 mL) was determined by the method of Moron et al[27]. Lipid peroxidation (Malondialdehyde (MDA) content) (2.0 mL) formation was estimated by the method of Slater and Sawyer[28]. Myloperoxidase (MPO content) (2.0 mL) assay was determined according to method described by Krawize et al[29]. Total protein estimation (0.1 mL) was determined the method of Lowry et al[30]. Gastric nitric oxide (NO) content (0.5 mL) was estimated as a nitrite according to method described by Miranda et al[31]. Histamine (0.5 mL) content was determined by Patange et al[32].
2.7. Histopathology
Gastric tissue samples were fixed in 10% formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin for histological examination using light microscopy.
2.8. Statistical analysis
The results are expressed as mean±SEM. The statistical analysis was done by using GraphPad prism 5.0. The statistical analysis of all the results was carried out using two way ANOVA followed by Bonferroni’s test and one way ANOVA followed by Dunnett’s test. P<0.05 was considered as significance.
3. Results
3.1. Acute toxicity test
In oral toxicity study administration of the extract of the graded doses 175, 550, 1 750 and 2 000 did not cause death of mice. MI-AQE was found to be safe up to a dose of 2 000 mg/kg, p. o.
3.2. Dose selection
Based upon toxicity studies and pilot studies (data not shown) three different doses of MI-AQE i.e. 100, 200 and 400 mg/kg were selected for antiulcer investigation.
3.3. Naproxen-induced ulcers
Oral administration of naproxen (30 mg/kg, p. o.) produced small erosion throughout the glandular portion of rat stomach (Figure 1). However, pretreatment with MI-AQE reduced severity of naproxen-induced gastric ulcer. After 10 and 20 days of pretreatment period, MI-AQE at the dose of 400 mg/kg, p. o. showed significant (P<0.01) reduction in ulcerated area (7.06±0.38) mm2and (5.41±0.48 mm)2, respectively and ulcer index (1.00±0.05) and (0.78±0.07), respectively as compared with naproxen control group (10.96±1.54) mm2and (9.48±1.53) mm2. At the dose of 200 mg/kg of MI-AQE produced significant (P<0.05) reduction in ulcerated area (6.68±0.23) mm2and ulcer index (0.94±0.02) after 20 days pretreatment period when compared with naproxen control group. On the other hand, after 30 days of pretreatment period, MIAQE produced significant (P<0.01), (P<0.001) reduction in ulcerated area (3.92±0.83) mm2and (2.89±0.56) mm2respectively and ulcer index (0.56±0.11) and (0.40±0.07) respectively at the doses of 200 and 400 mg/kg, p. o, although the level of significance was different when compared against naproxen control groups (8.73±1.79) mm2. At the dose of 100 mg/kg did not show any significance result from 10, 20 and 30 days treatment period. The reference standard omeprazole (30 mg/kg, p.o.) after 10, 20 and 30 days pretreatment was found to be more significantly (P<0.001) reduction in ulcerated area (0.83±0.05) mm2, (0.54±0.05) mm2and (0.15±0.03) mm2when compared against naproxen control group (Table 1).
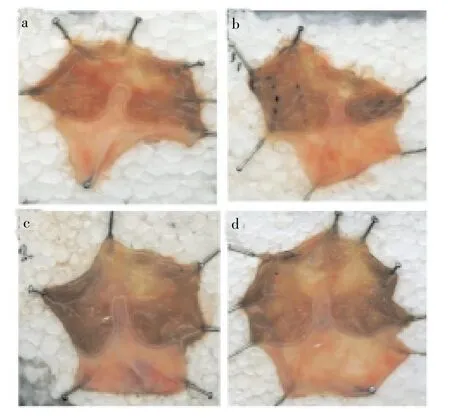
Figure 1. Representative stomachs images of rats after naproxen induced ulcer.
3.4. Effect of aqueous extract of M. indica Leaves on biochemical parameters
The gastro protective effect of 100, 200 and 400 mg/kg doses of MI-AQE on the naproxen-induced gastric ulcer in various gastric parameters are shown in Table 2. There were remarkable changes in the gastric parameters of MI-AQE treated group as compared with vehicle treated animals. The stomach SOD in naproxen treated rats decreased significantly (5.08±0.34) U/mg of protein compared with the normal group (8.74±0.28) U/mg of protein, while administration of theMI-AQE at the dose of 200 and 400 mg/kg in rat resulted in a significantly (6.81±0.37) U/mg of protein, P<0.05 and (7.44±0.50) U/mg of protein, P<0.01 increased SOD enzymes in stomach when compared with naproxen control group. Significantly reduction (0.81±0.10)µg/mg protein of stomach GSH was observed in naproxen treated rats when compared with (3.88±0.24) µg/mg protein normal group, whereas, pretreatment with MI-AQE at the dose of 200 and 400 mg/kg, significantly (2.23±0.17), (2.99±0.24) µg/mg protein, P<0.001 protect stomach from reduction.
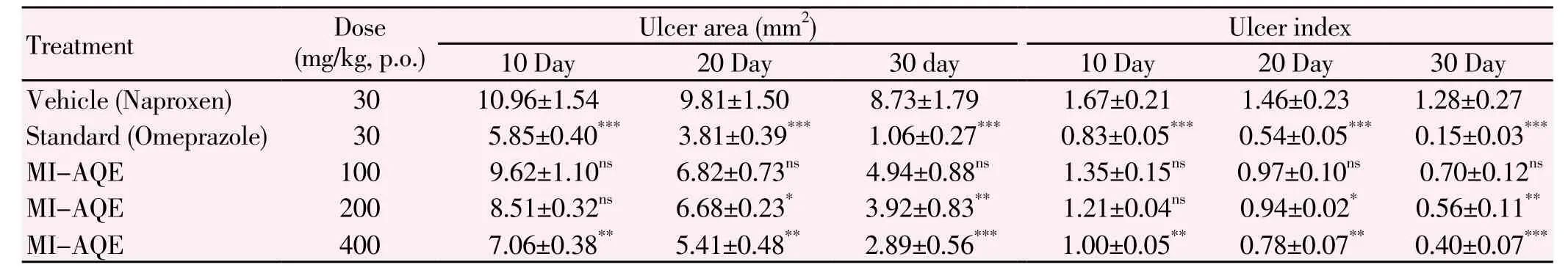
Table 1Effect of MI-AQE on naproxen induced ulcer area and ulcer index in rat.
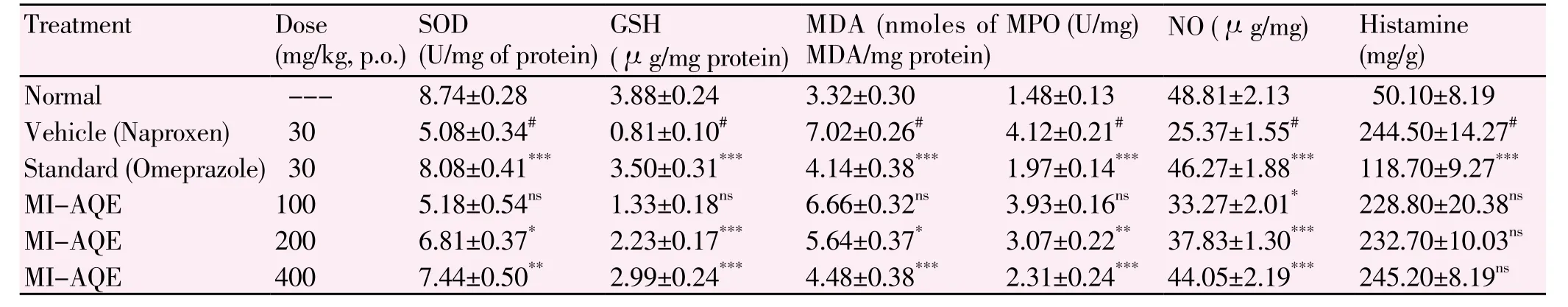
Table 2Effect of MI-AQE on various biochemical parameters in stomach of naproxen induced ulcer in rat.
In naproxen control rat the nitrite level was significantly lowered (25.37±1.55) µg/mg as compared to normal group rats (48.81±2.13) µg/mg. However significantly inhibited this reduction in nitrite level was found at the dose of 100, 200 and 400 mg/kg (33.27±2.01), (37.83±1.30) and (44.05±2.19) µg/mg, P<0.001 as compared to naproxen control group. Significantly increased in MDA concentration in naproxen control group (7.02±0.26) nmoles of MDA/mg protein as compared with normal group (3.32±0.30) nmoles of MDA/mg protein whereas this increase in concentration of MDA was reduced in dose dependent manner in MI-AQE pretreated rat with 200 and 400 mg/kg (5.64±0.37) nmoles of MDA/mg protein, P<0.001), (4.48±0.38) nmoles of MDA/mg protein, P<0.05.
The concentration of MPO in the naproxen control rat was significantly higher (4.12±0.21) U/mg as compared to normal rats (1.48±0.13) U/mg whereas, this increased in the concentration of MPO was reduced at the dose 200 and 400 mg/kg of MI-AQE was found significantly reduced (3.07±0.22), P<0.01 and 2.31±0.24 U/mg, P<0.001 MPO content. Oral administration of MI-AQE at all the three doses did not bring about any significant change in histamine content.
3.5. Result of histopathology
From histopathology it was found that, the rats treated with naproxen showed loss of gland architecture with erosion, loss of the epithelial layer, evident edema and inflammation cell. The total aqueous extract showed no ulcers in gastric mucosa, glands were regular and no inflammation was observed (Figure 2).
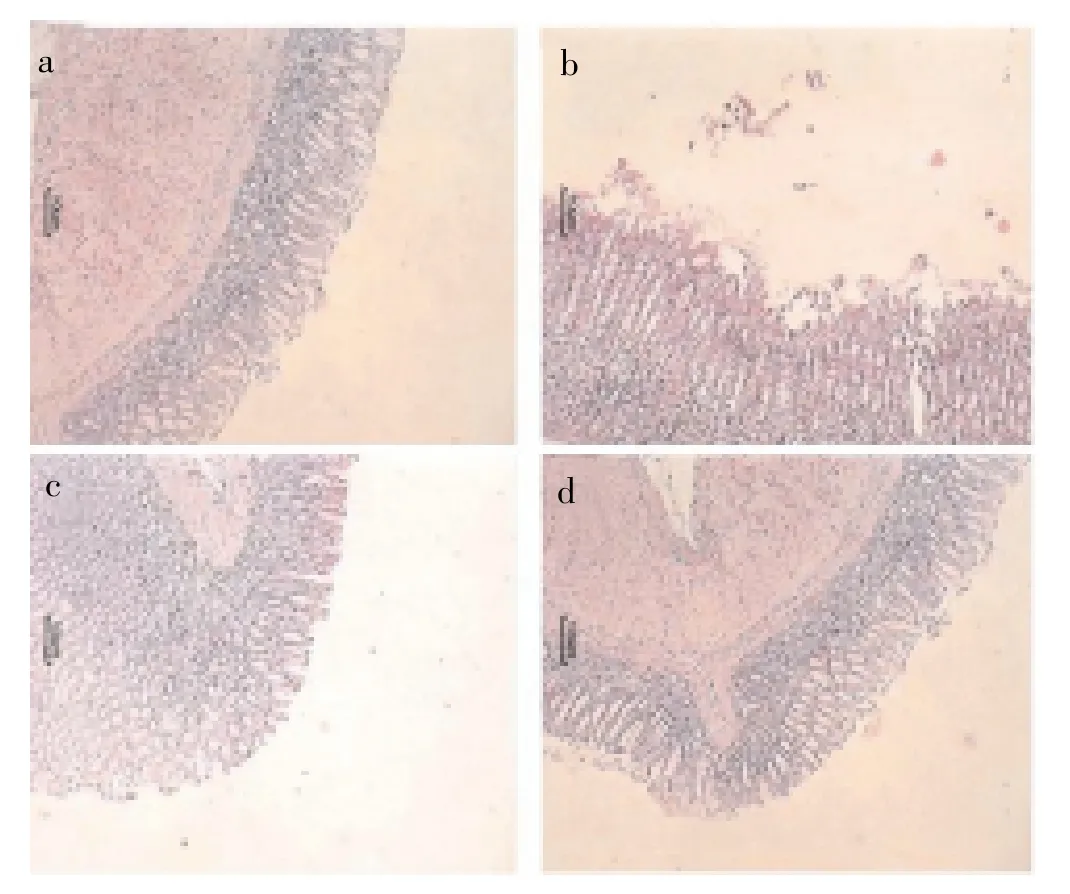
Figure 2. Photomicrographs of sections of stomach after naproxen induced ulcer stained with H&E.
4. Discussion
The present investigation revealed significant antiulcer effect of aqueous extract of M. indica leaves (MI-AQE) in experimental models of gastric ulcers induced by naproxen. Chronic administration of NSAIDs leads to gastric damage occurs mainly in the corpus region of the stomach and tends to be mostly in the form of erosion rather than ulcer. This is unlike situation in humans, where NSAID-induced gastric ulceration occurs mainly in gastric antrum[33]. Naproxen, aspirin and indomethacin are the most commonly used NSAIDs leading to hemorrhages and perforation. Naproxen is a non-corticosteroid drug with anti-inflammatory, antipyretic and pain-relieving properties, which is known to produce erosion, antral ulceration, and petechial bleeding in the mucosa in the stomach as an adverse effect[34,35]. Its ulcerogenic potential was exhibited due to non selective inhibition of cycloxygenase I and II (COX I and II) leading to reduced PGE2synthesis decreased mucus secretion. Inhibition of prostaglandin synthesis can exert injurious actions on gastric and duodenal mucosa as it abrogates a number of prostaglandin dependent defense mechanism[36]. In addition, non steroidal anti-inflammatory drugs NSAIDs have now become a regular prescriptive drug in the family, which is a powerful cause of gastric ulcer. Hence, a widespread search has been necessary to identify new anti-ulcer therapies from natural resources. Several clinical researches have confirmed the gastro protection activities conferred by plants on patients. In the present study, we used a simple, reproducible and relevant naproxen-induced gastric antral model, which is suitable for the human situation[37].
Naproxen was administered at a dose of 30 mg/kg, p. o. consecutively for 3 days clearly showed gastric antral ulcer. The oral administration of MI-AQE showed a significant protection against naproxen-induced gastric antral ulcer. A 30 mg/kg dose of naproxen for 3 consecutive days decreased SOD, GSH and NO as well as increased MDA, MPO, histamine level dramatically. It is widely accepted that a major underlying factor of this disorder is the generation of free radicals. There is substantial evidence that oxygen derived free radicals play an important role in the pathogenesis of the injury of various tissues, including the digestive system[38,39]. SOD and GSH are the important endogenous antioxidant enzyme playing an important role in protecting gastric mucosal tissue against oxidative damage. SODs superoxide radical to form hydrogen peroxide and water[40]. GSH is also an important inhibitor of free radical mediated lipid peroxidation[41,42]. MI-AQE resulted in a significant increase in the stomach SOD, reduced GSH levels as compared to the control animals.
Nitrate and nitrite are hallmarks of endogenous produced potential antioxidant. NO is a potent chain-breaking antioxidant in free radical-mediated lipid peroxidation[43-45]. It reported that gastric mucosal NO activity in the ulcerated region decreased with development of naproxen induced chronic ulcer in rats, although MI-AQE that decreased activity is gradually recovered with healing of gastric ulcer. Lipid peroxidation is a free radical mediated process, which has been implicated in a variety of disease states. It involves the formation and propagation of lipid radicals, the uptake of oxygen and rearrangement of double bonds in unsaturated fatty acids. Therefore it is not surprising that membrane lipids are susceptible to peroxidative attack[46-48]. MI-AQE significantly attenuates the elevated lipid peroxidation level in naproxen induced ulcer, which reveals its gastroprotective effect.
MPO is an enzyme present in neutrophils and at a much lower concentration in monocytes and macrophages. The level of MPO activity is directly proportional to the neutrophil concentration in the inflamed tissue[49-52]. In the present study, rat with daily oral administration of MIAQE showed both decreased gastric mucosal MPO activity, an index of tissue neutrophil infiltration and increased gastric mucosal adherent mucus content in ulcerated and intact regions at the healing stage of naproxen induced chronic ulcer when compared with rats without MI-AQE administration.
Oral administration of MI-AQE at all the three doses did not bring about any significant change in histamine content.
Plant extracts are the important sources for the new drug development due to their greater safety and high antioxidant composition. The medicinal properties of many plants are attributed mainly to the presence of flavonoids, coumarins, alkaloids, gaycoside, terpenoids, tannins, phenolic acids. Accordingly, the new teracyclic triterpenoid 19-(10-9β)-abeo-10αlanost-5-ene isolated from seeds of Cucurbita pepo showed maximum antioxidant as well as anti-ulcerogenic activity[53]. The main sesquiterpene derivatives from the aerial part of Fabiana imbricate plant was identified as 11-hydroxy-4-amorphen-15-oic acid showed gastroprotective effect[54]. The plant of Utleria salicifolia rhizome extract contains terpenoids exhibit anti-ulcer and antioxidant activity[55]. The main terpenoid from acetone extract of ginger showed anti-ulcer activity[56].
Myricetin-3-O-α-rahamnopyranoside, quercetin-3-O-α-L-arabinopyranoside, quercetin-3-O-β-D-galactopyranoside isolated from methanolic extract of Alchornea glandulosa leaves showed antiulcerogenic effect[57]. Aerial part of Bermuda grass herb was reported to contain flavonoids. The preliminary phytochemical investigation of the alcoholic extract of Bermuda grass showed the presence of flavonoids, which may be responsible for antiulcer activity[58]. Ficus religosa with phytoconstituents - saponin, flavonoids, steroids, terpenoids and cardiac glycosides was reported to have antiulcer activity[59].
Anti-ulcer activity of plant of M. indica could be linked to the presence of quercetin, myricitrin, triterpenoid, β-sitosterol and quercitrin. It is well known that many flavonoids, terpenoid display anti-secretory and cytoprotective properties in different experimental models of gastric ulcer[60]. Flavonoid possesses anti-ulcer properties in addition to strengthening the mucosal defence system through stimulation of gastric mucus secretion[61]. Flavonoids also protect ulcer development by improving microcirculation and increased capillary resistance, in turn, increasing gastric defensive factors[62].
In conclusion, the administration of naproxen (30 mg/kg) to rats proved to be a reliable and relevant method forevaluating NSAID-induced gastric antral ulceration. MIAQE showed a protective effect on naproxen induced gastric antral ulcer in a dose dependent manner. Our result also suggest that additional improvement of gastric mucosal function by elevating SOD, GSH and NO content, alleviation of inflammation by decreasing the level of MDA and MPO.
Therefore, we postulated that flavonoid present in the plant of M. indica leaves may correlate appropriately for the present activities. However, detailed study like isolation and identification of bioactive compound(s) are required to confirm the bioactive compound(s) responsible for the activity.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like acknowledge Dr. S. S. Kadam, Vice-Chancellor and Dr. K. R. Mahadik, Principal, Poona College Pharmacy, Bharati Vidyapeeth University, Pune, India, for providing necessary facilities to carry out the study. The authors are thankful to the Dr. A. S. Upadhye, Department of Botany, Agharkar Research Institute, Pune, India, for identification and authentication of plant. We are also thankful to the Council of Scientific industrial Research (CSIR) for providing financial support for the research work.
[1] Thirunavukkarasu P, Ramkumar L, Ramanathan T. Anti-ulcer activity of Excoecaria agallocha bark on NSAID-induced gastric ulcer in albino rats. Global J Pharmacol 2009; 3(3): 123-126.
[2] Ghosh P, Kandhare AD, Gauba D, Raygude KS, Bodhankar SL. Determination of efficacy, adverse drug reactions and cost effectiveness of three triple drug regimens for the treatment of H. pylori infected acid peptic disease patients. Asian Pac J Trop Dis 2012; 2: S783-S789.
[3] Ghosh P, Kandhare AD, V. Shiva K, Rajmane AR, Mohammad A, Bodhankar SL. Determination of clinical outcome and pharmacoeconomics of anti-rheumatoid arthritis therapy using CDAI, EQ-5D-3L and EQ-VAS as indices of disease amelioration. Asian Pac J Trop Dis 2012: 2: S671-S678.
[4] Soll AH, Weinstein WM, Kurata J, McCarthy D. Nonsteroidal antiinflammatory drug and peptic ulcer disease. Ann Intern Med 1991; 114: 307-319.
[5] Wallace JL. Nonsteroidal anti-inflammatory drug and gastroenteropathy: The second hundred years. Gastroenterology 1997; 112: 1000-1016.
[6] Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N Engl J Med 1999; 340(24): 1888-1899.
[7] Raygude KS, Kandhare AD, Ghosh P, Gosavi TP, Bodhankar SL. Consumption of alcohol and H. pylori infection: A cumulative meta-analysis of literature. Asian J Biochem Pharm Res 2011; 3(1): 338-345.
[8] Miedrer SE. Will anti-ulcer drugs soon differ only in their side effects? Fortschr Med 1986; 104: 918-920.
[9] Chan FK, Leung WK. Peptic ulcer disease. Lancet 2002; 360: 933-941.
[10] Dhuley JN. Anti-oxidant effect of cinnamon (Cinnamomum verum) bark and greater cardamom (Amomum subulatum) seeds in rats fed with high fat diet. Ind J Exp Biol 1999; 37: 238-242.
[11] Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacol 2012; 20(6): 331-341.
[12] Patil MVK, Kandhare AD, Bhise SD. Effect of aqueous extract of Cucumis sativus Linn. fruit in ulcerative colitis in laboratory animals. Asian Pac J Trop Biomed 2012; 2: S962-S969.
[13] Patil MK, Kandhare AD, Bhise SD. Pharmacological evaluation of ameliorative effect of aqueous extract of Cucumis sativus L. fruit formulation on wound healing in Wistar rats. Chron Young Sci 2011; 2: 207-213.
[14] Kandhare AD, V. Shiva K, Mohammad A, Rajmane AR, Ghosh P, Bodhankar SL. Investigation of gastro protective activity of Xanthium strumarium L. by modulation of cellular and biochemical marker. Orient Pharm Exp Med 2012; 12(4): 287-299.
[15] Kandhare AD, Raygude KS, Ghosh P, Bodhankar SL. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int J Green Pharm 2011; 5: 236-243.
[16] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin induced diabetic neuropathy in rats. Biomed Aging Pathol 2012; 2: 173-186.
[17] Mun Fei Yam, Rusliza B, Mohd ZA, Rosidah, Mariam A, Gabriel AA. Antioxidant and hepatoprotective activities of Elephantopus tomentous ethanol extract. Pharm Biol 2008; 46(3): 199-206.
[18] Ghani A. Medicinal Plants of Bangladesh, 1st ed. Dhaka, Bangladesh: The Asiatic Society of Bangladesh; 1998, p. 134-135, 202-204.
[19] Kirtikar KR, Basu BD. In: Indian medicinal plants, 2nd ed, Dehradun, India: International Book Distributors and Book sellers; 1987, p. 350-353.
[20] Nadkarni KM. Indian Materia Medica, 3rd edition. Bombay, India: Popular Books; 1954, p. 253-56.
[21] Subramanian SS, Nair AG. Sapotacea. Myricetin and quercetin glycosides from the leaves of four sapotaceous plants. Curr Sci 1973; 42: 746.
[22] Chatterjee A, Pakrashi SC. The treatise on indian medicinal plants. 1993; 4: 56-58.
[23] Siddiqui BS, Khan S, Nadeem Kardar M. A new isoflavone from the fruits of Madhuca latifolia. Nat Prod Res 2010; 24(1): 76-80.
[24] Lanza FL. Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal antiinflammatory agents. Am J Med 1984; 13: 19-24.
[25] Beck WS, Schneider HT, Dietzel K, Nuernberg B, Brune K. Gastrointestinal ulcerations induced by anti-inflammatory drugs in rats. Arch Toxicol 1990; 64: 210-217.
[26] Mishra HP, Fridovich I. The role of superoxide anion in the autooxidation of Epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972; 247: 3170-3175.
[27] Moron MS, Depierre JW, Mannervik B. Level of glutathione, glutathione reductases and glutathione S-transferase activities inrat lung and liver. Biochimica et Biophysica ACTA 1979; 582: 67-78.
[28] Slater TF, Sawyer BC. The stimulatiory effect of carbon tetrachloride and other halogenoalkanes or peroxidative reaction in rat liver fraction in vitro. Biochem J 1971; 123: 805-814.
[29] Krawize JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroentrology 1984; 87: 1344-1350.
[30] Lowry OH, Rosenbrough NJ, Farr AC, Randell RJ. Protein measurement with folin-phenol reagent. J Biol Chem 1951; 193: 265-275.
[31] Miranda K, Espy MG, Wink DA. A rapid and simple spectrophotometric method for simultaneous method detection of nitrate and nitrite. Nitric Oxide 2001; 5: 62-71.
[32] Patange SB, Mukundan MK, Ashok Kumar K. A simple and rapid method for colorimetric determination of histamine in fish flesh. Food Control 2005; 16: 465-472.
[33] Halter F, Schassman A. Cyclooxygenase 2-Implaication on maintenance of gastric mucosal integrity and ulcer healing: controversial issue and perspectives. Gut 2001; 49: 443-453.
[34] Roth SH, Bennett RE. Non-steriodal anti-inflammatory drug gastropathy: recognition and response. Arch Intern Med 1987; 147: 2093-2100.
[35] McCarthy DM. NSAIDs gastro-intestinal damage-acritical review of prophylaxis and therapy. J Clin Gastroenterol 1990; 12: S13-S20.
[36] McCarthy DM. Mechanism of mucosal injury and healing: the role of nonsteriodal anti-inflammatory drugs. Scand J Gastroenerol 1995; 208: 24-29.
[37] Kim HJ, Kim SY, Song GG, Park JJ. Chang H. Protective effect of astaxnthin on naproxen induced gastric anteral ulceration in rats. Eur J Pahrmcol 2005; 514: 53-59.
[38] Rastogi L, Patnaik GK, Dikshit M. Free radicals and antioxidant status following pylorus ligation induced gastric mucosal injury in rats. Pharmacol Res 1998; 38: 125-132.
[39] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2012; 83: 650-659.
[40] Hemnani T, Parihar MS. Reactive oxygen species and oxidative damage. Indian J Physiol Pharmacol 1998; 42: 440-452.
[41] Meistor A. Selective modification of glutathione metabolism. Science 1983; 220: 472-477.
[42] Gosavi TP, Ghosh P, Kandhare AD, V. Shiva K, Mohammad A, Rajmane AR, et al. Therapeutic effect of H. pylori nosode in healing of chronic H. pylori infected ulcers in laboratory animals. Asian Pac J Trop Dis 2012; 2: S603-S611.
[43] Slomiany BL, Pitorowski J, Slomiany A. Role of basic fibroblast growth factor in the suppression of aptotic caspase-3 during chronic gastric ulcer healing. J Physiol Pharmacol 1998; 49: 489-500.
[44] Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol induced neuropathic pain. Fund Clin Pharmacol 2012; DOI: 10.1111/fcp.12003.
[45] Patil MVK, Kandhare AD, Bhise SD. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci 2012; 4(1): 337-343.
[46] Cheesman KH. Lipid peroxidation in biological system. In: Halliwell B, Aruoma OI (Eds.), DNA and free radicals. London: Ellis Horwood; 1993, p. 12-17.
[47] Visnagri A, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of ameliorative effect of Co-enzyme Q10 in streptozotocin induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol 2012; 2: 157-172.
[48] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Ameliorative effect of aqueous extract of Phyllanthus amarus in acetic acid induced ulcerative colitis in laboratory animals. Apollo Medicine 2013; 10: 87-97.
[49] Fabia R, Rajab AR, Willen R, Marklunt S, Andersson R. The role of transient mucosal ischemia in acetic acid-induced colitis in the rat. J Surg Res 1996; 63: 406-412.
[50] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Gosavi TP, Badole SL, et al. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed 2012; 5: 337-344.
[51] Patil MVK, Kandhare AD, Bhise SD. Pharmacological evaluation of ethanolic extract of Daucus carota Linn root formulated cream on wound healing using excision and incision wound model. Asian Pac J Trop Biomed 2012; 2: S646-S655.
[52] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett 2012; 511: 18-22.
[53] Gill NS, Bali M. Isolation of anti ulcer cucurbitane type of triterpenoid from the seeds of Cucurbita pepo. Res J Phytochem 2011: 5: 1-10.
[54] Reyes M, Schmeda-Hirschmann G, Razmilic I, Theoduloz C, Yáñez T, Rodríguez JA. Gastroprotective activity of sesquiterpene derivatives from Fabiana imbricata. Phytother Res 2005; 19(12): 1038-1042.
[55] Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, et al. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol 2004; 91(2-3): 243-249.
[56] Yamahara J, Mochizuki M, Rong HQ, Matsuda H, Fujimura H. The anti-ulcer effect in rats of ginger constituents. J Ethnopharmacol 1988; 23(2-3): 299-304.
[57] Calvo TR, Lima ZP, Silva JS, Ballesteros KV, Pellizzon CH, Hiruma-Lima CA, et al. Constituents and antiulcer effect of Alchornea glandulosa: activation of cell proliferation in gastric mucosa during the healing process. Biol Pharm Bull 2007; 30(3): 451-459.
[58] Nair GA. Seminar in Ayurveda and Siddha. J Ethnopharmacol 1995; (57): 20-22
[59] Khan M SA, Hussain SA, Jais A MM, Zakaria ZA, Khan M. Antiulcer activity of Ficus religosa stem bark of ethanolic extract in rats. J Med Plants Res 2011; 5(3): 354-359.
[60] Zayachkivska OS, Konturek SJ, Drozdowicz D, Konturek PC, Brzozowaski T, Chegotsky MR. Gastroprotective effects of flavonoids in plant extracts. J Physiol Pharmacol 2005; 56: 219-231.
[61] Martin MJ, Marhuenda E, Perez-Guerrero C, Franco JM. Antiulcer effect of naringin on gastric lesion induced by ethanol in rats. Pharmacology 1994; 49: 144-150.
[62] Gonzaler FG, Di Stasi LC. Anti-ulcerogenic and analgesic activites of the leaves of Wilbrandia ebracteata in mice. Phytomedicine 2002; 9(2): 125-134.
21 February 2013
*Corresponding author: Dr. S. L. Bodhankar, Professor & Head, Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Erandwane, Pune-411 038, Maharashtra, India.
Tel: +91-20- 25437237
Fax: +91-20-25231831
E-mail: sbodh@yahoo.com
ARTICLE INFO
Article history:
Received in revised form 26 February 2013
Accepted 15 March 2013
Available online 20 June 2013
 Journal of Acute Disease2013年2期
Journal of Acute Disease2013年2期
- Journal of Acute Disease的其它文章
- Development of bioanalytical parameters for the standardization of Zingiber officinale
- Medicinal significance, pharmacological activities, and analytical aspects of solasodine: A concise report of current scientific literature
- A review on antimicrobial efficacy of some traditional medicinal plants in Tamilnadu
- Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed
- Anti-inflammatory activity of leaves of Jatropha gossypifolia L. by hrbc membrane stabilization method
- An overview of the current methodologies used for evaluation of aphrodisiac agents
